User login
European Society of Cardiology (ESC): Annual Congress
NHLBI hands off hypertension guidelines to ACC, AHA
The two U.S. groups most active in issuing guidelines and recommendations for cardiovascular disease diagnosis and management, the American College of Cardiology and American Heart Association, received a surprise in June when the National Heart, Lung, and Blood Institute suddenly announced that it would shift to these and other "partner organizations" primary responsibility for the next updates of U.S. hypertension guidelines, national cholesterol-management guidelines, and the other cardiovascular disease–related management recommendations that the institute has had in the works.
The NHLBI launched "a collaborative relationship with the ACC, AHA, and other organizations because they said they are not in a position to endorse guidelines, they must be endorsed by other organizations," said Dr. Sidney C. Smith Jr., professor of medicine at the University of North Carolina in Chapel Hill. Dr. Smith is a member of the panel that’s been writing the Eighth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8), and has been active for a long time in the ACC and AHA guidelines-development process.
On June 19, Dr. Gary H. Gibbons, NHLBI director, and his associates announced that effective immediately the institute was getting out of the guidelines-issuing business (Circulation 2013; doi: 10.1161/CIRCULATIONAHA.113.004587).
"Just over the past couple of months we began to look at how this will be done. Everyone wants the process to move quickly. How quickly can these organizations put it together? That’s the limiting factor right now," Dr. Smith said in an interview in early September.
While the ACC and AHA have on record some 20 sets of practice guidelines that cover most facets of cardiology, their list omits areas that the NHLBI covered in the past, notably hypertension and hypercholesterolemia assessment and management.
"The ACC and AHA guideline process is very expensive, and we wouldn’t dream of duplicating something when people you trust were commissioned by someone else [NHLBI] to do the work," said Dr. Kim Allan Williams Sr. of Wayne State University, Detroit. Dr. Williams will take the position of professor of medicine and chief of cardiovascular services at Rush University Medical Center in Chicago on Nov. 1. He serves as vice-president of the ACC. "We have all been under the impression that JNC 8 was being put together and getting published soon," he said in an interview.
Dr. Williams stressed that he and other ACC officials have pledged not to talk about the JNC 8 process until transition from the NHLBI works itself out, but he offered this succinct observation: The ACC "has made a commitment to go forward with the JNC process. There will be a publication from that panel, although it may not have that name."
Dr. Smith and Dr. Williams said that they had no relevant disclosures.
On Twitter @mitchelzoler
The two U.S. groups most active in issuing guidelines and recommendations for cardiovascular disease diagnosis and management, the American College of Cardiology and American Heart Association, received a surprise in June when the National Heart, Lung, and Blood Institute suddenly announced that it would shift to these and other "partner organizations" primary responsibility for the next updates of U.S. hypertension guidelines, national cholesterol-management guidelines, and the other cardiovascular disease–related management recommendations that the institute has had in the works.
The NHLBI launched "a collaborative relationship with the ACC, AHA, and other organizations because they said they are not in a position to endorse guidelines, they must be endorsed by other organizations," said Dr. Sidney C. Smith Jr., professor of medicine at the University of North Carolina in Chapel Hill. Dr. Smith is a member of the panel that’s been writing the Eighth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8), and has been active for a long time in the ACC and AHA guidelines-development process.
On June 19, Dr. Gary H. Gibbons, NHLBI director, and his associates announced that effective immediately the institute was getting out of the guidelines-issuing business (Circulation 2013; doi: 10.1161/CIRCULATIONAHA.113.004587).
"Just over the past couple of months we began to look at how this will be done. Everyone wants the process to move quickly. How quickly can these organizations put it together? That’s the limiting factor right now," Dr. Smith said in an interview in early September.
While the ACC and AHA have on record some 20 sets of practice guidelines that cover most facets of cardiology, their list omits areas that the NHLBI covered in the past, notably hypertension and hypercholesterolemia assessment and management.
"The ACC and AHA guideline process is very expensive, and we wouldn’t dream of duplicating something when people you trust were commissioned by someone else [NHLBI] to do the work," said Dr. Kim Allan Williams Sr. of Wayne State University, Detroit. Dr. Williams will take the position of professor of medicine and chief of cardiovascular services at Rush University Medical Center in Chicago on Nov. 1. He serves as vice-president of the ACC. "We have all been under the impression that JNC 8 was being put together and getting published soon," he said in an interview.
Dr. Williams stressed that he and other ACC officials have pledged not to talk about the JNC 8 process until transition from the NHLBI works itself out, but he offered this succinct observation: The ACC "has made a commitment to go forward with the JNC process. There will be a publication from that panel, although it may not have that name."
Dr. Smith and Dr. Williams said that they had no relevant disclosures.
On Twitter @mitchelzoler
The two U.S. groups most active in issuing guidelines and recommendations for cardiovascular disease diagnosis and management, the American College of Cardiology and American Heart Association, received a surprise in June when the National Heart, Lung, and Blood Institute suddenly announced that it would shift to these and other "partner organizations" primary responsibility for the next updates of U.S. hypertension guidelines, national cholesterol-management guidelines, and the other cardiovascular disease–related management recommendations that the institute has had in the works.
The NHLBI launched "a collaborative relationship with the ACC, AHA, and other organizations because they said they are not in a position to endorse guidelines, they must be endorsed by other organizations," said Dr. Sidney C. Smith Jr., professor of medicine at the University of North Carolina in Chapel Hill. Dr. Smith is a member of the panel that’s been writing the Eighth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8), and has been active for a long time in the ACC and AHA guidelines-development process.
On June 19, Dr. Gary H. Gibbons, NHLBI director, and his associates announced that effective immediately the institute was getting out of the guidelines-issuing business (Circulation 2013; doi: 10.1161/CIRCULATIONAHA.113.004587).
"Just over the past couple of months we began to look at how this will be done. Everyone wants the process to move quickly. How quickly can these organizations put it together? That’s the limiting factor right now," Dr. Smith said in an interview in early September.
While the ACC and AHA have on record some 20 sets of practice guidelines that cover most facets of cardiology, their list omits areas that the NHLBI covered in the past, notably hypertension and hypercholesterolemia assessment and management.
"The ACC and AHA guideline process is very expensive, and we wouldn’t dream of duplicating something when people you trust were commissioned by someone else [NHLBI] to do the work," said Dr. Kim Allan Williams Sr. of Wayne State University, Detroit. Dr. Williams will take the position of professor of medicine and chief of cardiovascular services at Rush University Medical Center in Chicago on Nov. 1. He serves as vice-president of the ACC. "We have all been under the impression that JNC 8 was being put together and getting published soon," he said in an interview.
Dr. Williams stressed that he and other ACC officials have pledged not to talk about the JNC 8 process until transition from the NHLBI works itself out, but he offered this succinct observation: The ACC "has made a commitment to go forward with the JNC process. There will be a publication from that panel, although it may not have that name."
Dr. Smith and Dr. Williams said that they had no relevant disclosures.
On Twitter @mitchelzoler
Inherently low triglycerides may lower mortality
AMSTERDAM – People with congenitally reduced triglyceride levels had about a 20% reduced risk of all-cause death compared with people without inherently low triglyceride levels during more than 30-years follow-up of nearly 14,000 people enrolled in the Copenhagen City Heart Study.
Data from the study also showed that people with higher triglyceride (TG) levels at the time they entered the study had significantly higher all-cause mortality during more than 30 years of follow-up than did people who entered with lower TG levels, Dr. Børge G. Nordestgaard said at the European Society of Cardiology annual congress.
But the result from the genetic analysis provided even more persuasive evidence that it is time to run a large, prospective intervention trial aimed at testing the preventive efficacy of TG lowering in patients selected based on elevated TGs, he said.
The genetic analysis "took advantage of nature’s randomized trial," said Dr. Nordestgaard, professor and chief of clinical biochemistry at Copenhagen University Hospital. The genetic analysis looked at long-term survival relative to the number of TG-lowering alleles each person had in their lipoprotein lipase genes. "These data suggest a causal relationship between low TGs and improved survival. Lots of drugs are available to lower triglycerides, such as atorvastatin and rosuvastatin, but so far all the trials only focused on high LDL or low HDL cholesterol. No trial enrolled patients on the basis of high triglycerides.
"A lot of people have normal LDL levels and high TGs, and right now they are ignored," he said. "Before statins, everyone talked about high LDL and high TGs as being equal risk factors, but now TGs are completely forgotten. A big mistake has been interpreting results from the statin trials, because the participants in those studies did not enter the trials because of their TG levels."
Dr. Nordestgaard and his associates performed an analysis of the hazard ratio for all-cause death among 13,957 people enrolled in the Copenhagen study. Compared with people who entered the study with a nonfasting serum triglyceride level of 266-442 mg/dL, 8% of everyone enrolled, those with a level of 177-265 mg/dL, 17% of enrollees, had an all-cause mortality rate that was relatively reduced by 10%, a statistically significant difference. People who entered with a TG level of 89-176 mg/dL, about half of the enrollees, had their relative mortality rate cut by 25% compared with the reference group, and those who entered with a level of less than 89 mg/dL, 22% of the enrollees, had a mortality rate that ran half that of the reference group, also statistically significant differences.
To better document an effect from reduced TG levels, the investigators used data that had been collected on six different genetic alleles in the gene coding for lipoprotein lipase linked to reduced TG levels. Data on allele numbers were available for 10,208 of the study participants, and those with 0-3 of the alleles were used as the reference group. People with four, five, or six of these alleles showed progressively larger drops in TG level; those with 4 of the alleles had a level about 10% below the reference group, those with five alleles were about 20% below, and those with six alleles had a TG level that averaged 30% below the reference group.
Mortality during follow-up tracked with TG levels at baseline and the number of TG-lowering alleles. People with 4 alleles had a mortality rate 15% below the rate among those with 0-3 alleles. People with five TG-lowering alleles had a 20% cut in mortality, and those with six alleles had a 22% reduction.
Dr. Nordestgaard said that he has been a consultant to 11 drug companies, including AstraZeneca, Merck, and Pfizer.
On Twitter @mitchelzoler
AMSTERDAM – People with congenitally reduced triglyceride levels had about a 20% reduced risk of all-cause death compared with people without inherently low triglyceride levels during more than 30-years follow-up of nearly 14,000 people enrolled in the Copenhagen City Heart Study.
Data from the study also showed that people with higher triglyceride (TG) levels at the time they entered the study had significantly higher all-cause mortality during more than 30 years of follow-up than did people who entered with lower TG levels, Dr. Børge G. Nordestgaard said at the European Society of Cardiology annual congress.
But the result from the genetic analysis provided even more persuasive evidence that it is time to run a large, prospective intervention trial aimed at testing the preventive efficacy of TG lowering in patients selected based on elevated TGs, he said.
The genetic analysis "took advantage of nature’s randomized trial," said Dr. Nordestgaard, professor and chief of clinical biochemistry at Copenhagen University Hospital. The genetic analysis looked at long-term survival relative to the number of TG-lowering alleles each person had in their lipoprotein lipase genes. "These data suggest a causal relationship between low TGs and improved survival. Lots of drugs are available to lower triglycerides, such as atorvastatin and rosuvastatin, but so far all the trials only focused on high LDL or low HDL cholesterol. No trial enrolled patients on the basis of high triglycerides.
"A lot of people have normal LDL levels and high TGs, and right now they are ignored," he said. "Before statins, everyone talked about high LDL and high TGs as being equal risk factors, but now TGs are completely forgotten. A big mistake has been interpreting results from the statin trials, because the participants in those studies did not enter the trials because of their TG levels."
Dr. Nordestgaard and his associates performed an analysis of the hazard ratio for all-cause death among 13,957 people enrolled in the Copenhagen study. Compared with people who entered the study with a nonfasting serum triglyceride level of 266-442 mg/dL, 8% of everyone enrolled, those with a level of 177-265 mg/dL, 17% of enrollees, had an all-cause mortality rate that was relatively reduced by 10%, a statistically significant difference. People who entered with a TG level of 89-176 mg/dL, about half of the enrollees, had their relative mortality rate cut by 25% compared with the reference group, and those who entered with a level of less than 89 mg/dL, 22% of the enrollees, had a mortality rate that ran half that of the reference group, also statistically significant differences.
To better document an effect from reduced TG levels, the investigators used data that had been collected on six different genetic alleles in the gene coding for lipoprotein lipase linked to reduced TG levels. Data on allele numbers were available for 10,208 of the study participants, and those with 0-3 of the alleles were used as the reference group. People with four, five, or six of these alleles showed progressively larger drops in TG level; those with 4 of the alleles had a level about 10% below the reference group, those with five alleles were about 20% below, and those with six alleles had a TG level that averaged 30% below the reference group.
Mortality during follow-up tracked with TG levels at baseline and the number of TG-lowering alleles. People with 4 alleles had a mortality rate 15% below the rate among those with 0-3 alleles. People with five TG-lowering alleles had a 20% cut in mortality, and those with six alleles had a 22% reduction.
Dr. Nordestgaard said that he has been a consultant to 11 drug companies, including AstraZeneca, Merck, and Pfizer.
On Twitter @mitchelzoler
AMSTERDAM – People with congenitally reduced triglyceride levels had about a 20% reduced risk of all-cause death compared with people without inherently low triglyceride levels during more than 30-years follow-up of nearly 14,000 people enrolled in the Copenhagen City Heart Study.
Data from the study also showed that people with higher triglyceride (TG) levels at the time they entered the study had significantly higher all-cause mortality during more than 30 years of follow-up than did people who entered with lower TG levels, Dr. Børge G. Nordestgaard said at the European Society of Cardiology annual congress.
But the result from the genetic analysis provided even more persuasive evidence that it is time to run a large, prospective intervention trial aimed at testing the preventive efficacy of TG lowering in patients selected based on elevated TGs, he said.
The genetic analysis "took advantage of nature’s randomized trial," said Dr. Nordestgaard, professor and chief of clinical biochemistry at Copenhagen University Hospital. The genetic analysis looked at long-term survival relative to the number of TG-lowering alleles each person had in their lipoprotein lipase genes. "These data suggest a causal relationship between low TGs and improved survival. Lots of drugs are available to lower triglycerides, such as atorvastatin and rosuvastatin, but so far all the trials only focused on high LDL or low HDL cholesterol. No trial enrolled patients on the basis of high triglycerides.
"A lot of people have normal LDL levels and high TGs, and right now they are ignored," he said. "Before statins, everyone talked about high LDL and high TGs as being equal risk factors, but now TGs are completely forgotten. A big mistake has been interpreting results from the statin trials, because the participants in those studies did not enter the trials because of their TG levels."
Dr. Nordestgaard and his associates performed an analysis of the hazard ratio for all-cause death among 13,957 people enrolled in the Copenhagen study. Compared with people who entered the study with a nonfasting serum triglyceride level of 266-442 mg/dL, 8% of everyone enrolled, those with a level of 177-265 mg/dL, 17% of enrollees, had an all-cause mortality rate that was relatively reduced by 10%, a statistically significant difference. People who entered with a TG level of 89-176 mg/dL, about half of the enrollees, had their relative mortality rate cut by 25% compared with the reference group, and those who entered with a level of less than 89 mg/dL, 22% of the enrollees, had a mortality rate that ran half that of the reference group, also statistically significant differences.
To better document an effect from reduced TG levels, the investigators used data that had been collected on six different genetic alleles in the gene coding for lipoprotein lipase linked to reduced TG levels. Data on allele numbers were available for 10,208 of the study participants, and those with 0-3 of the alleles were used as the reference group. People with four, five, or six of these alleles showed progressively larger drops in TG level; those with 4 of the alleles had a level about 10% below the reference group, those with five alleles were about 20% below, and those with six alleles had a TG level that averaged 30% below the reference group.
Mortality during follow-up tracked with TG levels at baseline and the number of TG-lowering alleles. People with 4 alleles had a mortality rate 15% below the rate among those with 0-3 alleles. People with five TG-lowering alleles had a 20% cut in mortality, and those with six alleles had a 22% reduction.
Dr. Nordestgaard said that he has been a consultant to 11 drug companies, including AstraZeneca, Merck, and Pfizer.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2013
Major finding:. People with five triglyceride-lowering alleles had 20% fewer long-term deaths compared with those who carried 0-3 alleles.
Data source: The Copenhagen City Heart Study, which enrolled 13,957 people in 1976-1978 and followed them for more than 30 years.
Disclosures: Dr. Nordestgaard said that he has been a consultant to 11 drug companies, including AstraZeneca, Merck, and Pfizer.
Study hints at obesity paradox in older women with coronary artery disease
AMSTERDAM – Older women with coronary artery disease who lost weight had an increased risk of death regardless of their body mass index, a finding that hints at the presence of an obesity paradox in this population, according to a registry-based retrospective study.
The unpublished abstract, which was presented at the annual congress of the European Society of Cardiology, also showed that, among patients who maintained their weight, underweight women had a twofold increase in risk of all-cause mortality while obese and overweight women had a nonsignificant but reduced risk of death, compared with normal-weight patients.
Although studies have shown that obesity is a risk factor for developing cardiovascular disease, and guidelines recommend weight loss for patients who are overweight and obese, the study’s key message is that advising weight loss to patients with heart disease is not so simple and straightforward, said Dr. Diethelm Tschoepe of Ruhr University in Bochum, Germany, who commented on the study.
Researchers analyzed data for 1,685 women with an average age of 64 years who were diagnosed with coronary artery disease (CAD) based on angiography between 2005 and 2011.
They stratified the cohort into three weight-change groups: no change (gain or loss of less than 2 kg/year), weight loss (loss of more than 2 kg/year), and weight gain (gain of more than 2 kg/year.)
Each group was then divided into four weight classes based on body mass index: underweight (BMI less than 20 kg/m2), normal weight (BMI 20-24.9 kg/m2), overweight (BMI 25-29.9 kg/m2), and obese (BMI more than 30 kg/m2).
The normal-weight group was used as reference to calculate the hazard ratios. Data were adjusted for age, smoking, diabetes, previous heart surgery, use of statins and antihypertensive drugs, degree of CAD, and previous percutaneous coronary intervention.
When comparing the BMI in weight-change groups, women who were underweight and maintained their weight had a twofold increase in death (hazard ratio, 2.15; P = .03), compared with normal-weight women. Also, when these women lost weight, their risk was increased by almost twofold, although it didn’t reach statistical significance.
Among the different BMI groups, obese women who lost weight had an eightfold increase in the risk of all-cause mortality, and obese women who gained weight had a more than fourfold risk increase, compared with obese women who maintained their weight.
Meanwhile, overweight and normal-weight women who lost weight had sixfold and threefold increases in risk of death, respectively, compared with their stable-weight counterparts.
Dr. Aziza Azimi of Gentofte Hospital, Copenhagen, who presented the study, said that the findings highlight the importance of individual-based weight management.
Researchers said that, since the study subjects had comorbidities, their weight management should be handled differently from the general population. They added that, because of the retrospective design of the study, it’s not clear why the women lost weight, although, given their condition, it was most probably unintentional and due to comorbidities or medications.
Dr. Azimi and Dr. Tschoepe had no disclosures.
On Twitter @NaseemSMiller
AMSTERDAM – Older women with coronary artery disease who lost weight had an increased risk of death regardless of their body mass index, a finding that hints at the presence of an obesity paradox in this population, according to a registry-based retrospective study.
The unpublished abstract, which was presented at the annual congress of the European Society of Cardiology, also showed that, among patients who maintained their weight, underweight women had a twofold increase in risk of all-cause mortality while obese and overweight women had a nonsignificant but reduced risk of death, compared with normal-weight patients.
Although studies have shown that obesity is a risk factor for developing cardiovascular disease, and guidelines recommend weight loss for patients who are overweight and obese, the study’s key message is that advising weight loss to patients with heart disease is not so simple and straightforward, said Dr. Diethelm Tschoepe of Ruhr University in Bochum, Germany, who commented on the study.
Researchers analyzed data for 1,685 women with an average age of 64 years who were diagnosed with coronary artery disease (CAD) based on angiography between 2005 and 2011.
They stratified the cohort into three weight-change groups: no change (gain or loss of less than 2 kg/year), weight loss (loss of more than 2 kg/year), and weight gain (gain of more than 2 kg/year.)
Each group was then divided into four weight classes based on body mass index: underweight (BMI less than 20 kg/m2), normal weight (BMI 20-24.9 kg/m2), overweight (BMI 25-29.9 kg/m2), and obese (BMI more than 30 kg/m2).
The normal-weight group was used as reference to calculate the hazard ratios. Data were adjusted for age, smoking, diabetes, previous heart surgery, use of statins and antihypertensive drugs, degree of CAD, and previous percutaneous coronary intervention.
When comparing the BMI in weight-change groups, women who were underweight and maintained their weight had a twofold increase in death (hazard ratio, 2.15; P = .03), compared with normal-weight women. Also, when these women lost weight, their risk was increased by almost twofold, although it didn’t reach statistical significance.
Among the different BMI groups, obese women who lost weight had an eightfold increase in the risk of all-cause mortality, and obese women who gained weight had a more than fourfold risk increase, compared with obese women who maintained their weight.
Meanwhile, overweight and normal-weight women who lost weight had sixfold and threefold increases in risk of death, respectively, compared with their stable-weight counterparts.
Dr. Aziza Azimi of Gentofte Hospital, Copenhagen, who presented the study, said that the findings highlight the importance of individual-based weight management.
Researchers said that, since the study subjects had comorbidities, their weight management should be handled differently from the general population. They added that, because of the retrospective design of the study, it’s not clear why the women lost weight, although, given their condition, it was most probably unintentional and due to comorbidities or medications.
Dr. Azimi and Dr. Tschoepe had no disclosures.
On Twitter @NaseemSMiller
AMSTERDAM – Older women with coronary artery disease who lost weight had an increased risk of death regardless of their body mass index, a finding that hints at the presence of an obesity paradox in this population, according to a registry-based retrospective study.
The unpublished abstract, which was presented at the annual congress of the European Society of Cardiology, also showed that, among patients who maintained their weight, underweight women had a twofold increase in risk of all-cause mortality while obese and overweight women had a nonsignificant but reduced risk of death, compared with normal-weight patients.
Although studies have shown that obesity is a risk factor for developing cardiovascular disease, and guidelines recommend weight loss for patients who are overweight and obese, the study’s key message is that advising weight loss to patients with heart disease is not so simple and straightforward, said Dr. Diethelm Tschoepe of Ruhr University in Bochum, Germany, who commented on the study.
Researchers analyzed data for 1,685 women with an average age of 64 years who were diagnosed with coronary artery disease (CAD) based on angiography between 2005 and 2011.
They stratified the cohort into three weight-change groups: no change (gain or loss of less than 2 kg/year), weight loss (loss of more than 2 kg/year), and weight gain (gain of more than 2 kg/year.)
Each group was then divided into four weight classes based on body mass index: underweight (BMI less than 20 kg/m2), normal weight (BMI 20-24.9 kg/m2), overweight (BMI 25-29.9 kg/m2), and obese (BMI more than 30 kg/m2).
The normal-weight group was used as reference to calculate the hazard ratios. Data were adjusted for age, smoking, diabetes, previous heart surgery, use of statins and antihypertensive drugs, degree of CAD, and previous percutaneous coronary intervention.
When comparing the BMI in weight-change groups, women who were underweight and maintained their weight had a twofold increase in death (hazard ratio, 2.15; P = .03), compared with normal-weight women. Also, when these women lost weight, their risk was increased by almost twofold, although it didn’t reach statistical significance.
Among the different BMI groups, obese women who lost weight had an eightfold increase in the risk of all-cause mortality, and obese women who gained weight had a more than fourfold risk increase, compared with obese women who maintained their weight.
Meanwhile, overweight and normal-weight women who lost weight had sixfold and threefold increases in risk of death, respectively, compared with their stable-weight counterparts.
Dr. Aziza Azimi of Gentofte Hospital, Copenhagen, who presented the study, said that the findings highlight the importance of individual-based weight management.
Researchers said that, since the study subjects had comorbidities, their weight management should be handled differently from the general population. They added that, because of the retrospective design of the study, it’s not clear why the women lost weight, although, given their condition, it was most probably unintentional and due to comorbidities or medications.
Dr. Azimi and Dr. Tschoepe had no disclosures.
On Twitter @NaseemSMiller
ESC CONGRESS 2013
Major finding: Women who were underweight and maintained their weight had a significant, 2.15-fold increase in mortality, compared with normal-weight women.
Data source: Danish registry data on 1,685 women with an average age of 64 years, diagnosed with CAD between 2005 and 2011.
Disclosures: Dr. Azimi and Dr. Tschoepe had no disclosures.
REGARDS study suggests optimal elderly SBP of 120-139 mm Hg
AMSTERDAM – The optimal level of systolic blood pressure in all patients above age 55 appears to be 120-139 mm Hg, according to controversial data from a large, observational, U.S. population–based cohort study.
Results from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study suggest that a systolic blood pressure (SBP) in that range is associated with the lowest rates of cardiovascular events, stroke, and all-cause mortality, even in individuals aged 75 years and up. Since REGARDS is an observational study, this proposed target SBP range should be viewed as a testable hypothesis worthy of confirmation in a large, randomized, antihypertensive treatment study in the elderly, Dr. Maciej Banach said at the annual congress of the European Society of Cardiology (ESC).
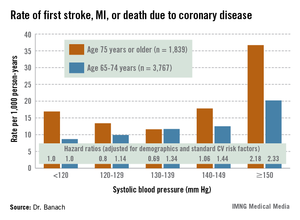
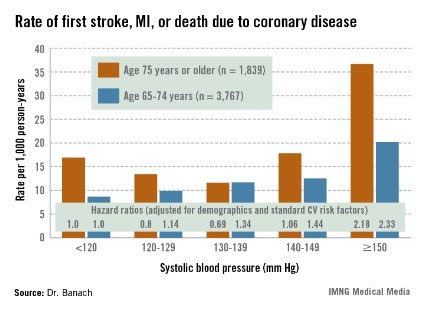
REGARDS also turned up evidence supportive of the existence of the long-controversial J-curve, with an increased rate of adverse events noted in subjects with an SBP below 120 mm Hg, added Dr. Banach, professor and head of the department of hypertension at the Medical University of Lodz (Poland).
But discussant Christi Deaton, Ph.D., was quick to slam on the brakes in response to Dr. Banach’s suggestion that 120-139 mm Hg looks like the new optimal in older patients. She noted that the REGARDS proposal is at odds with the latest ESC hypertension guidelines, issued just a couple of months ago.
The evidence-based ESC guidelines (J. Hypertens. 2013;31:1281-357) offer as a class I recommendation an SBP target of 140-150 mm Hg for elderly patients under age 80, with consideration of a target below 140 mm Hg for the subgroup of elderly patients who are fit, noted Dr. Deaton, professor of nursing at the University of Manchester (U.K.), and a member of the ESC Guidelines Committee.
The American College of Cardiology/American Heart Association guidelines, in contrast, recommend a target of less than 140/90 in 65- to 79-year-olds, and an SBP of 140-145 mm Hg, if tolerated, in those aged 80 years and up (Circulation 2011;123:2434-506).
"The hypothesis from this REGARDS analysis is a bold one," she observed. "But we need large, well-designed intervention trials in older patients, particularly in underrepresented groups, before we can set lower blood pressure targets than are currently recommended in our guidelines."
The REGARDS study is a National Institutes of Health–sponsored observational study of 30,329 U.S. subjects aged 45 years or older, with overrepresentation of blacks and residents of the nation’s southeastern "stroke belt." Dr. Banach presented an analysis of 13,948 REGARDS participants aged 55 years or older who were taking antihypertensive medications at baseline. They have thus far been followed for a median of 6 years for all-cause mortality, and slightly less for first occurrence of stroke or coronary heart disease.
The suggestion of a J-curve was strongest for the composite endpoint of a first stroke, nonfatal myocardial infarction (MI), or death due to coronary heart disease in subjects aged 75 years or older (see graphic). There was no significant relationship between stroke and SBP category for subjects under age 75; however, over age 75, stroke rates were highest in those with an SBP less than 120 or more than 150 mm Hg.
All-cause mortality showed a linear relationship with increasing SBP in subjects aged 55-74 years, but no relationship in those over age 75.
Dr. Deaton applauded the REGARDS investigators for focusing on a high-risk population that’s "certainly underrepresented in our clinical intervention trials," but she cited several major study limitations. One is that only baseline blood pressure measurements were available.
"Importantly," she observed, "we don’t know what happened to blood pressure control during these years of follow-up."
In addition, the incidence rates of stroke, MI, and other major adverse events were relatively low in some subgroups. The planned further follow-up in REGARDS should bring greater clarity, Dr. Deaton added.
Dr. Banach and Dr. Deaton reported having no financial conflicts of interest.
AMSTERDAM – The optimal level of systolic blood pressure in all patients above age 55 appears to be 120-139 mm Hg, according to controversial data from a large, observational, U.S. population–based cohort study.
Results from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study suggest that a systolic blood pressure (SBP) in that range is associated with the lowest rates of cardiovascular events, stroke, and all-cause mortality, even in individuals aged 75 years and up. Since REGARDS is an observational study, this proposed target SBP range should be viewed as a testable hypothesis worthy of confirmation in a large, randomized, antihypertensive treatment study in the elderly, Dr. Maciej Banach said at the annual congress of the European Society of Cardiology (ESC).

REGARDS also turned up evidence supportive of the existence of the long-controversial J-curve, with an increased rate of adverse events noted in subjects with an SBP below 120 mm Hg, added Dr. Banach, professor and head of the department of hypertension at the Medical University of Lodz (Poland).
But discussant Christi Deaton, Ph.D., was quick to slam on the brakes in response to Dr. Banach’s suggestion that 120-139 mm Hg looks like the new optimal in older patients. She noted that the REGARDS proposal is at odds with the latest ESC hypertension guidelines, issued just a couple of months ago.
The evidence-based ESC guidelines (J. Hypertens. 2013;31:1281-357) offer as a class I recommendation an SBP target of 140-150 mm Hg for elderly patients under age 80, with consideration of a target below 140 mm Hg for the subgroup of elderly patients who are fit, noted Dr. Deaton, professor of nursing at the University of Manchester (U.K.), and a member of the ESC Guidelines Committee.
The American College of Cardiology/American Heart Association guidelines, in contrast, recommend a target of less than 140/90 in 65- to 79-year-olds, and an SBP of 140-145 mm Hg, if tolerated, in those aged 80 years and up (Circulation 2011;123:2434-506).
"The hypothesis from this REGARDS analysis is a bold one," she observed. "But we need large, well-designed intervention trials in older patients, particularly in underrepresented groups, before we can set lower blood pressure targets than are currently recommended in our guidelines."
The REGARDS study is a National Institutes of Health–sponsored observational study of 30,329 U.S. subjects aged 45 years or older, with overrepresentation of blacks and residents of the nation’s southeastern "stroke belt." Dr. Banach presented an analysis of 13,948 REGARDS participants aged 55 years or older who were taking antihypertensive medications at baseline. They have thus far been followed for a median of 6 years for all-cause mortality, and slightly less for first occurrence of stroke or coronary heart disease.
The suggestion of a J-curve was strongest for the composite endpoint of a first stroke, nonfatal myocardial infarction (MI), or death due to coronary heart disease in subjects aged 75 years or older (see graphic). There was no significant relationship between stroke and SBP category for subjects under age 75; however, over age 75, stroke rates were highest in those with an SBP less than 120 or more than 150 mm Hg.
All-cause mortality showed a linear relationship with increasing SBP in subjects aged 55-74 years, but no relationship in those over age 75.
Dr. Deaton applauded the REGARDS investigators for focusing on a high-risk population that’s "certainly underrepresented in our clinical intervention trials," but she cited several major study limitations. One is that only baseline blood pressure measurements were available.
"Importantly," she observed, "we don’t know what happened to blood pressure control during these years of follow-up."
In addition, the incidence rates of stroke, MI, and other major adverse events were relatively low in some subgroups. The planned further follow-up in REGARDS should bring greater clarity, Dr. Deaton added.
Dr. Banach and Dr. Deaton reported having no financial conflicts of interest.
AMSTERDAM – The optimal level of systolic blood pressure in all patients above age 55 appears to be 120-139 mm Hg, according to controversial data from a large, observational, U.S. population–based cohort study.
Results from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study suggest that a systolic blood pressure (SBP) in that range is associated with the lowest rates of cardiovascular events, stroke, and all-cause mortality, even in individuals aged 75 years and up. Since REGARDS is an observational study, this proposed target SBP range should be viewed as a testable hypothesis worthy of confirmation in a large, randomized, antihypertensive treatment study in the elderly, Dr. Maciej Banach said at the annual congress of the European Society of Cardiology (ESC).

REGARDS also turned up evidence supportive of the existence of the long-controversial J-curve, with an increased rate of adverse events noted in subjects with an SBP below 120 mm Hg, added Dr. Banach, professor and head of the department of hypertension at the Medical University of Lodz (Poland).
But discussant Christi Deaton, Ph.D., was quick to slam on the brakes in response to Dr. Banach’s suggestion that 120-139 mm Hg looks like the new optimal in older patients. She noted that the REGARDS proposal is at odds with the latest ESC hypertension guidelines, issued just a couple of months ago.
The evidence-based ESC guidelines (J. Hypertens. 2013;31:1281-357) offer as a class I recommendation an SBP target of 140-150 mm Hg for elderly patients under age 80, with consideration of a target below 140 mm Hg for the subgroup of elderly patients who are fit, noted Dr. Deaton, professor of nursing at the University of Manchester (U.K.), and a member of the ESC Guidelines Committee.
The American College of Cardiology/American Heart Association guidelines, in contrast, recommend a target of less than 140/90 in 65- to 79-year-olds, and an SBP of 140-145 mm Hg, if tolerated, in those aged 80 years and up (Circulation 2011;123:2434-506).
"The hypothesis from this REGARDS analysis is a bold one," she observed. "But we need large, well-designed intervention trials in older patients, particularly in underrepresented groups, before we can set lower blood pressure targets than are currently recommended in our guidelines."
The REGARDS study is a National Institutes of Health–sponsored observational study of 30,329 U.S. subjects aged 45 years or older, with overrepresentation of blacks and residents of the nation’s southeastern "stroke belt." Dr. Banach presented an analysis of 13,948 REGARDS participants aged 55 years or older who were taking antihypertensive medications at baseline. They have thus far been followed for a median of 6 years for all-cause mortality, and slightly less for first occurrence of stroke or coronary heart disease.
The suggestion of a J-curve was strongest for the composite endpoint of a first stroke, nonfatal myocardial infarction (MI), or death due to coronary heart disease in subjects aged 75 years or older (see graphic). There was no significant relationship between stroke and SBP category for subjects under age 75; however, over age 75, stroke rates were highest in those with an SBP less than 120 or more than 150 mm Hg.
All-cause mortality showed a linear relationship with increasing SBP in subjects aged 55-74 years, but no relationship in those over age 75.
Dr. Deaton applauded the REGARDS investigators for focusing on a high-risk population that’s "certainly underrepresented in our clinical intervention trials," but she cited several major study limitations. One is that only baseline blood pressure measurements were available.
"Importantly," she observed, "we don’t know what happened to blood pressure control during these years of follow-up."
In addition, the incidence rates of stroke, MI, and other major adverse events were relatively low in some subgroups. The planned further follow-up in REGARDS should bring greater clarity, Dr. Deaton added.
Dr. Banach and Dr. Deaton reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: The adjusted risk of a first stroke, MI, or cardiovascular death in individuals aged 75 years or older on antihypertensive therapy was 20% lower in those with a systolic blood pressure of 120-129 mm Hg than in those with an SBP less than 120, and 31% lower in those with an SBP of 130-139 mm Hg. In contrast, the risk was 2.2-fold greater in those with an SBP of 150 mm Hg or more than in subjects with an SBP below 120 mm Hg.
Data source: This was a secondary analysis involving nearly 14,000 participants in the prospective observational REGARDS study, all at least 55 years old and on antihypertensive medication at baseline.
Disclosures: Dr. Banach and Dr. Deaton reported having no financial conflicts of interest.
TAVR shows large survival benefit in diabetes patients
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Transcatheter aortic valve replacement produced dramatically better 1-year survival, compared with surgical valve replacement, among high-risk patients with diabetes in a new, post hoc analysis of data collected in the first PARTNER trial.
The 145 patients with any type of diabetes who underwent transcatheter aortic valve replacement (TAVR) in the operable, cohort A of the first Placement of Aortic Transcatheter Valves (PARTNER) trial had an 18% all-cause mortality rate during 1-year follow-up, compared with a 27% rate among the 130 patients who underwent surgical aortic valve replacement (SAVR), a difference in this post hoc analysis that reached statistical significance.
The finding of a 9-percentage-point difference in mortality in the diabetic subgroup treated with TAVR, a 40% relative risk reduction compared with SAVR, contrasts with the overall, primary finding of the PARTNER I cohort A trial, which showed that TAVR and SAVR produced similar mortality rates in high surgical-risk patients after 1 year (N. Engl. J. Med. 2011;364:2187-98).
TAVR use in patients with diabetes also resulted in a statistically significant reduction in the 1-year incidence of renal failure requiring dialysis, a 4% rate compared with an 11% among the SAVR patients, Dr. Brian R. Lindman reported at the annual congress of the European Society of Cardiology. This also contrasted with the overall PARTNER results when patients without diabetes were included, which showed no difference in renal outcomes between TAVR and SAVR.
The results "raise the possibility that TAVR may be the preferred approach for patients with diabetes," said Dr. Lindman. But he cautioned that because this was a post hoc analysis, the results need confirmation in a prospective study.
Despite this caveat, Dr. Lindman said that the finding can’t be completely ignored when treatment options are discussed with patients who have severe aortic stenosis. "Going forward, I think this is something we’ll need to think about, and we might lean more toward TAVR for patients with diabetes. But we’d like to see some confirmatory evidence in the PARTNER II cohort A trial," he said in an interview. Investigators from the PARTNER II trial, which is randomizing patients with moderate surgical risk, have said that the cohort A results are expected in 2015.
"Although hypothesis-generating only, this result is good news for patients with diabetes," said Dr. William Wijns, codirector of the cardiovascular center at O.L.V. Hospital in Aalst, Belgium.
Dr. Lindman also cautioned that investigators collected limited data on patients’ diabetes status in PARTNER I. The data did not include information on diabetes type, hemoglobin A1c or blood glucose levels, or treatment received. He also acknowledged that the 42% prevalence of diabetes in the study cohort was unexpectedly high, but noted that if this meant that some patients with a questionable diabetes diagnosis entered the analysis, this should have diminished the mortality difference between TAVR and SAVR.
The 275 patients with diabetes in PARTNER I cohort A averaged 82 years of age, and just over a third were women. The subgroups of patients with diabetes randomized to TAVR and to SAVR showed no significant differences for any physiologic or cardiovascular measure or in the prevalence of various comorbidities.
The analysis also showed a statistically significant reduced rate of 1-year mortality in the subgroup treated with transfemoral TAVR compared with SAVR, and in the subgroup of patients with diabetes treated with transapical TAVR compared with SAVR. The 1-year rate of stroke was an identical 3.5% in the patients with diabetes treated with TAVR and in those treated with SAVR. Patients treated with SAVR had a higher incidence of a major bleeding event during follow-up compared with the TAVR patients, while the TAVR patients had a significantly higher rate of major vascular complications during follow-up. Both findings were consistent with the overall PARTNER I cohort A results, said Dr. Lindman, a cardiologist at Washington University in St. Louis.
The diabetes patients who underwent TAVR showed their striking reduction in 1-year mortality despite having the same problem with postprocedural aortic regurgitation as seen in the overall PARTNER I trial. At 6 months after treatment, 9% of the TAVR patients had moderate or severe aortic regurgitation, and 54% had mild regurgitation, compared with rates of 1% moderate or severe and 6% mild in the SAVR patients.
Dr. Lindman speculated that increased inflammation and oxidative stress in patients with diabetes may interact with the stresses of heart surgery to produce the excess mortality seen after SAVR in patients with diabetes. Results from prior studies had documented worsened survival in patients with diabetes who undergo heart surgery, he said.
The PARTNER trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER and said that he had no disclosures.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2013
Major finding: In patients with diabetes, 1-year mortality was 18% after TAVR and 27% after SAVR in a post hoc analysis.
Data source: The PARTNER I cohort A study, which enrolled 699 high-risk patients with severe aortic stenosis to treatment with aortic valve replacement by the transcatheter or surgical approach, including 275 patients with diabetes.
Disclosures: The trial was sponsored by Edwards Lifesciences, which markets the TAVR device used in the study. Dr. Lindman was an investigator in PARTNER, and said that he had no disclosures.
Full-dose beta-blockers still show heart failure benefit
AMSTERDAM – Patients with heart failure appeared to continue to benefit from full-dose beta-blocker therapy against a background of optimal, contemporary treatment, based on an analysis of data collected from an 1,800-patient trial of cardiac resynchronization therapy.
Dr. L. Brent Mitchell, who presented this finding at the annual congress of the European Society of Cardiology, acknowledged that assessing the efficacy of drugs in a study that did not randomize patients based on their use of those drugs had questionable validity and was vulnerable to unidentified confounding, but he also noted that data on the role of beta-blockers in contemporary heart failure management were unlikely to come from any other source.
"You need some tool to ask whether beta-blockers are still useful," said Dr. Mitchell, professor of cardiac sciences at the University of Calgary (Alta.). "The purpose of this analysis was to ask if beta-blockers at full dosages are still better than not using a full-dose beta-blocker despite all the other treatments" that patients received while in the study during 2003-2009. The analysis showed that those receiving full-dosage or nearly full-dosage beta-blocker regimens had a one-third cut in all-cause death or heart failure hospitalization, compared with patients receiving less than half the recommended dosage, after adjustment for many baseline differences between these two subgroups.
The analysis also showed that just half of the nearly 1,500 patients who received treatment with a beta-blocker in this cohort received an adequate dosage, defined as at least 50% of the level recommended by the European Society of Cardiology in heart failure treatment guidelines issued last year (Euro. Heart J. 2012;33:1787-847). Two-thirds of the patients on carvedilol received at least half of the recommended target dosage of 50 mg/day. In contrast, about a third of patients being treated with either bisoprolol or metoprolol received a dosage that was at least half the target dosage (10 mg/day for bisoprolol, 200 mg/day for metoprolol).
The analysis used data collected in the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT), which enrolled 1,798 patients primarily with New York Heart Association class II heart failure at 34 centers worldwide. When the primary endpoint of RAFT was evaluated, treatment with cardiac resynchronization therapy (CRT) with defibrillator capability was more effective at preventing death or heart failure hospitalization in the study population than was an implanted defibrillator alone against a background of optimal medical treatment (N. Engl. J. Med. 2010;363:2385-95).
Dr. Mitchell and his associates focused on 1,474 patients who all received beta-blocker treatment while in RAFT. Like the entire RAFT population, 97% of these patients also received treatment with an ACE inhibitor or angiotensin receptor blocker, and 42% received treatment with spironolactone, and their average left ventricular ejection fraction was 23%.
The patients in this group who were undertreated with beta-blockers were significantly older and had a significantly greater prevalence of New York Heart Association class III heart failure and heart failure with an ischemic etiology compared with those who received at least 50% of the target dosage.
In a regression model that adjusted for all measured baseline differences, patients treated with at least half of the recommended dosage had an incidence of mortality or heart failure hospitalization during an average 40 months of follow-up that was 33% below the rate among patients on lower beta-blocker dosages, a statistically significant difference. This 33% relative risk reduction with adequate beta-blocker treatment is "indistinguishable" from the risk reductions seen with placebo in the major beta-blocker efficacy trials in heart failure patients during the 1990s, Dr. Mitchell noted. "This suggests that beta-blocker treatment still has potency," he said.
Other significant, independent predictors of the primary outcome in this model were prior coronary artery bypass, which boosted the risk by 63%; a history of ischemic heart disease, which raised the risk by 39%; having peripheral vascular disease, which increased the risk by 36%; and receiving CRT, which cut the risk by 33%. In addition, adverse outcomes rose 10% for each reduction of 5 mL/min per 1.73 m2 in estimated glomerular filtration rate.
The impact of underdosing was roughly similar across all three beta-blockers, bisoprolol, carvedilol, and metoprolol. The adverse effect of underdosing was mitigated to some extent when patients also received CRT.
RAFT was partially funded by Medtronic of Canada. Dr. Mitchell said he had no relevant financial disclosures.
On Twitter @mitchelzoler
A post hoc analysis of the nonrandomized interventions that patients receive during randomized clinical trials is generally discouraged. Patients treated with lower dosages of hemodynamically active drugs like beta-blockers are often sicker and have a worse prognosis. This problem can be seen in the baseline characteristics of the two subgroups in this analysis, which revealed many significant differences between the two. Performing statistical compensations to counterbalance these differences is very uncertain.
In addition, a few years ago, my associates and I ran the Systolic Heart Failure Treatment With the If inhibitor Ivabradine Trial (SHIFT) (Lancet 2010;376:875-85) to assess the efficacy and safety of ivabradine to lower heart rate and improve outcomes in optimally treated heart failure patients. In SHIFT we saw a relationship similar to the one seen in RAFT by the current investigators: Patients on lower dosages of beta-blockers were sicker and at higher risk than patients on higher beta-blocker dosages. In our analyses, adjusting for differences in heart rate eliminated the link between beta-blocker dosage and outcomes. We found that the entire treatment effect could be explained by differences in patient heart rate.
Dr. Karl Swedberg is a professor of medicine at the University of Gothenburg (Sweden). He was lead investigator for the SHIFT trial, which was funded by Servier, the company that markets ivabradine (Procoralan). He made these comments as designated discussant for Dr. Mitchell’s report.
A post hoc analysis of the nonrandomized interventions that patients receive during randomized clinical trials is generally discouraged. Patients treated with lower dosages of hemodynamically active drugs like beta-blockers are often sicker and have a worse prognosis. This problem can be seen in the baseline characteristics of the two subgroups in this analysis, which revealed many significant differences between the two. Performing statistical compensations to counterbalance these differences is very uncertain.
In addition, a few years ago, my associates and I ran the Systolic Heart Failure Treatment With the If inhibitor Ivabradine Trial (SHIFT) (Lancet 2010;376:875-85) to assess the efficacy and safety of ivabradine to lower heart rate and improve outcomes in optimally treated heart failure patients. In SHIFT we saw a relationship similar to the one seen in RAFT by the current investigators: Patients on lower dosages of beta-blockers were sicker and at higher risk than patients on higher beta-blocker dosages. In our analyses, adjusting for differences in heart rate eliminated the link between beta-blocker dosage and outcomes. We found that the entire treatment effect could be explained by differences in patient heart rate.
Dr. Karl Swedberg is a professor of medicine at the University of Gothenburg (Sweden). He was lead investigator for the SHIFT trial, which was funded by Servier, the company that markets ivabradine (Procoralan). He made these comments as designated discussant for Dr. Mitchell’s report.
A post hoc analysis of the nonrandomized interventions that patients receive during randomized clinical trials is generally discouraged. Patients treated with lower dosages of hemodynamically active drugs like beta-blockers are often sicker and have a worse prognosis. This problem can be seen in the baseline characteristics of the two subgroups in this analysis, which revealed many significant differences between the two. Performing statistical compensations to counterbalance these differences is very uncertain.
In addition, a few years ago, my associates and I ran the Systolic Heart Failure Treatment With the If inhibitor Ivabradine Trial (SHIFT) (Lancet 2010;376:875-85) to assess the efficacy and safety of ivabradine to lower heart rate and improve outcomes in optimally treated heart failure patients. In SHIFT we saw a relationship similar to the one seen in RAFT by the current investigators: Patients on lower dosages of beta-blockers were sicker and at higher risk than patients on higher beta-blocker dosages. In our analyses, adjusting for differences in heart rate eliminated the link between beta-blocker dosage and outcomes. We found that the entire treatment effect could be explained by differences in patient heart rate.
Dr. Karl Swedberg is a professor of medicine at the University of Gothenburg (Sweden). He was lead investigator for the SHIFT trial, which was funded by Servier, the company that markets ivabradine (Procoralan). He made these comments as designated discussant for Dr. Mitchell’s report.
AMSTERDAM – Patients with heart failure appeared to continue to benefit from full-dose beta-blocker therapy against a background of optimal, contemporary treatment, based on an analysis of data collected from an 1,800-patient trial of cardiac resynchronization therapy.
Dr. L. Brent Mitchell, who presented this finding at the annual congress of the European Society of Cardiology, acknowledged that assessing the efficacy of drugs in a study that did not randomize patients based on their use of those drugs had questionable validity and was vulnerable to unidentified confounding, but he also noted that data on the role of beta-blockers in contemporary heart failure management were unlikely to come from any other source.
"You need some tool to ask whether beta-blockers are still useful," said Dr. Mitchell, professor of cardiac sciences at the University of Calgary (Alta.). "The purpose of this analysis was to ask if beta-blockers at full dosages are still better than not using a full-dose beta-blocker despite all the other treatments" that patients received while in the study during 2003-2009. The analysis showed that those receiving full-dosage or nearly full-dosage beta-blocker regimens had a one-third cut in all-cause death or heart failure hospitalization, compared with patients receiving less than half the recommended dosage, after adjustment for many baseline differences between these two subgroups.
The analysis also showed that just half of the nearly 1,500 patients who received treatment with a beta-blocker in this cohort received an adequate dosage, defined as at least 50% of the level recommended by the European Society of Cardiology in heart failure treatment guidelines issued last year (Euro. Heart J. 2012;33:1787-847). Two-thirds of the patients on carvedilol received at least half of the recommended target dosage of 50 mg/day. In contrast, about a third of patients being treated with either bisoprolol or metoprolol received a dosage that was at least half the target dosage (10 mg/day for bisoprolol, 200 mg/day for metoprolol).
The analysis used data collected in the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT), which enrolled 1,798 patients primarily with New York Heart Association class II heart failure at 34 centers worldwide. When the primary endpoint of RAFT was evaluated, treatment with cardiac resynchronization therapy (CRT) with defibrillator capability was more effective at preventing death or heart failure hospitalization in the study population than was an implanted defibrillator alone against a background of optimal medical treatment (N. Engl. J. Med. 2010;363:2385-95).
Dr. Mitchell and his associates focused on 1,474 patients who all received beta-blocker treatment while in RAFT. Like the entire RAFT population, 97% of these patients also received treatment with an ACE inhibitor or angiotensin receptor blocker, and 42% received treatment with spironolactone, and their average left ventricular ejection fraction was 23%.
The patients in this group who were undertreated with beta-blockers were significantly older and had a significantly greater prevalence of New York Heart Association class III heart failure and heart failure with an ischemic etiology compared with those who received at least 50% of the target dosage.
In a regression model that adjusted for all measured baseline differences, patients treated with at least half of the recommended dosage had an incidence of mortality or heart failure hospitalization during an average 40 months of follow-up that was 33% below the rate among patients on lower beta-blocker dosages, a statistically significant difference. This 33% relative risk reduction with adequate beta-blocker treatment is "indistinguishable" from the risk reductions seen with placebo in the major beta-blocker efficacy trials in heart failure patients during the 1990s, Dr. Mitchell noted. "This suggests that beta-blocker treatment still has potency," he said.
Other significant, independent predictors of the primary outcome in this model were prior coronary artery bypass, which boosted the risk by 63%; a history of ischemic heart disease, which raised the risk by 39%; having peripheral vascular disease, which increased the risk by 36%; and receiving CRT, which cut the risk by 33%. In addition, adverse outcomes rose 10% for each reduction of 5 mL/min per 1.73 m2 in estimated glomerular filtration rate.
The impact of underdosing was roughly similar across all three beta-blockers, bisoprolol, carvedilol, and metoprolol. The adverse effect of underdosing was mitigated to some extent when patients also received CRT.
RAFT was partially funded by Medtronic of Canada. Dr. Mitchell said he had no relevant financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Patients with heart failure appeared to continue to benefit from full-dose beta-blocker therapy against a background of optimal, contemporary treatment, based on an analysis of data collected from an 1,800-patient trial of cardiac resynchronization therapy.
Dr. L. Brent Mitchell, who presented this finding at the annual congress of the European Society of Cardiology, acknowledged that assessing the efficacy of drugs in a study that did not randomize patients based on their use of those drugs had questionable validity and was vulnerable to unidentified confounding, but he also noted that data on the role of beta-blockers in contemporary heart failure management were unlikely to come from any other source.
"You need some tool to ask whether beta-blockers are still useful," said Dr. Mitchell, professor of cardiac sciences at the University of Calgary (Alta.). "The purpose of this analysis was to ask if beta-blockers at full dosages are still better than not using a full-dose beta-blocker despite all the other treatments" that patients received while in the study during 2003-2009. The analysis showed that those receiving full-dosage or nearly full-dosage beta-blocker regimens had a one-third cut in all-cause death or heart failure hospitalization, compared with patients receiving less than half the recommended dosage, after adjustment for many baseline differences between these two subgroups.
The analysis also showed that just half of the nearly 1,500 patients who received treatment with a beta-blocker in this cohort received an adequate dosage, defined as at least 50% of the level recommended by the European Society of Cardiology in heart failure treatment guidelines issued last year (Euro. Heart J. 2012;33:1787-847). Two-thirds of the patients on carvedilol received at least half of the recommended target dosage of 50 mg/day. In contrast, about a third of patients being treated with either bisoprolol or metoprolol received a dosage that was at least half the target dosage (10 mg/day for bisoprolol, 200 mg/day for metoprolol).
The analysis used data collected in the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT), which enrolled 1,798 patients primarily with New York Heart Association class II heart failure at 34 centers worldwide. When the primary endpoint of RAFT was evaluated, treatment with cardiac resynchronization therapy (CRT) with defibrillator capability was more effective at preventing death or heart failure hospitalization in the study population than was an implanted defibrillator alone against a background of optimal medical treatment (N. Engl. J. Med. 2010;363:2385-95).
Dr. Mitchell and his associates focused on 1,474 patients who all received beta-blocker treatment while in RAFT. Like the entire RAFT population, 97% of these patients also received treatment with an ACE inhibitor or angiotensin receptor blocker, and 42% received treatment with spironolactone, and their average left ventricular ejection fraction was 23%.
The patients in this group who were undertreated with beta-blockers were significantly older and had a significantly greater prevalence of New York Heart Association class III heart failure and heart failure with an ischemic etiology compared with those who received at least 50% of the target dosage.
In a regression model that adjusted for all measured baseline differences, patients treated with at least half of the recommended dosage had an incidence of mortality or heart failure hospitalization during an average 40 months of follow-up that was 33% below the rate among patients on lower beta-blocker dosages, a statistically significant difference. This 33% relative risk reduction with adequate beta-blocker treatment is "indistinguishable" from the risk reductions seen with placebo in the major beta-blocker efficacy trials in heart failure patients during the 1990s, Dr. Mitchell noted. "This suggests that beta-blocker treatment still has potency," he said.
Other significant, independent predictors of the primary outcome in this model were prior coronary artery bypass, which boosted the risk by 63%; a history of ischemic heart disease, which raised the risk by 39%; having peripheral vascular disease, which increased the risk by 36%; and receiving CRT, which cut the risk by 33%. In addition, adverse outcomes rose 10% for each reduction of 5 mL/min per 1.73 m2 in estimated glomerular filtration rate.
The impact of underdosing was roughly similar across all three beta-blockers, bisoprolol, carvedilol, and metoprolol. The adverse effect of underdosing was mitigated to some extent when patients also received CRT.
RAFT was partially funded by Medtronic of Canada. Dr. Mitchell said he had no relevant financial disclosures.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2013
Major finding: Heart failure patients on recommended beta-blocker dosages had 33% fewer deaths or hospitalizations than did patients on lower dosages.
Data source: A post hoc subgroup analysis of results from RAFT, with 1,798 patients with heart failure at 34 worldwide sites.
Disclosures: RAFT was partially funded by Medtronic of Canada. Dr. Mitchell said he had no relevant financial disclosures.
HDL hypothesis takes yet another hit
AMSTERDAM – A novel oral drug that raises HDL cholesterol levels by upregulating hepatic synthesis of apolipoprotein A1 failed to significantly reduce coronary atheroma volume in a large phase II study.
The lack of efficacy for the drug known as RVX-208 in the ASSURE trial (ApoA1 Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation) was "disappointing and surprising, given promising earlier findings," Dr. Stephen J. Nicholls observed in presenting the trial results at the annual congress of the European Society of Cardiology.
The study involved 323 patients with symptomatic coronary artery disease and low HDL cholesterol who were randomized 3:1 to 26 weeks of RVX-208 at 100 mg twice daily or placebo. All subjects were on atorvastatin or rosuvastatin. The RVX-208 group experienced a mean 10.9% increase in HDL over baseline, a 12.8% rise in ApoA-1, and a 16% drop in LDL. But these beneficial lipid changes weren’t significantly different than in the placebo group, which showed a 7.7% increase in HDL, a 10.6% bump in ApoA-1, and a 17.6% fall in LDL.
The primary study endpoint was change from baseline in atheroma volume. The median drop was 40% in the RVX-208 group and not statistically different from the 30% decrease in placebo-treated controls. Nor was there a significant difference between the two study arms in reduction in atheroma volume within the most-diseased 10-mm coronary segment, reported Dr. Nicholls, professor of medicine at the University of Adelaide and deputy director of the South Australian Health and Medical Research Institute.
Moreover, RVX-208 was associated with liver enzyme elevations in the great majority of treated patients, exceeding three times the upper limit of normal in 7.1% of them.
"This drug had been developed with the thought that it would be a potent oral compound from the perspective of an HDL therapeutic and would be comparable to the HDL infusional approaches that were previously seen to regress plaque. That’s clearly not the case. We saw no biochemical or plaque improvement whatsoever above and beyond the placebo group. So RVX-208 does not appear to be a potent approach to HDL therapeutics," Dr. Nicholls concluded.
Nonetheless, he expressed some regret that the trial hadn’t been designed to be lengthier. It’s possible that with another 6 months or more of treatment the trends favoring the investigational agent might have achieved statistical significance.
Asked if the negative result in ASSURE, coming on top of a steady flow of consistently negative clinical trials for niacin, torcetrapib, and other HDL-raising agents, means that the HDL-raising hypothesis of cardiovascular prevention is dead, Dr. Deepak L. Bhatt replied, "I think that’s a good question. The hypothesis has always been a good one, but I believe that in patients who are statin treated, no one has really shown an incremental benefit of HDL-raising. Of course, there are older data in patients not on statins to show that drugs like niacin are useful, but these drugs also have an effect on other lipid parameters, like LDL.
"It does appear that low HDL is a risk marker. But it may not be a modifiable risk factor. I think for the time being, the HDL hypothesis remains a hypothesis with a lot of holes in it. Moving forward, we’ll probably have more to gain by reducing LDL incrementally beyond the levels obtained with statins than with solely trying to raise HDL, unless those HDL-raising compounds we study in the future also have LDL-reduction or other properties," added Dr. Bhatt, professor of medicine at Harvard Medical School, Boston, and chief of cardiology at Veterans Affairs Boston Healthcare System.
Dr. Nicholls conceded that "this continues to be a field with a lot of uncertainty." But he asserted "there continues to be immense interest in the development of therapies targeting HDL."
Noting that, in ASSURE, RVX-208 wasn’t significantly better at raising HDL than was state-of-the-art background medical therapy including statins in the placebo arm, he said, "I think we haven’t really tested the HDL hypothesis yet." Advancing an investigational agent to phase III testing with clinical outcomes will require that the drug first demonstrate atheroma regression in a solid phase II study, he added.
The ASSURE trial was sponsored by Resverlogix. Dr. Nicholls has received research support from, and serves as a consultant to, Resverlogix and more than half a dozen other pharmaceutical companies.
AMSTERDAM – A novel oral drug that raises HDL cholesterol levels by upregulating hepatic synthesis of apolipoprotein A1 failed to significantly reduce coronary atheroma volume in a large phase II study.
The lack of efficacy for the drug known as RVX-208 in the ASSURE trial (ApoA1 Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation) was "disappointing and surprising, given promising earlier findings," Dr. Stephen J. Nicholls observed in presenting the trial results at the annual congress of the European Society of Cardiology.
The study involved 323 patients with symptomatic coronary artery disease and low HDL cholesterol who were randomized 3:1 to 26 weeks of RVX-208 at 100 mg twice daily or placebo. All subjects were on atorvastatin or rosuvastatin. The RVX-208 group experienced a mean 10.9% increase in HDL over baseline, a 12.8% rise in ApoA-1, and a 16% drop in LDL. But these beneficial lipid changes weren’t significantly different than in the placebo group, which showed a 7.7% increase in HDL, a 10.6% bump in ApoA-1, and a 17.6% fall in LDL.
The primary study endpoint was change from baseline in atheroma volume. The median drop was 40% in the RVX-208 group and not statistically different from the 30% decrease in placebo-treated controls. Nor was there a significant difference between the two study arms in reduction in atheroma volume within the most-diseased 10-mm coronary segment, reported Dr. Nicholls, professor of medicine at the University of Adelaide and deputy director of the South Australian Health and Medical Research Institute.
Moreover, RVX-208 was associated with liver enzyme elevations in the great majority of treated patients, exceeding three times the upper limit of normal in 7.1% of them.
"This drug had been developed with the thought that it would be a potent oral compound from the perspective of an HDL therapeutic and would be comparable to the HDL infusional approaches that were previously seen to regress plaque. That’s clearly not the case. We saw no biochemical or plaque improvement whatsoever above and beyond the placebo group. So RVX-208 does not appear to be a potent approach to HDL therapeutics," Dr. Nicholls concluded.
Nonetheless, he expressed some regret that the trial hadn’t been designed to be lengthier. It’s possible that with another 6 months or more of treatment the trends favoring the investigational agent might have achieved statistical significance.
Asked if the negative result in ASSURE, coming on top of a steady flow of consistently negative clinical trials for niacin, torcetrapib, and other HDL-raising agents, means that the HDL-raising hypothesis of cardiovascular prevention is dead, Dr. Deepak L. Bhatt replied, "I think that’s a good question. The hypothesis has always been a good one, but I believe that in patients who are statin treated, no one has really shown an incremental benefit of HDL-raising. Of course, there are older data in patients not on statins to show that drugs like niacin are useful, but these drugs also have an effect on other lipid parameters, like LDL.
"It does appear that low HDL is a risk marker. But it may not be a modifiable risk factor. I think for the time being, the HDL hypothesis remains a hypothesis with a lot of holes in it. Moving forward, we’ll probably have more to gain by reducing LDL incrementally beyond the levels obtained with statins than with solely trying to raise HDL, unless those HDL-raising compounds we study in the future also have LDL-reduction or other properties," added Dr. Bhatt, professor of medicine at Harvard Medical School, Boston, and chief of cardiology at Veterans Affairs Boston Healthcare System.
Dr. Nicholls conceded that "this continues to be a field with a lot of uncertainty." But he asserted "there continues to be immense interest in the development of therapies targeting HDL."
Noting that, in ASSURE, RVX-208 wasn’t significantly better at raising HDL than was state-of-the-art background medical therapy including statins in the placebo arm, he said, "I think we haven’t really tested the HDL hypothesis yet." Advancing an investigational agent to phase III testing with clinical outcomes will require that the drug first demonstrate atheroma regression in a solid phase II study, he added.
The ASSURE trial was sponsored by Resverlogix. Dr. Nicholls has received research support from, and serves as a consultant to, Resverlogix and more than half a dozen other pharmaceutical companies.
AMSTERDAM – A novel oral drug that raises HDL cholesterol levels by upregulating hepatic synthesis of apolipoprotein A1 failed to significantly reduce coronary atheroma volume in a large phase II study.
The lack of efficacy for the drug known as RVX-208 in the ASSURE trial (ApoA1 Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation) was "disappointing and surprising, given promising earlier findings," Dr. Stephen J. Nicholls observed in presenting the trial results at the annual congress of the European Society of Cardiology.
The study involved 323 patients with symptomatic coronary artery disease and low HDL cholesterol who were randomized 3:1 to 26 weeks of RVX-208 at 100 mg twice daily or placebo. All subjects were on atorvastatin or rosuvastatin. The RVX-208 group experienced a mean 10.9% increase in HDL over baseline, a 12.8% rise in ApoA-1, and a 16% drop in LDL. But these beneficial lipid changes weren’t significantly different than in the placebo group, which showed a 7.7% increase in HDL, a 10.6% bump in ApoA-1, and a 17.6% fall in LDL.
The primary study endpoint was change from baseline in atheroma volume. The median drop was 40% in the RVX-208 group and not statistically different from the 30% decrease in placebo-treated controls. Nor was there a significant difference between the two study arms in reduction in atheroma volume within the most-diseased 10-mm coronary segment, reported Dr. Nicholls, professor of medicine at the University of Adelaide and deputy director of the South Australian Health and Medical Research Institute.
Moreover, RVX-208 was associated with liver enzyme elevations in the great majority of treated patients, exceeding three times the upper limit of normal in 7.1% of them.
"This drug had been developed with the thought that it would be a potent oral compound from the perspective of an HDL therapeutic and would be comparable to the HDL infusional approaches that were previously seen to regress plaque. That’s clearly not the case. We saw no biochemical or plaque improvement whatsoever above and beyond the placebo group. So RVX-208 does not appear to be a potent approach to HDL therapeutics," Dr. Nicholls concluded.
Nonetheless, he expressed some regret that the trial hadn’t been designed to be lengthier. It’s possible that with another 6 months or more of treatment the trends favoring the investigational agent might have achieved statistical significance.
Asked if the negative result in ASSURE, coming on top of a steady flow of consistently negative clinical trials for niacin, torcetrapib, and other HDL-raising agents, means that the HDL-raising hypothesis of cardiovascular prevention is dead, Dr. Deepak L. Bhatt replied, "I think that’s a good question. The hypothesis has always been a good one, but I believe that in patients who are statin treated, no one has really shown an incremental benefit of HDL-raising. Of course, there are older data in patients not on statins to show that drugs like niacin are useful, but these drugs also have an effect on other lipid parameters, like LDL.
"It does appear that low HDL is a risk marker. But it may not be a modifiable risk factor. I think for the time being, the HDL hypothesis remains a hypothesis with a lot of holes in it. Moving forward, we’ll probably have more to gain by reducing LDL incrementally beyond the levels obtained with statins than with solely trying to raise HDL, unless those HDL-raising compounds we study in the future also have LDL-reduction or other properties," added Dr. Bhatt, professor of medicine at Harvard Medical School, Boston, and chief of cardiology at Veterans Affairs Boston Healthcare System.
Dr. Nicholls conceded that "this continues to be a field with a lot of uncertainty." But he asserted "there continues to be immense interest in the development of therapies targeting HDL."
Noting that, in ASSURE, RVX-208 wasn’t significantly better at raising HDL than was state-of-the-art background medical therapy including statins in the placebo arm, he said, "I think we haven’t really tested the HDL hypothesis yet." Advancing an investigational agent to phase III testing with clinical outcomes will require that the drug first demonstrate atheroma regression in a solid phase II study, he added.
The ASSURE trial was sponsored by Resverlogix. Dr. Nicholls has received research support from, and serves as a consultant to, Resverlogix and more than half a dozen other pharmaceutical companies.
AT THE ESC CONGRESS 2013
Major finding: The investigational oral HDL-raising agent RVX-208 reduced percent atheroma volume as measured by IVUS by 40% from baseline, a nonsignificant difference from the 30% reduction in placebo patients.
Data source: ASSURE, a 26-week, randomized, placebo-controlled study in which 323 patients with symptomatic coronary artery disease were randomized 3:1 to RVX-208 or placebo.
Disclosures: The study was sponsored by Resverlogix. The presenter has received research funding from, and is a paid consultant to, the company.
Insulin-triggered hypoglycemia poses less danger
AMSTERDAM – When patients with dysglycemia had severe hypoglycemia while on treatment with basal insulin glargine, the episodes did not trigger as many adverse cardiovascular outcomes as did severe hypoglycemia during standard care with oral glucose-lowering drugs but no insulin.
"Hypoglycemia caused by insulin glargine–mediated glucose lowering is unlikely to cause cardiovascular outcomes," Dr. Linda G. Mellbin said at the European Society of Cardiology Congress 2013. The link between hypoglycemia and cardiovascular outcomes was up to two- to threefold lower in patients receiving insulin glargine treatment, compared with patients on standard oral regimens in data from more than 12,000 patients with early type 2 diabetes, impaired fasting glucose, or impaired glucose tolerance enrolled in a trial that compared insulin glargine with standard treatment, said Dr. Mellbin, a cardiologist at the Karolinska Institute in Stockholm.
"What was reassuring was that while insulin glargine caused more hypoglycemic events" compared with standard treatment in these patients, "the absolute number of fatal events was still lower with insulin, showing insulin is a very safe treatment," said Dr. Lars Rydén, professor of cardiology at the Karolinska Institute and a coinvestigator with Dr. Mellbin.
"The clinical implication is that you shouldn’t be afraid of insulin; patients should use it if they need it," Dr. Rydén said in an interview. "One reason people are afraid is because it causes hypoglycemia, but this [hypoglycemia] doesn’t seem to be very dangerous."
Dr. Mellbin, Dr. Rydén, and their associates studied data collected in the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial. Last year, the primary outcome of the study showed that in 12,537 patients with early type 2 diabetes or other indications of dysglycemia and at high risk for cardiovascular outcomes, treatment with basal insulin glargine (Lantus) had a neutral effect during a median 6 years follow-up, compared with standard care on the incidence of several cardiovascular outcomes: death, myocardial infarction, stroke, any revascularization procedure, or heart-failure hospitalization (N. Engl. J. Med. 2012;367:319-28). The new analysis focused on the 3,518 patients in ORIGIN who had at least one hypoglycemic episode during follow-up+ and included 472 patients with at least one severe hypoglycemic event.
Hypoglycemic episodes were more frequent in insulin-treated patients, who accounted for about three-quarters of all hypoglycemic episodes and as well as about three-quarters of the severe events.
The nonsevere hypoglycemic episodes were benign. In a propensity-score adjusted analysis, nonsevere hypoglycemia had no significant association with total mortality, cardiovascular death, arrhythmic death, or cardiovascular death plus nonfatal MI or stroke.
But severe hypoglycemic episodes, as well as severe nocturnal episodes, were significantly linked with increased rates for all these outcomes in the adjusted analysis. For example, patients who had at least one severe hypoglycemic event had a 58% increased incidence of cardiovascular death, MI, or stroke, and a 74% higher rate of total mortality, Dr. Mellbin reported at the meeting. Concurrent with her report, an article with the results was published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht332]).
Although more patients who received insulin had severe hypoglycemia, patients who had these episodes while on glargine had a substantially lower rate of death and other cardiovascular outcomes than did the patients who became hypoglycemic while on standard treatment. The standard-treatment patients who had a severe hypoglycemic episode were 70% more likely to have a subsequent primary-outcome event, compared with the patients who had severe hypoglycemia, while on glargine. The rate of all-cause death following severe hypoglycemia was more than twice as common in the standard care patients, compared with those who had severe hypoglycemia on glargine, and the rate of arrhythmia death was nearly threefold higher in the standard-care patients.
These findings explain why patients on insulin glargine did not have excess cardiovascular and mortality endpoints over standard treatment: Each hypoglycemic event while on insulin had less adverse consequence than when hypoglycemia occurred in patients on standard treatment. In the standard-treatment group, 29% of patients received a sulfonylurea, 28% received metformin, and 3% received another oral hypoglycemic drug. In the insulin glargine group use of oral drugs was very similar, but all patients also received insulin.
The finding that severe hypoglycemic events differ in their clinical impact depending on the treatment context in which they occur in the patients enrolled in ORIGIN could have several possible explanations, Dr. Rydén said. First, it’s possible that the more frequent, repeated episodes of hypoglycemia in the patients on insulin pre-conditioned their myocardium and prevented more severe reactions to subsequent hypoglycemic episodes.
Another possible explanation is an adverse effect from sulfonylurea drugs among patients on standard care. "Sulfonylureas may restrict the ability of the myocardium to adapt to ischemia, and it also prolongs the hypoglycemia," Dr. Rydén said. This explanation was endorsed by Dr. Peter Grant, a diabetologist and professor of medicine at the University of Leeds (U.K.). "It is difficult to believe that sulfonylureas are not having an adverse effect," he said as the designated discussant for Dr. Mellbin’s report. "These results tell us that hypoglycemia is an important determinant of outcome, and they raise questions about sulfonylureas," Dr. Grant said.
A third possible explanation is that increased mortality and poor cardiovascular prognosis and a tendency to develop hypoglycemia have a common, as-yet unidentified cause that has not been adjusted for in the models, Dr. Rydén said. "The relationship between severe hypoglycemia and cardiovascular outcomes is likely confounded by unmeasured risk factors," Dr. Mellbin said.
ORIGIN was funded by Sanofi, the company that markets insulin glargine (Lantus). Dr. Mellbin said that she has received lecture honoraria from Sanofi-Aventis and six other drug companies. Dr. Rydén said that he has received consulting and lecture honoraria from Sanofi-Aventis, Roche, Bristol-Myers Squibb, AstraZeneca, and Bayer. Dr. Grant said that he had no relevant disclosures.
On Twitter @mitchelzoler
AMSTERDAM – When patients with dysglycemia had severe hypoglycemia while on treatment with basal insulin glargine, the episodes did not trigger as many adverse cardiovascular outcomes as did severe hypoglycemia during standard care with oral glucose-lowering drugs but no insulin.
"Hypoglycemia caused by insulin glargine–mediated glucose lowering is unlikely to cause cardiovascular outcomes," Dr. Linda G. Mellbin said at the European Society of Cardiology Congress 2013. The link between hypoglycemia and cardiovascular outcomes was up to two- to threefold lower in patients receiving insulin glargine treatment, compared with patients on standard oral regimens in data from more than 12,000 patients with early type 2 diabetes, impaired fasting glucose, or impaired glucose tolerance enrolled in a trial that compared insulin glargine with standard treatment, said Dr. Mellbin, a cardiologist at the Karolinska Institute in Stockholm.
"What was reassuring was that while insulin glargine caused more hypoglycemic events" compared with standard treatment in these patients, "the absolute number of fatal events was still lower with insulin, showing insulin is a very safe treatment," said Dr. Lars Rydén, professor of cardiology at the Karolinska Institute and a coinvestigator with Dr. Mellbin.
"The clinical implication is that you shouldn’t be afraid of insulin; patients should use it if they need it," Dr. Rydén said in an interview. "One reason people are afraid is because it causes hypoglycemia, but this [hypoglycemia] doesn’t seem to be very dangerous."
Dr. Mellbin, Dr. Rydén, and their associates studied data collected in the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial. Last year, the primary outcome of the study showed that in 12,537 patients with early type 2 diabetes or other indications of dysglycemia and at high risk for cardiovascular outcomes, treatment with basal insulin glargine (Lantus) had a neutral effect during a median 6 years follow-up, compared with standard care on the incidence of several cardiovascular outcomes: death, myocardial infarction, stroke, any revascularization procedure, or heart-failure hospitalization (N. Engl. J. Med. 2012;367:319-28). The new analysis focused on the 3,518 patients in ORIGIN who had at least one hypoglycemic episode during follow-up+ and included 472 patients with at least one severe hypoglycemic event.
Hypoglycemic episodes were more frequent in insulin-treated patients, who accounted for about three-quarters of all hypoglycemic episodes and as well as about three-quarters of the severe events.
The nonsevere hypoglycemic episodes were benign. In a propensity-score adjusted analysis, nonsevere hypoglycemia had no significant association with total mortality, cardiovascular death, arrhythmic death, or cardiovascular death plus nonfatal MI or stroke.
But severe hypoglycemic episodes, as well as severe nocturnal episodes, were significantly linked with increased rates for all these outcomes in the adjusted analysis. For example, patients who had at least one severe hypoglycemic event had a 58% increased incidence of cardiovascular death, MI, or stroke, and a 74% higher rate of total mortality, Dr. Mellbin reported at the meeting. Concurrent with her report, an article with the results was published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht332]).
Although more patients who received insulin had severe hypoglycemia, patients who had these episodes while on glargine had a substantially lower rate of death and other cardiovascular outcomes than did the patients who became hypoglycemic while on standard treatment. The standard-treatment patients who had a severe hypoglycemic episode were 70% more likely to have a subsequent primary-outcome event, compared with the patients who had severe hypoglycemia, while on glargine. The rate of all-cause death following severe hypoglycemia was more than twice as common in the standard care patients, compared with those who had severe hypoglycemia on glargine, and the rate of arrhythmia death was nearly threefold higher in the standard-care patients.
These findings explain why patients on insulin glargine did not have excess cardiovascular and mortality endpoints over standard treatment: Each hypoglycemic event while on insulin had less adverse consequence than when hypoglycemia occurred in patients on standard treatment. In the standard-treatment group, 29% of patients received a sulfonylurea, 28% received metformin, and 3% received another oral hypoglycemic drug. In the insulin glargine group use of oral drugs was very similar, but all patients also received insulin.
The finding that severe hypoglycemic events differ in their clinical impact depending on the treatment context in which they occur in the patients enrolled in ORIGIN could have several possible explanations, Dr. Rydén said. First, it’s possible that the more frequent, repeated episodes of hypoglycemia in the patients on insulin pre-conditioned their myocardium and prevented more severe reactions to subsequent hypoglycemic episodes.
Another possible explanation is an adverse effect from sulfonylurea drugs among patients on standard care. "Sulfonylureas may restrict the ability of the myocardium to adapt to ischemia, and it also prolongs the hypoglycemia," Dr. Rydén said. This explanation was endorsed by Dr. Peter Grant, a diabetologist and professor of medicine at the University of Leeds (U.K.). "It is difficult to believe that sulfonylureas are not having an adverse effect," he said as the designated discussant for Dr. Mellbin’s report. "These results tell us that hypoglycemia is an important determinant of outcome, and they raise questions about sulfonylureas," Dr. Grant said.
A third possible explanation is that increased mortality and poor cardiovascular prognosis and a tendency to develop hypoglycemia have a common, as-yet unidentified cause that has not been adjusted for in the models, Dr. Rydén said. "The relationship between severe hypoglycemia and cardiovascular outcomes is likely confounded by unmeasured risk factors," Dr. Mellbin said.
ORIGIN was funded by Sanofi, the company that markets insulin glargine (Lantus). Dr. Mellbin said that she has received lecture honoraria from Sanofi-Aventis and six other drug companies. Dr. Rydén said that he has received consulting and lecture honoraria from Sanofi-Aventis, Roche, Bristol-Myers Squibb, AstraZeneca, and Bayer. Dr. Grant said that he had no relevant disclosures.
On Twitter @mitchelzoler
AMSTERDAM – When patients with dysglycemia had severe hypoglycemia while on treatment with basal insulin glargine, the episodes did not trigger as many adverse cardiovascular outcomes as did severe hypoglycemia during standard care with oral glucose-lowering drugs but no insulin.
"Hypoglycemia caused by insulin glargine–mediated glucose lowering is unlikely to cause cardiovascular outcomes," Dr. Linda G. Mellbin said at the European Society of Cardiology Congress 2013. The link between hypoglycemia and cardiovascular outcomes was up to two- to threefold lower in patients receiving insulin glargine treatment, compared with patients on standard oral regimens in data from more than 12,000 patients with early type 2 diabetes, impaired fasting glucose, or impaired glucose tolerance enrolled in a trial that compared insulin glargine with standard treatment, said Dr. Mellbin, a cardiologist at the Karolinska Institute in Stockholm.
"What was reassuring was that while insulin glargine caused more hypoglycemic events" compared with standard treatment in these patients, "the absolute number of fatal events was still lower with insulin, showing insulin is a very safe treatment," said Dr. Lars Rydén, professor of cardiology at the Karolinska Institute and a coinvestigator with Dr. Mellbin.
"The clinical implication is that you shouldn’t be afraid of insulin; patients should use it if they need it," Dr. Rydén said in an interview. "One reason people are afraid is because it causes hypoglycemia, but this [hypoglycemia] doesn’t seem to be very dangerous."
Dr. Mellbin, Dr. Rydén, and their associates studied data collected in the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial. Last year, the primary outcome of the study showed that in 12,537 patients with early type 2 diabetes or other indications of dysglycemia and at high risk for cardiovascular outcomes, treatment with basal insulin glargine (Lantus) had a neutral effect during a median 6 years follow-up, compared with standard care on the incidence of several cardiovascular outcomes: death, myocardial infarction, stroke, any revascularization procedure, or heart-failure hospitalization (N. Engl. J. Med. 2012;367:319-28). The new analysis focused on the 3,518 patients in ORIGIN who had at least one hypoglycemic episode during follow-up+ and included 472 patients with at least one severe hypoglycemic event.
Hypoglycemic episodes were more frequent in insulin-treated patients, who accounted for about three-quarters of all hypoglycemic episodes and as well as about three-quarters of the severe events.
The nonsevere hypoglycemic episodes were benign. In a propensity-score adjusted analysis, nonsevere hypoglycemia had no significant association with total mortality, cardiovascular death, arrhythmic death, or cardiovascular death plus nonfatal MI or stroke.
But severe hypoglycemic episodes, as well as severe nocturnal episodes, were significantly linked with increased rates for all these outcomes in the adjusted analysis. For example, patients who had at least one severe hypoglycemic event had a 58% increased incidence of cardiovascular death, MI, or stroke, and a 74% higher rate of total mortality, Dr. Mellbin reported at the meeting. Concurrent with her report, an article with the results was published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht332]).
Although more patients who received insulin had severe hypoglycemia, patients who had these episodes while on glargine had a substantially lower rate of death and other cardiovascular outcomes than did the patients who became hypoglycemic while on standard treatment. The standard-treatment patients who had a severe hypoglycemic episode were 70% more likely to have a subsequent primary-outcome event, compared with the patients who had severe hypoglycemia, while on glargine. The rate of all-cause death following severe hypoglycemia was more than twice as common in the standard care patients, compared with those who had severe hypoglycemia on glargine, and the rate of arrhythmia death was nearly threefold higher in the standard-care patients.
These findings explain why patients on insulin glargine did not have excess cardiovascular and mortality endpoints over standard treatment: Each hypoglycemic event while on insulin had less adverse consequence than when hypoglycemia occurred in patients on standard treatment. In the standard-treatment group, 29% of patients received a sulfonylurea, 28% received metformin, and 3% received another oral hypoglycemic drug. In the insulin glargine group use of oral drugs was very similar, but all patients also received insulin.
The finding that severe hypoglycemic events differ in their clinical impact depending on the treatment context in which they occur in the patients enrolled in ORIGIN could have several possible explanations, Dr. Rydén said. First, it’s possible that the more frequent, repeated episodes of hypoglycemia in the patients on insulin pre-conditioned their myocardium and prevented more severe reactions to subsequent hypoglycemic episodes.
Another possible explanation is an adverse effect from sulfonylurea drugs among patients on standard care. "Sulfonylureas may restrict the ability of the myocardium to adapt to ischemia, and it also prolongs the hypoglycemia," Dr. Rydén said. This explanation was endorsed by Dr. Peter Grant, a diabetologist and professor of medicine at the University of Leeds (U.K.). "It is difficult to believe that sulfonylureas are not having an adverse effect," he said as the designated discussant for Dr. Mellbin’s report. "These results tell us that hypoglycemia is an important determinant of outcome, and they raise questions about sulfonylureas," Dr. Grant said.
A third possible explanation is that increased mortality and poor cardiovascular prognosis and a tendency to develop hypoglycemia have a common, as-yet unidentified cause that has not been adjusted for in the models, Dr. Rydén said. "The relationship between severe hypoglycemia and cardiovascular outcomes is likely confounded by unmeasured risk factors," Dr. Mellbin said.
ORIGIN was funded by Sanofi, the company that markets insulin glargine (Lantus). Dr. Mellbin said that she has received lecture honoraria from Sanofi-Aventis and six other drug companies. Dr. Rydén said that he has received consulting and lecture honoraria from Sanofi-Aventis, Roche, Bristol-Myers Squibb, AstraZeneca, and Bayer. Dr. Grant said that he had no relevant disclosures.
On Twitter @mitchelzoler
AT ESC 2013
Major finding: Severe hypoglycemia during standard, oral dysglycemia treatment produced 70% more cardiovascular outcomes than did severe hypoglycemia during insulin treatment.
Data source: ORIGIN, a multicenter trial with 12,537 patients with early type 2 diabetes or dysglycemia randomized to treatment with basal insulin glargine or a standard, oral regimen.
Disclosures: ORIGIN was funded by Sanofi, the company that markets insulin glargine (Lantus). Dr. Mellbin said that she has received lecture honoraria from Sanofi-Aventis and six other drug companies. Dr. Rydén said that he has received consulting and lecture honoraria from Sanofi-Aventis, Roche, Bristol-Myers Squibb, AstraZeneca, and Bayer. Dr. Grant said that he had no relevant disclosures.
Losartan shown effective in Marfan syndrome
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.

|
Bruce Jancin/IMNG Medical Media
|
COMPARE is a very important study whose results are going to mean a paradigm shift for the management of Marfan syndrome.
It is intriguing to consider that the benefits of losartan might possibly also extend to patients with thoracic aortic disease in general, a worthy topic for future investigation.
Dr. John Gordon Harold is with Cedars-Sinai Heart Institute, Los Angeles, and president of the American College of Cardiology. He had no relevant financial disclosures.
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.
AMSTERDAM – Daily losartan significantly slowed the aortic root dilatation rate in adults with Marfan syndrome in a 3-year randomized clinical trial.
"I think we can be positive about this treatment. We can now recommend losartan in clinical practice," Dr. Maarten Groenink said at the annual congress of the European Society of Cardiology.
The COMPARE (Cozaar in Marfan Patients Reduces Aortic Enlargement) trial included 218 patients at all four university Marfan centers in the Netherlands. Patients were randomized to oral losartan at a target dose of 100 mg/day or no losartan in addition to standard-of-care treatment with beta-blockers. Roughly half of the patients in the losartan group were unable to tolerate the full dose of losartan in addition to a beta-blocker; those patients were maintained on losartan at 50 mg/day. Aortic root diameter was measured by MRI at enrollment and after 3 years of prospective follow-up. The aortic dilatation rate was significantly lower in the losartan group than in controls both in the patients with a native root and in those who had undergone aortic root replacement surgery, reported Dr. Groenink, a cardiologist at the Academic Medical Center, Amsterdam.
There were no aortic dissections in the losartan group and two in the control arm. Elective aortic replacement surgery was performed in a similar number of patients in both groups.
Blood pressure was lower in the losartan group, yet blood pressure didn’t correlate with the aortic dilatation rate. Dr. Groenink speculated that losartan’s chief mechanism of benefit in Marfan syndrome is its ability to curb overexpression of transforming growth factor-beta, which weakens the structure of the media layer of the aortic wall.
Dr. Groenink said it’s unknown whether losartan’s benefits are specific to that drug or are a class effect obtainable with other angiotensin II receptor antagonists, though he suspects it’s a class effect.
Ongoing clinical trials are evaluating losartan in children and adolescents with Marfan syndrome, he said, adding that there is a solid rationale for beginning treatment as early in life as possible.
"I believe the adverse effects on the aortic wall in Marfan syndrome are caused by the fibrillin defect but also by wear and tear due to cyclic stress by the beating heart. So you can hypothesize that the earlier you start treatment, the better the results," he explained.
Marfan syndrome is a genetic connective tissue disorder affecting multiple organ systems. The prognosis is mainly determined by the aortic complications, including dilatation, aneurysm formation, and possible acute dissection. Affected individuals tend to be tall, long-limbed, and have distinctively long, thin fingers. The prevalence of Marfan syndrome has been estimated at 1 in 5,000, but Dr. Groenink suspects the syndrome may actually be more common than that.
Simultaneous with Dr. Groenink’s presentation at the ESC, the COMPARE results were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht334]).
The COMPARE trial was funded by the Dutch Heart Association. Dr. Groenink reported having no relevant financial interests.
*CORRECTION 11/14/13: The first version of this story had Dr. Groenink's name misspelled.
AT THE ESC CONGRESS 2013
Major finding: The rate of aortic root enlargement during 3 years of prospective follow-up was 0.77 mm in losartan-treated patients with Marfan syndrome, significantly less than the 1.35 mm in patients on standard-of-care treatment with no losartan.
Data source: The COMPARE trial was a randomized, prospective, open-label multicenter study in which 218 patients with Marfan syndrome were randomized to losartan at a target dose of 100 mg or to no losartan and followed for 3 years with the aortic root dilatation rate as measured by MRI the primary endpoint.
Disclosures: The COMPARE trial was supported by the Dutch Heart Association. Dr. Groenink reported having no financial conflicts.
CLARIFY'ed: Angina's impact on outcomes in stable CAD
AMSTERDAM – Sixty percent of all myocardial infarctions and cardiovascular deaths in a large population of stable outpatients with coronary artery disease occurred in patients with neither anginal symptoms in daily life nor evidence of silent myocardial ischemia.
"This emphasizes the need to enforce secondary prevention measures, even in stable asymptomatic patients, Dr. Phillipe G. Steg observed at the annual congress of the European Society of Cardiology.
The risk of these adverse events was significantly greater in patients with stable coronary artery disease (CAD) who had angina than in those with neither angina nor silent myocardial ischemia, and higher still in those with both angina and silent ischemia. But in patients with silent ischemia and no angina, the risk of adverse clinical outcomes wasn’t significantly different than in patients with neither angina nor ischemia, added Dr. Steg, professor of cardiology at the University of Paris.
He presented an analysis from the CLARIFY registry, a prospective registry including 32,396 patients with stable CAD enrolled during 2009-2010 in 45 countries and followed up for 2 years. Participants had to have a baseline history of MI, chest pain with evidence of myocardial ischemia, evidence of CAD on coronary angiography, or prior PCI or CABG surgery.
For this analysis, Dr. Steg focused on the 20,402 CLARIFY participants who underwent noninvasive testing for myocardial ischemia within 1 year prior to enrollment and didn’t undergo revascularization as a result of the findings. These patients fell into four categories: 65% had neither angina nor myocardial ischemia at baseline, 9% had angina without ischemia, 15% had ischemia and no angina, and 11% had both angina and ischemia.
The absolute risk of the primary composite endpoint – cardiovascular death or nonfatal MI – was just under 4% in 2 years of follow-up in the patients with both angina and ischemia.
"I think that low rate corresponds to the modern environment of a well-treated patient population. Even though this was a global registry, patients were remarkably well treated, with a high rate of use of antiplatelet agents, lipid-lowering therapies, ACE inhibitors, and beta-blockers. I think it’s striking that two-thirds of the population had neither angina nor ischemia," he added.
In a multivariate analysis adjusted for demographics, smoking status, dyslipidemia, and diabetes, patients with angina but not silent ischemia had a statistically significant 46% greater risk of cardiovascular death or nonfatal MI than did those with neither angina nor ischemia. Patients with both angina and ischemia had a 76% greater risk than did the comparison group.
For the secondary composite endpoint consisting of cardiovascular death, MI, stroke, or revascularization, the angina-only group was at 38% increased risk and the angina-plus-ischemia group had a 58% greater risk than did patients with neither angina nor ischemia.
Importantly, major bleeding was not linked to the presence of ischemia, angina, or both.
The CLARIFY registry was supported by Servier. Dr. Steg has received research funding from and served as a consultant to the company. He has also consulted for a dozen other pharmaceutical or medical device companies.
AMSTERDAM – Sixty percent of all myocardial infarctions and cardiovascular deaths in a large population of stable outpatients with coronary artery disease occurred in patients with neither anginal symptoms in daily life nor evidence of silent myocardial ischemia.
"This emphasizes the need to enforce secondary prevention measures, even in stable asymptomatic patients, Dr. Phillipe G. Steg observed at the annual congress of the European Society of Cardiology.
The risk of these adverse events was significantly greater in patients with stable coronary artery disease (CAD) who had angina than in those with neither angina nor silent myocardial ischemia, and higher still in those with both angina and silent ischemia. But in patients with silent ischemia and no angina, the risk of adverse clinical outcomes wasn’t significantly different than in patients with neither angina nor ischemia, added Dr. Steg, professor of cardiology at the University of Paris.
He presented an analysis from the CLARIFY registry, a prospective registry including 32,396 patients with stable CAD enrolled during 2009-2010 in 45 countries and followed up for 2 years. Participants had to have a baseline history of MI, chest pain with evidence of myocardial ischemia, evidence of CAD on coronary angiography, or prior PCI or CABG surgery.
For this analysis, Dr. Steg focused on the 20,402 CLARIFY participants who underwent noninvasive testing for myocardial ischemia within 1 year prior to enrollment and didn’t undergo revascularization as a result of the findings. These patients fell into four categories: 65% had neither angina nor myocardial ischemia at baseline, 9% had angina without ischemia, 15% had ischemia and no angina, and 11% had both angina and ischemia.
The absolute risk of the primary composite endpoint – cardiovascular death or nonfatal MI – was just under 4% in 2 years of follow-up in the patients with both angina and ischemia.
"I think that low rate corresponds to the modern environment of a well-treated patient population. Even though this was a global registry, patients were remarkably well treated, with a high rate of use of antiplatelet agents, lipid-lowering therapies, ACE inhibitors, and beta-blockers. I think it’s striking that two-thirds of the population had neither angina nor ischemia," he added.
In a multivariate analysis adjusted for demographics, smoking status, dyslipidemia, and diabetes, patients with angina but not silent ischemia had a statistically significant 46% greater risk of cardiovascular death or nonfatal MI than did those with neither angina nor ischemia. Patients with both angina and ischemia had a 76% greater risk than did the comparison group.
For the secondary composite endpoint consisting of cardiovascular death, MI, stroke, or revascularization, the angina-only group was at 38% increased risk and the angina-plus-ischemia group had a 58% greater risk than did patients with neither angina nor ischemia.
Importantly, major bleeding was not linked to the presence of ischemia, angina, or both.
The CLARIFY registry was supported by Servier. Dr. Steg has received research funding from and served as a consultant to the company. He has also consulted for a dozen other pharmaceutical or medical device companies.
AMSTERDAM – Sixty percent of all myocardial infarctions and cardiovascular deaths in a large population of stable outpatients with coronary artery disease occurred in patients with neither anginal symptoms in daily life nor evidence of silent myocardial ischemia.
"This emphasizes the need to enforce secondary prevention measures, even in stable asymptomatic patients, Dr. Phillipe G. Steg observed at the annual congress of the European Society of Cardiology.
The risk of these adverse events was significantly greater in patients with stable coronary artery disease (CAD) who had angina than in those with neither angina nor silent myocardial ischemia, and higher still in those with both angina and silent ischemia. But in patients with silent ischemia and no angina, the risk of adverse clinical outcomes wasn’t significantly different than in patients with neither angina nor ischemia, added Dr. Steg, professor of cardiology at the University of Paris.
He presented an analysis from the CLARIFY registry, a prospective registry including 32,396 patients with stable CAD enrolled during 2009-2010 in 45 countries and followed up for 2 years. Participants had to have a baseline history of MI, chest pain with evidence of myocardial ischemia, evidence of CAD on coronary angiography, or prior PCI or CABG surgery.
For this analysis, Dr. Steg focused on the 20,402 CLARIFY participants who underwent noninvasive testing for myocardial ischemia within 1 year prior to enrollment and didn’t undergo revascularization as a result of the findings. These patients fell into four categories: 65% had neither angina nor myocardial ischemia at baseline, 9% had angina without ischemia, 15% had ischemia and no angina, and 11% had both angina and ischemia.
The absolute risk of the primary composite endpoint – cardiovascular death or nonfatal MI – was just under 4% in 2 years of follow-up in the patients with both angina and ischemia.
"I think that low rate corresponds to the modern environment of a well-treated patient population. Even though this was a global registry, patients were remarkably well treated, with a high rate of use of antiplatelet agents, lipid-lowering therapies, ACE inhibitors, and beta-blockers. I think it’s striking that two-thirds of the population had neither angina nor ischemia," he added.
In a multivariate analysis adjusted for demographics, smoking status, dyslipidemia, and diabetes, patients with angina but not silent ischemia had a statistically significant 46% greater risk of cardiovascular death or nonfatal MI than did those with neither angina nor ischemia. Patients with both angina and ischemia had a 76% greater risk than did the comparison group.
For the secondary composite endpoint consisting of cardiovascular death, MI, stroke, or revascularization, the angina-only group was at 38% increased risk and the angina-plus-ischemia group had a 58% greater risk than did patients with neither angina nor ischemia.
Importantly, major bleeding was not linked to the presence of ischemia, angina, or both.
The CLARIFY registry was supported by Servier. Dr. Steg has received research funding from and served as a consultant to the company. He has also consulted for a dozen other pharmaceutical or medical device companies.
AT THE ESC CONGRESS 2013
Major finding: Patients with stable coronary artery disease who had angina in their daily life but not silent ischemia had a 46% greater 2-year risk of cardiovascular death or nonfatal MI than did those with neither angina nor ischemia. Those with both angina and ischemia were at 76% increased risk of the composite endpoint.
Data source: This was a prospective registry with 2-year follow-up of more than 32,000 patients with stable coronary artery disease in 45 countries.
Disclosures: The CLARIFY registry was supported by Servier. The presenter is a consultant to the company.