User login
European College of Neuropsychopharmacology (ECNP): Annual Congress
New psychoactive drug nomenclature system devised
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.
"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
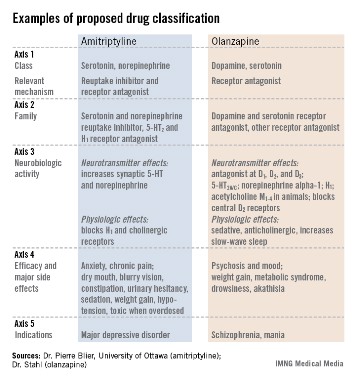
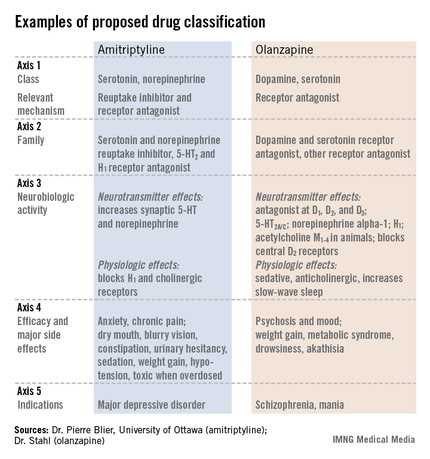
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.

"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
BARCELONA – Neuropsychopharmacologists want to change the way you describe psychoactive drugs.
A team of representatives from four international neuropsychopharmacology groups is finishing a first draft of its proposed new system for drug classification and nomenclature that – in its current form – describes each of approximately 120 psychoactive drugs by five different parameters, or axes, including neurobiologic activity, efficacy, and approved indications worldwide. The goal is a new, Web-based framework for classifying and identifying drugs that is more consistent, informative, and transparent than the existing nomenclature.

"We expect drug nomenclature to reflect current scientific knowledge, give useful pharmacologic information to clinicians, and give patients a rationale for using a drug," but the existing nomenclature does not accomplish those goals, said Dr. Joseph Zohar, director of psychiatry at Sheba Medical Center in Tel Hashomer, Israel, and a member of the nomenclature group. "Existing nomenclature dates from the 1960s; no wonder it does not reflect current understanding. Drugs called antidepressants are often prescribed to patients with anxiety disorders. Patients ask, why give me an antidepressant if I have anxiety? Antipsychotics are prescribed to patients with depression, and they ask why they are getting an antipsychotic? The nomenclature is confusing," Dr. Zohar said at a session at the annual congress of the European College of Neuropsychopharmacology devoted to presenting the new nomenclature system to congress participants.
"We want to rationalize classification and have it better reflect science. We want to focus on mechanisms; knowing the entire profile of medications will help make more informed choices," he said.
"It’s getting people to think more about what drugs do. If people understood drugs better, they would use them better," said Dr. David J. Nutt, professor of neuropsychopharmacology at Imperial College London and another member of the nomenclature group. "The shorthands we currently use [for classifying psychoactive drugs] may be useful, but we need to determine what’s not useful" and get rid of it.
Leadership from the European College of Neuropsychopharmacology (ECNP) arranged for a panel with two representatives each from the American College of Neuropsychopharmacology, the International College of Neuropsychopharmacology, the Asian College of Neuropsychopharmacology, and the ECNP. A draft version of the nomenclature document should appear for comment on the ECNP website by early next year, and the current plan is to publish a finalized version before the end of next year. But the panel members stressed that since the document will primarily be Web-based, it will be open to frequent revision.
"This is the first step in a long process. We’re trying to wake up a 50-year-old nomenclature into a living document that will be used over the next decades and may someday merge with DSM [Diagnostic and Statistical Manual of Mental Disorders]," said Dr. Stephen M. Stahl, a panel member and psychiatrist affiliated with the University of California, San Diego.
The format the panel has in place currently describes every psychoactive drug in terms of five axes: class and relevant mechanisms; family; neurobiologic activity, neurotransmitter effects, and physiologic effects; efficacy and major side effects; and approved and licenses indications in the major countries and regions that perform drug licensing. Some of the collaborators on the panel published an explanation of the five axes template online in September (Euro. Neuropsychopharmacol. 2013 [doi:10.1016/j.euroneuro.2013.08.004]).
All this information for each drug "should tell you everything you need to know to make a rational decision about what to do with the drug," Dr. Nutt said.
"We need to be very humble about these medications and what they do. You have multiple things going on simultaneously," said Dr. Stahl, also founder of the Neuroscience Education Institute in San Diego. "The second-generation antipsychotics are the most complicated drugs in medicine; one patient’s side effect is another’s therapeutic effect.
"We propose that it is no longer appropriate to name all drugs that treat psychosis as ‘antipsychotics.’ We propose a multiaxial classification system based on pharmacologic mechanisms of action. A mechanism-based nomenclature may clarify the differing mechanisms of individual agents, especially actions in psychosis versus mood disorders. This approach has the potential to better inform those who work with drugs that treat depression, and prevent confusion with other drugs that treat both psychosis and multiple additional disorders such as depression and mania. This approach is also a strategy for naming drugs yet to be discovered that target novel mechanisms of action."
"What we don’t want is industry or a regulator to define a new glycine-reuptake blocker as an antipsychotic," Dr. Nutt said.
Several panel members stressed that the five-axis format will allow a diverse list of useful information.
"We don’t want to be constrained by the approved indications," Dr. Nutt said. "This is an opportunity to document off-label indications and to link to the evidence for off-label uses," said Dr. Guy Goodwin, chairman of psychiatry at the University of Oxford (England) and another collaborator on the panel.
The audience of about 150 congress attendees at the session seemed persuaded by the speakers. At the session’s end, in an electronic vote, 67% said they fully agreed, and another 26% said they partly agreed, that the existing nomenclature needs revision. Asked whether the proposed nomenclature was a step in the right direction, 48% said they fully agreed, and another 39% said they partly agreed. Another question asked what feature should drive a drug’s top-line definition. Sixty percent of the audience said pharmacologic action, and an additional 29% voted in favor of clinical indication.
Dr. Zohar said he has been an adviser or consultant to or received research support from eight drug companies. Dr. Nutt said he has been an adviser or consultant to eight drug companies. Dr. Stahl said he has been an adviser or consultant to more than 30 drug companies. Dr. Goodwin said he had been an adviser or consultant to 12 drug companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Second-generation antipsychotics cause modest extrapyramidal symptoms
BARCELONA – The greatest risk that patients with schizophrenia will develop extrapyramidal symptoms while on treatment with a second-generation antipsychotic drug comes during the first few weeks of treatment, and then substantially resolves during the rest of the first year on treatment, according to findings from a multicenter European trial with 498 patients.
In addition, the risk level varies from drug to drug, and some extrapyramidal symptoms are rare, with no treatment-related episodes of dystonia and dyskinesia, including tardive dyskinesia, during the year on treatment, Dr. Janusz K. Rybakowski said at the annual congress of the European College of Neuropsychopharmacology.
"Generally, it can be stated that extrapyramidal symptoms are not much of a problem for patients taking second-generation antipsychotic drugs except for the problem of akathisia with ziprasidone treatment, which should be taken into account" when considering which drug to prescribe, said Dr. Rybakowski, professor and head of adult psychiatry at Poznañ (Poland) University.
In the study, 24% of newly diagnosed schizophrenia patients who received ziprasidone as their initial treatment developed akathisia by 1 month after starting treatment. However, among patients who remained in the study and on ziprasidone treatment for 1 year, the prevalence of akathisia fell to less than 9%, he reported. The analysis also documented the rates at which patients developed parkinsonian symptoms.
Second-generation antipsychotics "have to be looked at for their risk-benefit profile," commented Dr. W. Wolfgang Fleischhacker, professor and director of psychiatry at the Medical University of Innsbruck, Austria, and a EUFEST (European First Episode of Schizophrenia Trial) coinvestigator. "Some of these drugs have problems, but they are also effective. Some may have problems causing metabolic syndrome but not extrapyramidal symptoms, and vise versa. And we lack good predictors" for which patients will develop significant adverse effects on treatment. "Even if olanzapine is the biggest offender with regard to metabolic disturbances, probably only 40% of patients are affected. So we can’t judge these drugs generally; we need to closely monitor patients" to see which ones actually develop a significant adverse effect on treatment, Dr. Fleischhacker said.
Dr. Rybakowski and his associates used data collected in EUFEST, which enrolled 498 patients at 50 centers in Europe and in Israel (Lancet 2008;371:1085-97). The investigators randomized patients to treatment with olanzapine, quetiapine, amisulpride, ziprasidone, or to the first-generation antipsychotic haloperidol.
After 1 month, the incidence of parkinsonian symptoms ranged from 4% among those on olanzapine to 13% of those on ziprasidone (and 26% for those on haloperidol). But by 12 months, the rate had dropped to zero among patients on olanzapine and also dropped among all other patients. At 1 year, the highest rate, 9%, was among patients on haloperidol. After 1 month, the incidence of akathisia ranged from 3% among those on olanzapine to 24% for those on ziprasidone (and 21% for patients on haloperidol); after 12 months, the rate fell to zero in patients on olanzapine or amisulpride and was highest, at 7%, among patients on quetiapine.
Although no patients on any second-generation drug developed new symptoms of dystonia or dyskinesia, a few on haloperidol developed dyskinesia.
The percent of patients receiving an anticholinergic drug at 1 month to treat their extrapyramidal symptoms ranged from 13% of those on ziprasidone, 10% on amisulpride, 3% on olanzapine, 2% on quetiapine, and 24% on haloperidol. After 12 months, the rate of anticholinergic drug use fell to a high of 11% on haloperidol and 6% for amisulpride. Patients on all the other second-generation antipsychotics had lower rates of anticholinergic drug use.
EUFEST was sponsored by AstraZeneca, Sanofi-Aventis, and Pfizer. Dr. Rybakowski said he has been a consultant to Adamed, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Sanofi-Aventis, and Servier. Dr. Fleischhacker has received honoraria as a speaker or consultant to Bristol-Myers Squibb, Janssen, Lundbeck, MedAvante, Merck, Otsuka, Pfizer, and Roche.
On Twitter @mitchelzoler
BARCELONA – The greatest risk that patients with schizophrenia will develop extrapyramidal symptoms while on treatment with a second-generation antipsychotic drug comes during the first few weeks of treatment, and then substantially resolves during the rest of the first year on treatment, according to findings from a multicenter European trial with 498 patients.
In addition, the risk level varies from drug to drug, and some extrapyramidal symptoms are rare, with no treatment-related episodes of dystonia and dyskinesia, including tardive dyskinesia, during the year on treatment, Dr. Janusz K. Rybakowski said at the annual congress of the European College of Neuropsychopharmacology.
"Generally, it can be stated that extrapyramidal symptoms are not much of a problem for patients taking second-generation antipsychotic drugs except for the problem of akathisia with ziprasidone treatment, which should be taken into account" when considering which drug to prescribe, said Dr. Rybakowski, professor and head of adult psychiatry at Poznañ (Poland) University.
In the study, 24% of newly diagnosed schizophrenia patients who received ziprasidone as their initial treatment developed akathisia by 1 month after starting treatment. However, among patients who remained in the study and on ziprasidone treatment for 1 year, the prevalence of akathisia fell to less than 9%, he reported. The analysis also documented the rates at which patients developed parkinsonian symptoms.
Second-generation antipsychotics "have to be looked at for their risk-benefit profile," commented Dr. W. Wolfgang Fleischhacker, professor and director of psychiatry at the Medical University of Innsbruck, Austria, and a EUFEST (European First Episode of Schizophrenia Trial) coinvestigator. "Some of these drugs have problems, but they are also effective. Some may have problems causing metabolic syndrome but not extrapyramidal symptoms, and vise versa. And we lack good predictors" for which patients will develop significant adverse effects on treatment. "Even if olanzapine is the biggest offender with regard to metabolic disturbances, probably only 40% of patients are affected. So we can’t judge these drugs generally; we need to closely monitor patients" to see which ones actually develop a significant adverse effect on treatment, Dr. Fleischhacker said.
Dr. Rybakowski and his associates used data collected in EUFEST, which enrolled 498 patients at 50 centers in Europe and in Israel (Lancet 2008;371:1085-97). The investigators randomized patients to treatment with olanzapine, quetiapine, amisulpride, ziprasidone, or to the first-generation antipsychotic haloperidol.
After 1 month, the incidence of parkinsonian symptoms ranged from 4% among those on olanzapine to 13% of those on ziprasidone (and 26% for those on haloperidol). But by 12 months, the rate had dropped to zero among patients on olanzapine and also dropped among all other patients. At 1 year, the highest rate, 9%, was among patients on haloperidol. After 1 month, the incidence of akathisia ranged from 3% among those on olanzapine to 24% for those on ziprasidone (and 21% for patients on haloperidol); after 12 months, the rate fell to zero in patients on olanzapine or amisulpride and was highest, at 7%, among patients on quetiapine.
Although no patients on any second-generation drug developed new symptoms of dystonia or dyskinesia, a few on haloperidol developed dyskinesia.
The percent of patients receiving an anticholinergic drug at 1 month to treat their extrapyramidal symptoms ranged from 13% of those on ziprasidone, 10% on amisulpride, 3% on olanzapine, 2% on quetiapine, and 24% on haloperidol. After 12 months, the rate of anticholinergic drug use fell to a high of 11% on haloperidol and 6% for amisulpride. Patients on all the other second-generation antipsychotics had lower rates of anticholinergic drug use.
EUFEST was sponsored by AstraZeneca, Sanofi-Aventis, and Pfizer. Dr. Rybakowski said he has been a consultant to Adamed, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Sanofi-Aventis, and Servier. Dr. Fleischhacker has received honoraria as a speaker or consultant to Bristol-Myers Squibb, Janssen, Lundbeck, MedAvante, Merck, Otsuka, Pfizer, and Roche.
On Twitter @mitchelzoler
BARCELONA – The greatest risk that patients with schizophrenia will develop extrapyramidal symptoms while on treatment with a second-generation antipsychotic drug comes during the first few weeks of treatment, and then substantially resolves during the rest of the first year on treatment, according to findings from a multicenter European trial with 498 patients.
In addition, the risk level varies from drug to drug, and some extrapyramidal symptoms are rare, with no treatment-related episodes of dystonia and dyskinesia, including tardive dyskinesia, during the year on treatment, Dr. Janusz K. Rybakowski said at the annual congress of the European College of Neuropsychopharmacology.
"Generally, it can be stated that extrapyramidal symptoms are not much of a problem for patients taking second-generation antipsychotic drugs except for the problem of akathisia with ziprasidone treatment, which should be taken into account" when considering which drug to prescribe, said Dr. Rybakowski, professor and head of adult psychiatry at Poznañ (Poland) University.
In the study, 24% of newly diagnosed schizophrenia patients who received ziprasidone as their initial treatment developed akathisia by 1 month after starting treatment. However, among patients who remained in the study and on ziprasidone treatment for 1 year, the prevalence of akathisia fell to less than 9%, he reported. The analysis also documented the rates at which patients developed parkinsonian symptoms.
Second-generation antipsychotics "have to be looked at for their risk-benefit profile," commented Dr. W. Wolfgang Fleischhacker, professor and director of psychiatry at the Medical University of Innsbruck, Austria, and a EUFEST (European First Episode of Schizophrenia Trial) coinvestigator. "Some of these drugs have problems, but they are also effective. Some may have problems causing metabolic syndrome but not extrapyramidal symptoms, and vise versa. And we lack good predictors" for which patients will develop significant adverse effects on treatment. "Even if olanzapine is the biggest offender with regard to metabolic disturbances, probably only 40% of patients are affected. So we can’t judge these drugs generally; we need to closely monitor patients" to see which ones actually develop a significant adverse effect on treatment, Dr. Fleischhacker said.
Dr. Rybakowski and his associates used data collected in EUFEST, which enrolled 498 patients at 50 centers in Europe and in Israel (Lancet 2008;371:1085-97). The investigators randomized patients to treatment with olanzapine, quetiapine, amisulpride, ziprasidone, or to the first-generation antipsychotic haloperidol.
After 1 month, the incidence of parkinsonian symptoms ranged from 4% among those on olanzapine to 13% of those on ziprasidone (and 26% for those on haloperidol). But by 12 months, the rate had dropped to zero among patients on olanzapine and also dropped among all other patients. At 1 year, the highest rate, 9%, was among patients on haloperidol. After 1 month, the incidence of akathisia ranged from 3% among those on olanzapine to 24% for those on ziprasidone (and 21% for patients on haloperidol); after 12 months, the rate fell to zero in patients on olanzapine or amisulpride and was highest, at 7%, among patients on quetiapine.
Although no patients on any second-generation drug developed new symptoms of dystonia or dyskinesia, a few on haloperidol developed dyskinesia.
The percent of patients receiving an anticholinergic drug at 1 month to treat their extrapyramidal symptoms ranged from 13% of those on ziprasidone, 10% on amisulpride, 3% on olanzapine, 2% on quetiapine, and 24% on haloperidol. After 12 months, the rate of anticholinergic drug use fell to a high of 11% on haloperidol and 6% for amisulpride. Patients on all the other second-generation antipsychotics had lower rates of anticholinergic drug use.
EUFEST was sponsored by AstraZeneca, Sanofi-Aventis, and Pfizer. Dr. Rybakowski said he has been a consultant to Adamed, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Sanofi-Aventis, and Servier. Dr. Fleischhacker has received honoraria as a speaker or consultant to Bristol-Myers Squibb, Janssen, Lundbeck, MedAvante, Merck, Otsuka, Pfizer, and Roche.
On Twitter @mitchelzoler
AT THE ECNP CONGRESS
Major finding: Ziprasidone caused the highest incidence of extrapyramidal symptoms: a 24% akathisia rate and 13% parkinsonian-symptom rate after 1 month.
Data source: EUFEST, a multicenter, randomized study with 498 patients with first-episode schizophrenia.
Disclosures: EUFEST was sponsored by AstraZeneca, Sanofi-Aventis, and Pfizer. Dr. Rybakowski said that he has been a consultant to Adamed, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Sanofi-Aventis, and Servier. Dr. Fleischhacker has received honoraria as a speaker or consultant to Bristol-Myers Squibb, Janssen, Lundbeck, MedAvante, Merck, Otsuka, Pfizer, and Roche.
Fibromyalgia patients may harbor fewer small-nerve fibers
BARCELONA – Many patients with fibromyalgia syndrome have an unusually low number of small-nerve fibers in their skin, a finding that may provide important new insights into the pathology, etiology, and possible treatment of what has been a poorly understood disorder, according to Dr. Claudia Sommer.
"With three different methods, we showed pathologic changes in small-nerve fibers" in the skin of patients diagnosed with fibromyalgia, she said at the annual congress of the European College of Neuropsychopharmacology. These somatic findings are too strong to allow classification of these patients with fibromyalgia as having a "somatoform pain disorder." She suggested that the nerve deficits in patients’ skin could reflect systemic nerve damage that causes the deep pain that fibromyalgia patients have in muscles, tendons, and joints. "What we see in the skin may also exist elsewhere," said Dr. Sommer, professor of neurology at the University Clinic of Würzburg (Germany).
Three other, independent research groups have recently made similar findings of small-nerve deficits in the skin of fibromyalgia patients, including researchers at Massachusetts General Hospital in Boston (Pain 2013;154:2310-6), further boosting the likelihood that impaired small-nerve function is real and a key feature of at least some fibromyalgia patients, Dr. Sommer said.
The finding led her to develop a new model for the pathogenesis of these cases: An adverse life event or other predisposing factor leads to a more proinflammatory cytokine profile that causes small-nerve fiber damage and ongoing pain system activity that produces central sensitization and changes in central pain processing.
"This is just a model, but we can work on this and test hypotheses," she said. "We are a long way from finding new treatments, but trying to understand what causes damage to nerve fibers may give a start, something a new treatment could be directed against."
Dr. Sommer and her associates studied 25 patients who had been diagnosed with fibromyalgia, 10 diagnosed with unipolar depression, and 25 healthy controls who were matched by age and sex with the fibromyalgia patients. The participants ranged from 39 to 75 years old, and those with either depression or fibromyalgia had been diagnosed for an average of more than 20 years.
Compared with the controls, patients with fibromyalgia had significantly increased detection thresholds to warm and cold when quantitative sensory testing was used, while the depressed patients had no significant difference compared with the controls. Measurement of pain-related evoked potentials showed increased latency on stimulation of the feet and reduced amplitudes on stimulation of the feet, hands, and face in fibromyalgia patients compared with both the controls and the patients with depression.
The researchers also found reduced numbers of total and regenerating intraepidermal, unmyelinated nerve fibers in skin biopsies from the lower leg and upper thigh of the patients with fibromyalgia compared with the controls and the patients with depression. The nerve fiber count averaged 5 fibers/mm2 in the patients with fibromyalgia, about 7/mm2 in patients with depression, and about 8/mm2 in the controls. The difference between the fibromyalgia patients and the controls was statistically significant.
Dr. Sommer and her associates also published these results in Brain (2013;136:1857-67).
Dr. Sommer said she has received honoraria for being a speaker on behalf of Allergan, Astella, Baxter, CSL Behring, Eli Lilly, Genzyme, GlaxoSmithKline, and Pfizer.
On Twitter @mitchelzoler
BARCELONA – Many patients with fibromyalgia syndrome have an unusually low number of small-nerve fibers in their skin, a finding that may provide important new insights into the pathology, etiology, and possible treatment of what has been a poorly understood disorder, according to Dr. Claudia Sommer.
"With three different methods, we showed pathologic changes in small-nerve fibers" in the skin of patients diagnosed with fibromyalgia, she said at the annual congress of the European College of Neuropsychopharmacology. These somatic findings are too strong to allow classification of these patients with fibromyalgia as having a "somatoform pain disorder." She suggested that the nerve deficits in patients’ skin could reflect systemic nerve damage that causes the deep pain that fibromyalgia patients have in muscles, tendons, and joints. "What we see in the skin may also exist elsewhere," said Dr. Sommer, professor of neurology at the University Clinic of Würzburg (Germany).
Three other, independent research groups have recently made similar findings of small-nerve deficits in the skin of fibromyalgia patients, including researchers at Massachusetts General Hospital in Boston (Pain 2013;154:2310-6), further boosting the likelihood that impaired small-nerve function is real and a key feature of at least some fibromyalgia patients, Dr. Sommer said.
The finding led her to develop a new model for the pathogenesis of these cases: An adverse life event or other predisposing factor leads to a more proinflammatory cytokine profile that causes small-nerve fiber damage and ongoing pain system activity that produces central sensitization and changes in central pain processing.
"This is just a model, but we can work on this and test hypotheses," she said. "We are a long way from finding new treatments, but trying to understand what causes damage to nerve fibers may give a start, something a new treatment could be directed against."
Dr. Sommer and her associates studied 25 patients who had been diagnosed with fibromyalgia, 10 diagnosed with unipolar depression, and 25 healthy controls who were matched by age and sex with the fibromyalgia patients. The participants ranged from 39 to 75 years old, and those with either depression or fibromyalgia had been diagnosed for an average of more than 20 years.
Compared with the controls, patients with fibromyalgia had significantly increased detection thresholds to warm and cold when quantitative sensory testing was used, while the depressed patients had no significant difference compared with the controls. Measurement of pain-related evoked potentials showed increased latency on stimulation of the feet and reduced amplitudes on stimulation of the feet, hands, and face in fibromyalgia patients compared with both the controls and the patients with depression.
The researchers also found reduced numbers of total and regenerating intraepidermal, unmyelinated nerve fibers in skin biopsies from the lower leg and upper thigh of the patients with fibromyalgia compared with the controls and the patients with depression. The nerve fiber count averaged 5 fibers/mm2 in the patients with fibromyalgia, about 7/mm2 in patients with depression, and about 8/mm2 in the controls. The difference between the fibromyalgia patients and the controls was statistically significant.
Dr. Sommer and her associates also published these results in Brain (2013;136:1857-67).
Dr. Sommer said she has received honoraria for being a speaker on behalf of Allergan, Astella, Baxter, CSL Behring, Eli Lilly, Genzyme, GlaxoSmithKline, and Pfizer.
On Twitter @mitchelzoler
BARCELONA – Many patients with fibromyalgia syndrome have an unusually low number of small-nerve fibers in their skin, a finding that may provide important new insights into the pathology, etiology, and possible treatment of what has been a poorly understood disorder, according to Dr. Claudia Sommer.
"With three different methods, we showed pathologic changes in small-nerve fibers" in the skin of patients diagnosed with fibromyalgia, she said at the annual congress of the European College of Neuropsychopharmacology. These somatic findings are too strong to allow classification of these patients with fibromyalgia as having a "somatoform pain disorder." She suggested that the nerve deficits in patients’ skin could reflect systemic nerve damage that causes the deep pain that fibromyalgia patients have in muscles, tendons, and joints. "What we see in the skin may also exist elsewhere," said Dr. Sommer, professor of neurology at the University Clinic of Würzburg (Germany).
Three other, independent research groups have recently made similar findings of small-nerve deficits in the skin of fibromyalgia patients, including researchers at Massachusetts General Hospital in Boston (Pain 2013;154:2310-6), further boosting the likelihood that impaired small-nerve function is real and a key feature of at least some fibromyalgia patients, Dr. Sommer said.
The finding led her to develop a new model for the pathogenesis of these cases: An adverse life event or other predisposing factor leads to a more proinflammatory cytokine profile that causes small-nerve fiber damage and ongoing pain system activity that produces central sensitization and changes in central pain processing.
"This is just a model, but we can work on this and test hypotheses," she said. "We are a long way from finding new treatments, but trying to understand what causes damage to nerve fibers may give a start, something a new treatment could be directed against."
Dr. Sommer and her associates studied 25 patients who had been diagnosed with fibromyalgia, 10 diagnosed with unipolar depression, and 25 healthy controls who were matched by age and sex with the fibromyalgia patients. The participants ranged from 39 to 75 years old, and those with either depression or fibromyalgia had been diagnosed for an average of more than 20 years.
Compared with the controls, patients with fibromyalgia had significantly increased detection thresholds to warm and cold when quantitative sensory testing was used, while the depressed patients had no significant difference compared with the controls. Measurement of pain-related evoked potentials showed increased latency on stimulation of the feet and reduced amplitudes on stimulation of the feet, hands, and face in fibromyalgia patients compared with both the controls and the patients with depression.
The researchers also found reduced numbers of total and regenerating intraepidermal, unmyelinated nerve fibers in skin biopsies from the lower leg and upper thigh of the patients with fibromyalgia compared with the controls and the patients with depression. The nerve fiber count averaged 5 fibers/mm2 in the patients with fibromyalgia, about 7/mm2 in patients with depression, and about 8/mm2 in the controls. The difference between the fibromyalgia patients and the controls was statistically significant.
Dr. Sommer and her associates also published these results in Brain (2013;136:1857-67).
Dr. Sommer said she has received honoraria for being a speaker on behalf of Allergan, Astella, Baxter, CSL Behring, Eli Lilly, Genzyme, GlaxoSmithKline, and Pfizer.
On Twitter @mitchelzoler
AT THE ECNP CONGRESS
Major finding: Skin biopsies from patients with fibromyalgia averaged 5 fibers/mm2 compared with 8 fibers/mm2 in matched control patients.
Data source: A single-center study of 25 adults diagnosed with fibromyalgia syndrome and 25 healthy controls matched by age and sex.
Disclosures: Dr. Sommer that she has received honoraria for being a speaker on behalf of Allergan, Astella, Baxter, CSL Behring, Eli Lilly, Genzyme, GlaxoSmithKline, and Pfizer.
Did the DSM-5 pathologize what’s normal?
Some elements of the DSM-5, released nearly 6 months ago now, follow a tricky path between giving the psychiatric community useful, new diagnostic tools and pathologizing what is part of the normal range of human behavior, but despite that, the manual seems to generally succeed in "better classifying the patients who come to psychiatrists," said Dr. Guy Goodwin, head of the psychiatry department at the University of Oxford (England).
Dr. Goodwin spoke in an unusual press conference during the annual Congress of the European College of Neuropsychopharmacology (ECNP) in Barcelona in October. The topic was the still somewhat new Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), and the role of diagnosis in psychiatry, which did not link to any report or talk at the meeting. The press conference seemed mostly a way for Dr. Goodwin, who became president of the ECNP during the meeting, and two other participants to give their thoughts on the DSM-5 and shifting views on psychiatric diagnoses, especially in children.
Dr. Goodwin ultimately called himself "sort of pro-DSM-5" and said that the key test of whether the diagnostic changes made by the DSM-5 were a step forward or not will come with time, as psychiatrists determine whether following the DSM-5 results in better diagnosis and patient care.
He offered an example of what he said some have characterized as a disorder "invented" by the DSM-5, disruptive mood dysregulation disorder (DMDD). It was an interesting choice for Dr. Goodwin to highlight, as he is not a pediatric specialist, although he noted it ties to his practice of treating patients with bipolar disorder because DMDD was designed as a replacement diagnosis for many children previously diagnosed with bipolar disorder. DMDD also has a history of controversy going back several years to when the DSM-5 writing group struggled with how to deal with what many saw as overdiagnosis of bipolar disease in children.
The DSM-5 "is trying to stop" the diagnosis of bipolar disease by U.S. psychiatrists in children as young as 3 years old "by inventing a disorder that describes these problematic children." DMDD is "a diagnosis that takes you into areas of judgment that many people are uncomfortable with," such as deciding whether outbursts by children are "grossly out of proportion" to the situation, Dr. Goodwin said. "It’s specific to the United States, and I think the rest of the world has difficulty with this. You’ve added another diagnosis that’s carved out of normal childhood experience. This is the difficulty for people with the perspective that all diagnoses are bad labels."
But Dr. Goodwin also noted that the DSM-5 "set the bar high" when designing the diagnosis. "U.S. psychiatrists see this as a way to stop the pathologizing of normal life" by creating a high threshold for diagnosis that is rarely met.
Dr. Goodwin also stressed that psychiatric diagnoses are not inherently bad for patients. Research findings, including work he collaborated on, showed that patients generally welcome a diagnosis and don’t feel stigmatized by it.
"Diagnoses are useful, because they give patients a starting point for understanding their problems and give clinicians a starting point for understanding" the best way to manage each patient, he said. "What patients want is an explanation of their situation."
A second speaker at the press conference, Dr. Celso Arango, head of child and adolescent psychiatry at a university hospital in Madrid and president-elect of ECNP, offered his own take on diagnosing psychiatric disorders in children that indirectly added another dimension to questions about DMDD’s validity and role.
Although psychiatric disorders are usually first diagnosed in adults, "they start much earlier. More than 70% of mental illnesses present first symptoms during childhood," said Dr. Arango. "The earlier we intervene, the lower the risk of a more severe disorder." He cited recent study results documenting the cost effectiveness of early intervention in children diagnosed with psychosis.
The DMDD diagnosis "may or may not be useful. If you count kids with this new diagnosis and find that it’s useful, that it’s a precursor for later problems, and is something we need to take seriously and treat, then it may be a prelude to successful intervention," Dr. Goodwin said.
On Twitter @mitchelzoler
Some elements of the DSM-5, released nearly 6 months ago now, follow a tricky path between giving the psychiatric community useful, new diagnostic tools and pathologizing what is part of the normal range of human behavior, but despite that, the manual seems to generally succeed in "better classifying the patients who come to psychiatrists," said Dr. Guy Goodwin, head of the psychiatry department at the University of Oxford (England).
Dr. Goodwin spoke in an unusual press conference during the annual Congress of the European College of Neuropsychopharmacology (ECNP) in Barcelona in October. The topic was the still somewhat new Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), and the role of diagnosis in psychiatry, which did not link to any report or talk at the meeting. The press conference seemed mostly a way for Dr. Goodwin, who became president of the ECNP during the meeting, and two other participants to give their thoughts on the DSM-5 and shifting views on psychiatric diagnoses, especially in children.
Dr. Goodwin ultimately called himself "sort of pro-DSM-5" and said that the key test of whether the diagnostic changes made by the DSM-5 were a step forward or not will come with time, as psychiatrists determine whether following the DSM-5 results in better diagnosis and patient care.
He offered an example of what he said some have characterized as a disorder "invented" by the DSM-5, disruptive mood dysregulation disorder (DMDD). It was an interesting choice for Dr. Goodwin to highlight, as he is not a pediatric specialist, although he noted it ties to his practice of treating patients with bipolar disorder because DMDD was designed as a replacement diagnosis for many children previously diagnosed with bipolar disorder. DMDD also has a history of controversy going back several years to when the DSM-5 writing group struggled with how to deal with what many saw as overdiagnosis of bipolar disease in children.
The DSM-5 "is trying to stop" the diagnosis of bipolar disease by U.S. psychiatrists in children as young as 3 years old "by inventing a disorder that describes these problematic children." DMDD is "a diagnosis that takes you into areas of judgment that many people are uncomfortable with," such as deciding whether outbursts by children are "grossly out of proportion" to the situation, Dr. Goodwin said. "It’s specific to the United States, and I think the rest of the world has difficulty with this. You’ve added another diagnosis that’s carved out of normal childhood experience. This is the difficulty for people with the perspective that all diagnoses are bad labels."
But Dr. Goodwin also noted that the DSM-5 "set the bar high" when designing the diagnosis. "U.S. psychiatrists see this as a way to stop the pathologizing of normal life" by creating a high threshold for diagnosis that is rarely met.
Dr. Goodwin also stressed that psychiatric diagnoses are not inherently bad for patients. Research findings, including work he collaborated on, showed that patients generally welcome a diagnosis and don’t feel stigmatized by it.
"Diagnoses are useful, because they give patients a starting point for understanding their problems and give clinicians a starting point for understanding" the best way to manage each patient, he said. "What patients want is an explanation of their situation."
A second speaker at the press conference, Dr. Celso Arango, head of child and adolescent psychiatry at a university hospital in Madrid and president-elect of ECNP, offered his own take on diagnosing psychiatric disorders in children that indirectly added another dimension to questions about DMDD’s validity and role.
Although psychiatric disorders are usually first diagnosed in adults, "they start much earlier. More than 70% of mental illnesses present first symptoms during childhood," said Dr. Arango. "The earlier we intervene, the lower the risk of a more severe disorder." He cited recent study results documenting the cost effectiveness of early intervention in children diagnosed with psychosis.
The DMDD diagnosis "may or may not be useful. If you count kids with this new diagnosis and find that it’s useful, that it’s a precursor for later problems, and is something we need to take seriously and treat, then it may be a prelude to successful intervention," Dr. Goodwin said.
On Twitter @mitchelzoler
Some elements of the DSM-5, released nearly 6 months ago now, follow a tricky path between giving the psychiatric community useful, new diagnostic tools and pathologizing what is part of the normal range of human behavior, but despite that, the manual seems to generally succeed in "better classifying the patients who come to psychiatrists," said Dr. Guy Goodwin, head of the psychiatry department at the University of Oxford (England).
Dr. Goodwin spoke in an unusual press conference during the annual Congress of the European College of Neuropsychopharmacology (ECNP) in Barcelona in October. The topic was the still somewhat new Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), and the role of diagnosis in psychiatry, which did not link to any report or talk at the meeting. The press conference seemed mostly a way for Dr. Goodwin, who became president of the ECNP during the meeting, and two other participants to give their thoughts on the DSM-5 and shifting views on psychiatric diagnoses, especially in children.
Dr. Goodwin ultimately called himself "sort of pro-DSM-5" and said that the key test of whether the diagnostic changes made by the DSM-5 were a step forward or not will come with time, as psychiatrists determine whether following the DSM-5 results in better diagnosis and patient care.
He offered an example of what he said some have characterized as a disorder "invented" by the DSM-5, disruptive mood dysregulation disorder (DMDD). It was an interesting choice for Dr. Goodwin to highlight, as he is not a pediatric specialist, although he noted it ties to his practice of treating patients with bipolar disorder because DMDD was designed as a replacement diagnosis for many children previously diagnosed with bipolar disorder. DMDD also has a history of controversy going back several years to when the DSM-5 writing group struggled with how to deal with what many saw as overdiagnosis of bipolar disease in children.
The DSM-5 "is trying to stop" the diagnosis of bipolar disease by U.S. psychiatrists in children as young as 3 years old "by inventing a disorder that describes these problematic children." DMDD is "a diagnosis that takes you into areas of judgment that many people are uncomfortable with," such as deciding whether outbursts by children are "grossly out of proportion" to the situation, Dr. Goodwin said. "It’s specific to the United States, and I think the rest of the world has difficulty with this. You’ve added another diagnosis that’s carved out of normal childhood experience. This is the difficulty for people with the perspective that all diagnoses are bad labels."
But Dr. Goodwin also noted that the DSM-5 "set the bar high" when designing the diagnosis. "U.S. psychiatrists see this as a way to stop the pathologizing of normal life" by creating a high threshold for diagnosis that is rarely met.
Dr. Goodwin also stressed that psychiatric diagnoses are not inherently bad for patients. Research findings, including work he collaborated on, showed that patients generally welcome a diagnosis and don’t feel stigmatized by it.
"Diagnoses are useful, because they give patients a starting point for understanding their problems and give clinicians a starting point for understanding" the best way to manage each patient, he said. "What patients want is an explanation of their situation."
A second speaker at the press conference, Dr. Celso Arango, head of child and adolescent psychiatry at a university hospital in Madrid and president-elect of ECNP, offered his own take on diagnosing psychiatric disorders in children that indirectly added another dimension to questions about DMDD’s validity and role.
Although psychiatric disorders are usually first diagnosed in adults, "they start much earlier. More than 70% of mental illnesses present first symptoms during childhood," said Dr. Arango. "The earlier we intervene, the lower the risk of a more severe disorder." He cited recent study results documenting the cost effectiveness of early intervention in children diagnosed with psychosis.
The DMDD diagnosis "may or may not be useful. If you count kids with this new diagnosis and find that it’s useful, that it’s a precursor for later problems, and is something we need to take seriously and treat, then it may be a prelude to successful intervention," Dr. Goodwin said.
On Twitter @mitchelzoler
NMDA blocker lanicemine safely reduces depression
BARCELONA – Lanicemine, a new drug active in the glutamate neurotransmitter system, showed safety and efficacy for treating major depressive disorder during 3 weeks of treatment in a multicenter, U.S. phase II study with 152 patients.
In addition, 3 weeks of lanicemine treatment did not result in any marked psychomimetic effects such as those produced by ketamine, an anesthetic and related drug that first showed clinical antidepressant effects more than a decade ago and led to lanicemine’s testing, Dr. Gerard Sanacora said at the annual congress of the European College of Neuropsychopharmacology.
With the new lanicemine results, "I think we’re getting closer to a proof of concept that an NMDA [N-methyl-D-aspartate] receptor antagonist could be useful for development of antidepressant drugs," said Dr. Sanacora, professor of psychiatry and director of the depression research program at Yale University in New Haven, Conn.
"The results are encouraging. The study adds a lot" to addressing whether treatments targeted at the NMDA receptor can help patients with mood disorder, he said in an interview.
The study enrolled 152 patients diagnosed with major depressive disorder and poor responses to at least two antidepressants at 30 U.S. centers. While patients remained on their entry drugs, the researchers administered 100 or 150 mg lanicemine or placebo as an intravenous infusion three times a week for 3 weeks. At 3 weeks, the change from baseline in total Montgomery Åsberg Depression Rating Scale (MADRS) score, the primary endpoint, was about a 13-point decline with either dosage of lanicemine and a decline of about 8 points with placebo, a 5-point increased reduction with either lanicemine dosage that was statistically significant for each dosage.
The 100-mg dosage showed more consistent efficacy than the higher dosage. The 100-mg dosage produced significantly greater reductions from baseline, compared with placebo after 2 weeks on treatment, and also at 4 and 5 weeks after treatment began, when patients no longer received infusions. The higher lanicemine dosage did not show significant effects compared with placebo at these other time points.
Lanicemine treatment, especially the 100-mg dosage, also showed efficacy by several secondary measures. For example, the proportion of patients who recorded a clinical global impression of much improved or very much improved was 65% with the 100-mg dosage at weeks 3, 4, and 5 after treatment began, significantly better than the 25%-30% rates at the same time points in the placebo group. Dr. Sanacora and his associates also published their results online (Mol. Psychiatry 2013 [doi:10.1038/mp.2013.130]).
During the study, neither lanicemine dosage produced any clinically meaningful difference compared with placebo for dissociative symptoms, and lanicemine was generally well tolerated. The most common adverse effect was dizziness around the time of infusion, which occurred in a third to half of the lanicemine patients, compared with 12% of those on placebo. Lanicemine "looked dramatically different from ketamine," Dr. Sanacora said.
He also downplayed the barrier that treatment by infusion presents. "There is such an unmet need" for patients with severe depression who fail to respond to existing drugs that infusion is not a major issue, he said. But "several companies are trying to develop novel formulations of NMDA receptor–targeted drugs" that might be active orally or intranasally.
The study was funded by AstraZeneca, the company developing lanicemine. Dr. Sanacora said he has been a consultant to and has received research support from AstraZeneca and several other drug companies. Some of Dr. Sanacora’s associates on this study were AstraZeneca employees.
On Twitter @mitchelzoler
BARCELONA – Lanicemine, a new drug active in the glutamate neurotransmitter system, showed safety and efficacy for treating major depressive disorder during 3 weeks of treatment in a multicenter, U.S. phase II study with 152 patients.
In addition, 3 weeks of lanicemine treatment did not result in any marked psychomimetic effects such as those produced by ketamine, an anesthetic and related drug that first showed clinical antidepressant effects more than a decade ago and led to lanicemine’s testing, Dr. Gerard Sanacora said at the annual congress of the European College of Neuropsychopharmacology.
With the new lanicemine results, "I think we’re getting closer to a proof of concept that an NMDA [N-methyl-D-aspartate] receptor antagonist could be useful for development of antidepressant drugs," said Dr. Sanacora, professor of psychiatry and director of the depression research program at Yale University in New Haven, Conn.
"The results are encouraging. The study adds a lot" to addressing whether treatments targeted at the NMDA receptor can help patients with mood disorder, he said in an interview.
The study enrolled 152 patients diagnosed with major depressive disorder and poor responses to at least two antidepressants at 30 U.S. centers. While patients remained on their entry drugs, the researchers administered 100 or 150 mg lanicemine or placebo as an intravenous infusion three times a week for 3 weeks. At 3 weeks, the change from baseline in total Montgomery Åsberg Depression Rating Scale (MADRS) score, the primary endpoint, was about a 13-point decline with either dosage of lanicemine and a decline of about 8 points with placebo, a 5-point increased reduction with either lanicemine dosage that was statistically significant for each dosage.
The 100-mg dosage showed more consistent efficacy than the higher dosage. The 100-mg dosage produced significantly greater reductions from baseline, compared with placebo after 2 weeks on treatment, and also at 4 and 5 weeks after treatment began, when patients no longer received infusions. The higher lanicemine dosage did not show significant effects compared with placebo at these other time points.
Lanicemine treatment, especially the 100-mg dosage, also showed efficacy by several secondary measures. For example, the proportion of patients who recorded a clinical global impression of much improved or very much improved was 65% with the 100-mg dosage at weeks 3, 4, and 5 after treatment began, significantly better than the 25%-30% rates at the same time points in the placebo group. Dr. Sanacora and his associates also published their results online (Mol. Psychiatry 2013 [doi:10.1038/mp.2013.130]).
During the study, neither lanicemine dosage produced any clinically meaningful difference compared with placebo for dissociative symptoms, and lanicemine was generally well tolerated. The most common adverse effect was dizziness around the time of infusion, which occurred in a third to half of the lanicemine patients, compared with 12% of those on placebo. Lanicemine "looked dramatically different from ketamine," Dr. Sanacora said.
He also downplayed the barrier that treatment by infusion presents. "There is such an unmet need" for patients with severe depression who fail to respond to existing drugs that infusion is not a major issue, he said. But "several companies are trying to develop novel formulations of NMDA receptor–targeted drugs" that might be active orally or intranasally.
The study was funded by AstraZeneca, the company developing lanicemine. Dr. Sanacora said he has been a consultant to and has received research support from AstraZeneca and several other drug companies. Some of Dr. Sanacora’s associates on this study were AstraZeneca employees.
On Twitter @mitchelzoler
BARCELONA – Lanicemine, a new drug active in the glutamate neurotransmitter system, showed safety and efficacy for treating major depressive disorder during 3 weeks of treatment in a multicenter, U.S. phase II study with 152 patients.
In addition, 3 weeks of lanicemine treatment did not result in any marked psychomimetic effects such as those produced by ketamine, an anesthetic and related drug that first showed clinical antidepressant effects more than a decade ago and led to lanicemine’s testing, Dr. Gerard Sanacora said at the annual congress of the European College of Neuropsychopharmacology.
With the new lanicemine results, "I think we’re getting closer to a proof of concept that an NMDA [N-methyl-D-aspartate] receptor antagonist could be useful for development of antidepressant drugs," said Dr. Sanacora, professor of psychiatry and director of the depression research program at Yale University in New Haven, Conn.
"The results are encouraging. The study adds a lot" to addressing whether treatments targeted at the NMDA receptor can help patients with mood disorder, he said in an interview.
The study enrolled 152 patients diagnosed with major depressive disorder and poor responses to at least two antidepressants at 30 U.S. centers. While patients remained on their entry drugs, the researchers administered 100 or 150 mg lanicemine or placebo as an intravenous infusion three times a week for 3 weeks. At 3 weeks, the change from baseline in total Montgomery Åsberg Depression Rating Scale (MADRS) score, the primary endpoint, was about a 13-point decline with either dosage of lanicemine and a decline of about 8 points with placebo, a 5-point increased reduction with either lanicemine dosage that was statistically significant for each dosage.
The 100-mg dosage showed more consistent efficacy than the higher dosage. The 100-mg dosage produced significantly greater reductions from baseline, compared with placebo after 2 weeks on treatment, and also at 4 and 5 weeks after treatment began, when patients no longer received infusions. The higher lanicemine dosage did not show significant effects compared with placebo at these other time points.
Lanicemine treatment, especially the 100-mg dosage, also showed efficacy by several secondary measures. For example, the proportion of patients who recorded a clinical global impression of much improved or very much improved was 65% with the 100-mg dosage at weeks 3, 4, and 5 after treatment began, significantly better than the 25%-30% rates at the same time points in the placebo group. Dr. Sanacora and his associates also published their results online (Mol. Psychiatry 2013 [doi:10.1038/mp.2013.130]).
During the study, neither lanicemine dosage produced any clinically meaningful difference compared with placebo for dissociative symptoms, and lanicemine was generally well tolerated. The most common adverse effect was dizziness around the time of infusion, which occurred in a third to half of the lanicemine patients, compared with 12% of those on placebo. Lanicemine "looked dramatically different from ketamine," Dr. Sanacora said.
He also downplayed the barrier that treatment by infusion presents. "There is such an unmet need" for patients with severe depression who fail to respond to existing drugs that infusion is not a major issue, he said. But "several companies are trying to develop novel formulations of NMDA receptor–targeted drugs" that might be active orally or intranasally.
The study was funded by AstraZeneca, the company developing lanicemine. Dr. Sanacora said he has been a consultant to and has received research support from AstraZeneca and several other drug companies. Some of Dr. Sanacora’s associates on this study were AstraZeneca employees.
On Twitter @mitchelzoler
AT THE ANNUAL ENCP CONGRESS
Major finding: Treatment with lanicemine produced an average 5-point greater reduction in depression score from baseline, compared with placebo.
Data source: A phase II study that randomized 152 patients with major depressive disorder to either of two lanicemine dosages or placebo for 3 weeks at 30 U.S. centers.
Disclosures: The study was funded by AstraZeneca, the company developing lanicemine. Dr. Sanacora said he has been a consultant to and has received research support from AstraZeneca and several other drug companies. Some of Dr. Sanacora’s associates in the study were AstraZeneca employees.
Atomoxetine may lead to improved driving for adults with ADHD
BARCELONA – Adults with attention-deficit/hyperactivity disorder who undergo treatment for their most intrusive symptoms also might benefit by having their driving improve.
Most adults with attention-deficit/hyperactivity disorder (ADHD) do not seek treatment because of their driving; most usually want their disorder to intrude less into their work life, education, or relationships. However, a poor driving record is common among ADHD patients. In addition, driving performance improved significantly during treatment with atomoxetine in a controlled study with 43 patients.
These are the first study results to show that atomoxetine treatment can improve driving performance in real traffic in adults with ADHD, Dr. Esther Sobanski said at the annual congress of the European College of Neuropsychopharmacology.
Atomoxetine, which is approved in the United States and in Europe for treating ADHD in adults and children, also has shown efficacy in controlled studies for treating patients with ADHD and social anxiety disorder (Depress. Anxiety 2009;26:212-21), and partial efficacy for patients with ADHD and alcohol-use disorder (Drug Alcohol Depend. 2008;96:145-54). The drug probably is similar in efficacy and safety to methylphenidate but without methylphenidate’s long clinical track record, Dr. Sobanski said.
"We are trying to figure out whom to treat with atomoxetine and whom to treat with methylphenidate," she said in an interview. "There is no evidence base now, so we are trying to figure out" whether certain types of ADHD patients respond better to one drug or the other.
The study enrolled adults diagnosed with ADHD at an outpatient mental health clinic in Mannheim, Germany, who were 18-50 years old and had a valid German driver’s license. After a 4-week tapering-dosage washout of entry medications, patients underwent baseline testing and then randomized to 18 mg/day atomoxetine or placebo. The atomoxetine dosage gradually ramped up over 4 weeks to 80 mg/day, which continued for 8 weeks until follow-up testing occurred. Enrolled patients averaged about 35 years old.
After 8 weeks of full treatment, the 22 atomoxetine patients showed statistically significant reductions in their average rates of false reactions for three different measures: orientation to traffic, attention to traffic, and driver skills. For each of these, the rate of false reactions fell by more than half, compared with the baseline rate, reported Dr. Sobanski, a psychiatrist at the Central Institute of Mental Health in Mannheim. False reactions by a fourth measure, risk-related self-control, also fell by more than half, but this trend was not statistically significant. In contrast, the placebo group showed virtually no change from baseline by all four measures. The rate of "critical traffic situations" recorded by participants in their daily diaries also fell by about half in the atomoxetine patients but not in the placebo group. Dr. Sobanski and her associates recently published their findings (Eur. Psychiatry 2013;28:379-85).
Prior research documented that adults with ADHD have three to four times more traffic accidents and double the traffic violations (especially speeding) than does the general adult population, Dr. Sobanski said. But driving problems usually are not what bring patients to treatment. "Most patients with ADHD get interventions for other reasons, but our findings show that their driving benefits, too."
The study was funded by Eli Lilly, which markets atomoxetine (Strattera). Dr. Sobanski said she has been an adviser to and received support from Eli Lilly and also from Medice, Shire, and Novartis.
On Twitter @mitchelzoler
BARCELONA – Adults with attention-deficit/hyperactivity disorder who undergo treatment for their most intrusive symptoms also might benefit by having their driving improve.
Most adults with attention-deficit/hyperactivity disorder (ADHD) do not seek treatment because of their driving; most usually want their disorder to intrude less into their work life, education, or relationships. However, a poor driving record is common among ADHD patients. In addition, driving performance improved significantly during treatment with atomoxetine in a controlled study with 43 patients.
These are the first study results to show that atomoxetine treatment can improve driving performance in real traffic in adults with ADHD, Dr. Esther Sobanski said at the annual congress of the European College of Neuropsychopharmacology.
Atomoxetine, which is approved in the United States and in Europe for treating ADHD in adults and children, also has shown efficacy in controlled studies for treating patients with ADHD and social anxiety disorder (Depress. Anxiety 2009;26:212-21), and partial efficacy for patients with ADHD and alcohol-use disorder (Drug Alcohol Depend. 2008;96:145-54). The drug probably is similar in efficacy and safety to methylphenidate but without methylphenidate’s long clinical track record, Dr. Sobanski said.
"We are trying to figure out whom to treat with atomoxetine and whom to treat with methylphenidate," she said in an interview. "There is no evidence base now, so we are trying to figure out" whether certain types of ADHD patients respond better to one drug or the other.
The study enrolled adults diagnosed with ADHD at an outpatient mental health clinic in Mannheim, Germany, who were 18-50 years old and had a valid German driver’s license. After a 4-week tapering-dosage washout of entry medications, patients underwent baseline testing and then randomized to 18 mg/day atomoxetine or placebo. The atomoxetine dosage gradually ramped up over 4 weeks to 80 mg/day, which continued for 8 weeks until follow-up testing occurred. Enrolled patients averaged about 35 years old.
After 8 weeks of full treatment, the 22 atomoxetine patients showed statistically significant reductions in their average rates of false reactions for three different measures: orientation to traffic, attention to traffic, and driver skills. For each of these, the rate of false reactions fell by more than half, compared with the baseline rate, reported Dr. Sobanski, a psychiatrist at the Central Institute of Mental Health in Mannheim. False reactions by a fourth measure, risk-related self-control, also fell by more than half, but this trend was not statistically significant. In contrast, the placebo group showed virtually no change from baseline by all four measures. The rate of "critical traffic situations" recorded by participants in their daily diaries also fell by about half in the atomoxetine patients but not in the placebo group. Dr. Sobanski and her associates recently published their findings (Eur. Psychiatry 2013;28:379-85).
Prior research documented that adults with ADHD have three to four times more traffic accidents and double the traffic violations (especially speeding) than does the general adult population, Dr. Sobanski said. But driving problems usually are not what bring patients to treatment. "Most patients with ADHD get interventions for other reasons, but our findings show that their driving benefits, too."
The study was funded by Eli Lilly, which markets atomoxetine (Strattera). Dr. Sobanski said she has been an adviser to and received support from Eli Lilly and also from Medice, Shire, and Novartis.
On Twitter @mitchelzoler
BARCELONA – Adults with attention-deficit/hyperactivity disorder who undergo treatment for their most intrusive symptoms also might benefit by having their driving improve.
Most adults with attention-deficit/hyperactivity disorder (ADHD) do not seek treatment because of their driving; most usually want their disorder to intrude less into their work life, education, or relationships. However, a poor driving record is common among ADHD patients. In addition, driving performance improved significantly during treatment with atomoxetine in a controlled study with 43 patients.
These are the first study results to show that atomoxetine treatment can improve driving performance in real traffic in adults with ADHD, Dr. Esther Sobanski said at the annual congress of the European College of Neuropsychopharmacology.
Atomoxetine, which is approved in the United States and in Europe for treating ADHD in adults and children, also has shown efficacy in controlled studies for treating patients with ADHD and social anxiety disorder (Depress. Anxiety 2009;26:212-21), and partial efficacy for patients with ADHD and alcohol-use disorder (Drug Alcohol Depend. 2008;96:145-54). The drug probably is similar in efficacy and safety to methylphenidate but without methylphenidate’s long clinical track record, Dr. Sobanski said.
"We are trying to figure out whom to treat with atomoxetine and whom to treat with methylphenidate," she said in an interview. "There is no evidence base now, so we are trying to figure out" whether certain types of ADHD patients respond better to one drug or the other.
The study enrolled adults diagnosed with ADHD at an outpatient mental health clinic in Mannheim, Germany, who were 18-50 years old and had a valid German driver’s license. After a 4-week tapering-dosage washout of entry medications, patients underwent baseline testing and then randomized to 18 mg/day atomoxetine or placebo. The atomoxetine dosage gradually ramped up over 4 weeks to 80 mg/day, which continued for 8 weeks until follow-up testing occurred. Enrolled patients averaged about 35 years old.
After 8 weeks of full treatment, the 22 atomoxetine patients showed statistically significant reductions in their average rates of false reactions for three different measures: orientation to traffic, attention to traffic, and driver skills. For each of these, the rate of false reactions fell by more than half, compared with the baseline rate, reported Dr. Sobanski, a psychiatrist at the Central Institute of Mental Health in Mannheim. False reactions by a fourth measure, risk-related self-control, also fell by more than half, but this trend was not statistically significant. In contrast, the placebo group showed virtually no change from baseline by all four measures. The rate of "critical traffic situations" recorded by participants in their daily diaries also fell by about half in the atomoxetine patients but not in the placebo group. Dr. Sobanski and her associates recently published their findings (Eur. Psychiatry 2013;28:379-85).
Prior research documented that adults with ADHD have three to four times more traffic accidents and double the traffic violations (especially speeding) than does the general adult population, Dr. Sobanski said. But driving problems usually are not what bring patients to treatment. "Most patients with ADHD get interventions for other reasons, but our findings show that their driving benefits, too."
The study was funded by Eli Lilly, which markets atomoxetine (Strattera). Dr. Sobanski said she has been an adviser to and received support from Eli Lilly and also from Medice, Shire, and Novartis.
On Twitter @mitchelzoler
AT THE ANNUAL ECNP CONGRESS
Major finding: After 8 weeks treatment with atomoxetine, attention-deficit/hyperactivity disorder patients showed significant improvement over placebo in three measures of driving attention.
Data source: The study randomized 43 patients to treatment with 80 mg/day atomoxetine or placebo for 8 weeks in a single-center German study.
Disclosures: The study was funded by Eli Lilly, which markets atomoxetine (Strattera). Dr. Sobanski said she has been an adviser to and received support from Eli Lilly and also from Medice, Shire, and Novartis.
Armodafinil shows bipolar depression efficacy
BARCELONA – Armodafinil, a drug with Food and Drug Administration approval to treat excessive sleepiness and narcolepsy, showed efficacy for improving concentration, energy, and appetite in a controlled study of nearly 400 patients with bipolar I depression.
"I think [armodafinil] is clinically useful because its efficacy appears complementary to the efficacy we see from other compounds," Dr. Joseph R. Calabrese said at the annual congress of the European College of Neuropsychopharmacology.
In addition to showing useful efficacy compared with placebo as adjunctive treatment, armodafinil also had a "very, very benign" adverse effect profile – its 6% dropout rate because of adverse effects was not statistically significant from the 4% rate in placebo patients.
Armodafinil is the R-enantiomer of modafinil, which means the two agents are essentially the same. "I think off-label use [of armodafinil] is okay, because there are a lot of [safety] data for modafinil," Dr. Calabrese said in an interview.
Armodafinil (Nuvigil) has FDA approval for treating shift-work disorder, narcolepsy, and treated obstructed sleep apnea. He foresees no push for formal labeling of armodafinil for bipolar I depression because three phase III trials were run and only one was positive for efficacy. The two trials that failed to prove efficacy fell short not because of a blunted drug effect but because of an unexpectedly high response in the placebo group. This design flaw in two of the three pivotal trials likely doomed any hope of getting armodafinil labeled for this indication, he said.
"There is a role for armodafinil. It is something to try for patients" who need help in the things it affects, said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland. "It has stimulant-like properties that can improve concentration" and can help patients who are on another drug causing sedation, he added.
The study he ran enrolled patients who had cycled into a new major depressive episode after treatment with antimanic drugs for at least 4 weeks. Most patients were on antimanic monotherapy during the study, about 10% were on two antimanic drugs, and two patients were on three drugs. The researchers randomized 198 patients to receive 150 mg of armodafinil daily and 199 to placebo. An additional 32 patients initially received 200 mg armodafinil daily, but that arm was discontinued because of adverse effects.
After 8 weeks, the study’s primary endpoint – change in average, total clinician-rated, 30-item Inventory of Depressive Symptomatology (IDS) score – showed a 21.7 point drop with armodafinil and a 17.9 point drop with placebo, a statistically significant difference. The incidence of responder patients, those with at least a 50% reduction from their baseline IDS score, was 46% in the armodafinil arm and 34% in the placebo arm, a statistically significant difference and indicating a number needed to treat of nine to achieve one additional response over placebo.
The results also showed that the improvements in IDS score focused on five of the score’s 30 individual items: panic and phobic symptoms, appetite, concentration and decision making, energy and fatigability, and leaden paralysis and physical energy. In contrast, armodafinil had no apparent effect on core symptoms of depression. Armodafinil also produced no worsening of anxiety, sleep, or switch rates, and no meaningful trends toward gain or loss of weight.
The study was sponsored by Teva, which markets armodafinil (Nuvigil). Dr. Calabrese said that he has been a consultant to and received research support from Teva and more than a dozen other drug companies.
On Twitter @mitchelzoler
BARCELONA – Armodafinil, a drug with Food and Drug Administration approval to treat excessive sleepiness and narcolepsy, showed efficacy for improving concentration, energy, and appetite in a controlled study of nearly 400 patients with bipolar I depression.
"I think [armodafinil] is clinically useful because its efficacy appears complementary to the efficacy we see from other compounds," Dr. Joseph R. Calabrese said at the annual congress of the European College of Neuropsychopharmacology.
In addition to showing useful efficacy compared with placebo as adjunctive treatment, armodafinil also had a "very, very benign" adverse effect profile – its 6% dropout rate because of adverse effects was not statistically significant from the 4% rate in placebo patients.
Armodafinil is the R-enantiomer of modafinil, which means the two agents are essentially the same. "I think off-label use [of armodafinil] is okay, because there are a lot of [safety] data for modafinil," Dr. Calabrese said in an interview.
Armodafinil (Nuvigil) has FDA approval for treating shift-work disorder, narcolepsy, and treated obstructed sleep apnea. He foresees no push for formal labeling of armodafinil for bipolar I depression because three phase III trials were run and only one was positive for efficacy. The two trials that failed to prove efficacy fell short not because of a blunted drug effect but because of an unexpectedly high response in the placebo group. This design flaw in two of the three pivotal trials likely doomed any hope of getting armodafinil labeled for this indication, he said.
"There is a role for armodafinil. It is something to try for patients" who need help in the things it affects, said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland. "It has stimulant-like properties that can improve concentration" and can help patients who are on another drug causing sedation, he added.
The study he ran enrolled patients who had cycled into a new major depressive episode after treatment with antimanic drugs for at least 4 weeks. Most patients were on antimanic monotherapy during the study, about 10% were on two antimanic drugs, and two patients were on three drugs. The researchers randomized 198 patients to receive 150 mg of armodafinil daily and 199 to placebo. An additional 32 patients initially received 200 mg armodafinil daily, but that arm was discontinued because of adverse effects.
After 8 weeks, the study’s primary endpoint – change in average, total clinician-rated, 30-item Inventory of Depressive Symptomatology (IDS) score – showed a 21.7 point drop with armodafinil and a 17.9 point drop with placebo, a statistically significant difference. The incidence of responder patients, those with at least a 50% reduction from their baseline IDS score, was 46% in the armodafinil arm and 34% in the placebo arm, a statistically significant difference and indicating a number needed to treat of nine to achieve one additional response over placebo.
The results also showed that the improvements in IDS score focused on five of the score’s 30 individual items: panic and phobic symptoms, appetite, concentration and decision making, energy and fatigability, and leaden paralysis and physical energy. In contrast, armodafinil had no apparent effect on core symptoms of depression. Armodafinil also produced no worsening of anxiety, sleep, or switch rates, and no meaningful trends toward gain or loss of weight.
The study was sponsored by Teva, which markets armodafinil (Nuvigil). Dr. Calabrese said that he has been a consultant to and received research support from Teva and more than a dozen other drug companies.
On Twitter @mitchelzoler
BARCELONA – Armodafinil, a drug with Food and Drug Administration approval to treat excessive sleepiness and narcolepsy, showed efficacy for improving concentration, energy, and appetite in a controlled study of nearly 400 patients with bipolar I depression.
"I think [armodafinil] is clinically useful because its efficacy appears complementary to the efficacy we see from other compounds," Dr. Joseph R. Calabrese said at the annual congress of the European College of Neuropsychopharmacology.
In addition to showing useful efficacy compared with placebo as adjunctive treatment, armodafinil also had a "very, very benign" adverse effect profile – its 6% dropout rate because of adverse effects was not statistically significant from the 4% rate in placebo patients.
Armodafinil is the R-enantiomer of modafinil, which means the two agents are essentially the same. "I think off-label use [of armodafinil] is okay, because there are a lot of [safety] data for modafinil," Dr. Calabrese said in an interview.
Armodafinil (Nuvigil) has FDA approval for treating shift-work disorder, narcolepsy, and treated obstructed sleep apnea. He foresees no push for formal labeling of armodafinil for bipolar I depression because three phase III trials were run and only one was positive for efficacy. The two trials that failed to prove efficacy fell short not because of a blunted drug effect but because of an unexpectedly high response in the placebo group. This design flaw in two of the three pivotal trials likely doomed any hope of getting armodafinil labeled for this indication, he said.
"There is a role for armodafinil. It is something to try for patients" who need help in the things it affects, said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland. "It has stimulant-like properties that can improve concentration" and can help patients who are on another drug causing sedation, he added.
The study he ran enrolled patients who had cycled into a new major depressive episode after treatment with antimanic drugs for at least 4 weeks. Most patients were on antimanic monotherapy during the study, about 10% were on two antimanic drugs, and two patients were on three drugs. The researchers randomized 198 patients to receive 150 mg of armodafinil daily and 199 to placebo. An additional 32 patients initially received 200 mg armodafinil daily, but that arm was discontinued because of adverse effects.
After 8 weeks, the study’s primary endpoint – change in average, total clinician-rated, 30-item Inventory of Depressive Symptomatology (IDS) score – showed a 21.7 point drop with armodafinil and a 17.9 point drop with placebo, a statistically significant difference. The incidence of responder patients, those with at least a 50% reduction from their baseline IDS score, was 46% in the armodafinil arm and 34% in the placebo arm, a statistically significant difference and indicating a number needed to treat of nine to achieve one additional response over placebo.
The results also showed that the improvements in IDS score focused on five of the score’s 30 individual items: panic and phobic symptoms, appetite, concentration and decision making, energy and fatigability, and leaden paralysis and physical energy. In contrast, armodafinil had no apparent effect on core symptoms of depression. Armodafinil also produced no worsening of anxiety, sleep, or switch rates, and no meaningful trends toward gain or loss of weight.
The study was sponsored by Teva, which markets armodafinil (Nuvigil). Dr. Calabrese said that he has been a consultant to and received research support from Teva and more than a dozen other drug companies.
On Twitter @mitchelzoler
AT THE ECNP CONGRESS
Major finding: Adjunctive treatment of bipolar I depression with armodafinil produced a 46% depression response rate, compared with a 34% rate from placebo.
Data source: Randomized, placebo-controlled, multicenter study involving 397 patients with bipolar I depression treated for 8 weeks.
Disclosures: The study was sponsored by Teva, which markets armodafinil (Nuvigil). Dr. Calabrese said that he has been a consultant to and received research support from Teva and more than a dozen other drug companies.
Deep brain stimulation shows early promise for depression
BARCELONA – Assessment of deep brain stimulation for patients with treatment-resistant depression has inched forward over the past decade with promising results but remains in its early days with a total worldwide experience of roughly 60 patients.
That total includes patients who have received deep brain stimulation (DBS) leads placed in six different brain regions. However, the results suggest that one of the better target locations for DBS in patients with treatment-resistant depression (TRD) is in the nucleus accumbens, Dr. Bruno Millet said at the annual congress of the European College of Neuropsychopharmacology.
"The nucleus accumbens is a very good target that we will continue to study. It is very safe, and the surgery is easy and rapid. The nucleus accumbens has a good benefit-to-risk ratio," said Dr. Millet, professor of psychiatry at the University of Rennes (France). "This is a strength of this target."
The nucleus accumbens became a target because it is "at the center of a circuit involved in major depressive disorder," and plays a "central role in reward circuitry and its dysfunctions," he added.
All patients who have received DBS had stage 5 TRD, which meant that their depression was unresponsive to all possible drug classes, including monamine oxidase inhibitors. These patients also failed to adequately respond to electroconvulsive therapy.
Dr. Millet and his associates at nine other French centers ran a pilot study with four patients who received DBS in the nuclear accumbens starting in 2009. Patients received stimulation at a level of 1.5-4 V. All four patients showed gradual improvement in their depression during the first 6 months after treatment. After 1 year of follow-up, all patients had responded, with response defined as at least a 50% decline from the baseline Hamilton Rating Scale for Depression (HRSD) score. One patient went into remission, defined as an HRSD score of less than 10. None of the patients had somatic adverse effects during 15 months of follow-up, but one patient had worsening mood and anxiety and made a suicide attempt; one patient had worsening mood and sleep and increased food intake; and one patient developed paresthesia.
The largest patient series and the longest follow-up of patients who received DBS in their nucleus accumbens for TRD involved 11 patients treated in Germany. The patients had minimal adverse effects, and 5 of the 11 responded, with the response remaining stable during 4 years of follow-up (Neuropsychopharmacology 2012;37:1975-85).
Another target that produced notable results is the medial forebrain bundle, which led to good DBS results in seven patients treated by the same German team. Earlier this year, the investigators reported that six of the seven patients had responded and four patients had reached remission of their depression after 3-7 months of follow-up (Biol. Psychiatry 2013;73:1204-12). Two other brain targets that have been used by other groups with success are the subgenual cingulate gyrus, and the ventral capsule and ventral striatum (World Neurosurg. 2013;80:S27.e17-24).
But "one of the striking things in DBS for the moment is the lack of any randomized controlled trials," Dr. Millet said. He and his French associates have expanded their network to 12 centers, including one in Geneva, and they have designed and are now starting a trial that will place DBS leads in all patients but will withhold active stimulation in half the patients for the first 7 months after lead placement. This trial is sponsored by Medtronic, a company that markets the DBS device Reclaim.
Medtronic primarily markets its DBS device for the treatment of Parkinson’s disease, essential tremor, and dystonia, but in 2009, the Food and Drug Administration also approved its use to treat obsessive-compulsive disorder under a humanitarian device exemption. Some medical ethicists, psychiatrists, and neurosurgeons have questioned the growing use of DBS for obsessive-compulsive disorder in the absence of evidence for its efficacy in a randomized controlled trial, calling this practice "misuse" of the device (Health Aff. 2011;30:302-11). One of these critics, Dr. Thomas E. Schlaepfer of Bonn, Germany, also is head of the group with perhaps the largest experience in using DBS to treat patients with depression.
Dr. Millet said he has received grant support from Medtronic, marketer of the Reclaim DBS therapy device. He also said he has been a consultant to, has been a speaker for, or has received research funding from nine other drug or device companies.
On Twitter @mitchelzoler
BARCELONA – Assessment of deep brain stimulation for patients with treatment-resistant depression has inched forward over the past decade with promising results but remains in its early days with a total worldwide experience of roughly 60 patients.
That total includes patients who have received deep brain stimulation (DBS) leads placed in six different brain regions. However, the results suggest that one of the better target locations for DBS in patients with treatment-resistant depression (TRD) is in the nucleus accumbens, Dr. Bruno Millet said at the annual congress of the European College of Neuropsychopharmacology.
"The nucleus accumbens is a very good target that we will continue to study. It is very safe, and the surgery is easy and rapid. The nucleus accumbens has a good benefit-to-risk ratio," said Dr. Millet, professor of psychiatry at the University of Rennes (France). "This is a strength of this target."
The nucleus accumbens became a target because it is "at the center of a circuit involved in major depressive disorder," and plays a "central role in reward circuitry and its dysfunctions," he added.
All patients who have received DBS had stage 5 TRD, which meant that their depression was unresponsive to all possible drug classes, including monamine oxidase inhibitors. These patients also failed to adequately respond to electroconvulsive therapy.
Dr. Millet and his associates at nine other French centers ran a pilot study with four patients who received DBS in the nuclear accumbens starting in 2009. Patients received stimulation at a level of 1.5-4 V. All four patients showed gradual improvement in their depression during the first 6 months after treatment. After 1 year of follow-up, all patients had responded, with response defined as at least a 50% decline from the baseline Hamilton Rating Scale for Depression (HRSD) score. One patient went into remission, defined as an HRSD score of less than 10. None of the patients had somatic adverse effects during 15 months of follow-up, but one patient had worsening mood and anxiety and made a suicide attempt; one patient had worsening mood and sleep and increased food intake; and one patient developed paresthesia.
The largest patient series and the longest follow-up of patients who received DBS in their nucleus accumbens for TRD involved 11 patients treated in Germany. The patients had minimal adverse effects, and 5 of the 11 responded, with the response remaining stable during 4 years of follow-up (Neuropsychopharmacology 2012;37:1975-85).
Another target that produced notable results is the medial forebrain bundle, which led to good DBS results in seven patients treated by the same German team. Earlier this year, the investigators reported that six of the seven patients had responded and four patients had reached remission of their depression after 3-7 months of follow-up (Biol. Psychiatry 2013;73:1204-12). Two other brain targets that have been used by other groups with success are the subgenual cingulate gyrus, and the ventral capsule and ventral striatum (World Neurosurg. 2013;80:S27.e17-24).
But "one of the striking things in DBS for the moment is the lack of any randomized controlled trials," Dr. Millet said. He and his French associates have expanded their network to 12 centers, including one in Geneva, and they have designed and are now starting a trial that will place DBS leads in all patients but will withhold active stimulation in half the patients for the first 7 months after lead placement. This trial is sponsored by Medtronic, a company that markets the DBS device Reclaim.
Medtronic primarily markets its DBS device for the treatment of Parkinson’s disease, essential tremor, and dystonia, but in 2009, the Food and Drug Administration also approved its use to treat obsessive-compulsive disorder under a humanitarian device exemption. Some medical ethicists, psychiatrists, and neurosurgeons have questioned the growing use of DBS for obsessive-compulsive disorder in the absence of evidence for its efficacy in a randomized controlled trial, calling this practice "misuse" of the device (Health Aff. 2011;30:302-11). One of these critics, Dr. Thomas E. Schlaepfer of Bonn, Germany, also is head of the group with perhaps the largest experience in using DBS to treat patients with depression.
Dr. Millet said he has received grant support from Medtronic, marketer of the Reclaim DBS therapy device. He also said he has been a consultant to, has been a speaker for, or has received research funding from nine other drug or device companies.
On Twitter @mitchelzoler
BARCELONA – Assessment of deep brain stimulation for patients with treatment-resistant depression has inched forward over the past decade with promising results but remains in its early days with a total worldwide experience of roughly 60 patients.
That total includes patients who have received deep brain stimulation (DBS) leads placed in six different brain regions. However, the results suggest that one of the better target locations for DBS in patients with treatment-resistant depression (TRD) is in the nucleus accumbens, Dr. Bruno Millet said at the annual congress of the European College of Neuropsychopharmacology.
"The nucleus accumbens is a very good target that we will continue to study. It is very safe, and the surgery is easy and rapid. The nucleus accumbens has a good benefit-to-risk ratio," said Dr. Millet, professor of psychiatry at the University of Rennes (France). "This is a strength of this target."
The nucleus accumbens became a target because it is "at the center of a circuit involved in major depressive disorder," and plays a "central role in reward circuitry and its dysfunctions," he added.
All patients who have received DBS had stage 5 TRD, which meant that their depression was unresponsive to all possible drug classes, including monamine oxidase inhibitors. These patients also failed to adequately respond to electroconvulsive therapy.
Dr. Millet and his associates at nine other French centers ran a pilot study with four patients who received DBS in the nuclear accumbens starting in 2009. Patients received stimulation at a level of 1.5-4 V. All four patients showed gradual improvement in their depression during the first 6 months after treatment. After 1 year of follow-up, all patients had responded, with response defined as at least a 50% decline from the baseline Hamilton Rating Scale for Depression (HRSD) score. One patient went into remission, defined as an HRSD score of less than 10. None of the patients had somatic adverse effects during 15 months of follow-up, but one patient had worsening mood and anxiety and made a suicide attempt; one patient had worsening mood and sleep and increased food intake; and one patient developed paresthesia.
The largest patient series and the longest follow-up of patients who received DBS in their nucleus accumbens for TRD involved 11 patients treated in Germany. The patients had minimal adverse effects, and 5 of the 11 responded, with the response remaining stable during 4 years of follow-up (Neuropsychopharmacology 2012;37:1975-85).
Another target that produced notable results is the medial forebrain bundle, which led to good DBS results in seven patients treated by the same German team. Earlier this year, the investigators reported that six of the seven patients had responded and four patients had reached remission of their depression after 3-7 months of follow-up (Biol. Psychiatry 2013;73:1204-12). Two other brain targets that have been used by other groups with success are the subgenual cingulate gyrus, and the ventral capsule and ventral striatum (World Neurosurg. 2013;80:S27.e17-24).
But "one of the striking things in DBS for the moment is the lack of any randomized controlled trials," Dr. Millet said. He and his French associates have expanded their network to 12 centers, including one in Geneva, and they have designed and are now starting a trial that will place DBS leads in all patients but will withhold active stimulation in half the patients for the first 7 months after lead placement. This trial is sponsored by Medtronic, a company that markets the DBS device Reclaim.
Medtronic primarily markets its DBS device for the treatment of Parkinson’s disease, essential tremor, and dystonia, but in 2009, the Food and Drug Administration also approved its use to treat obsessive-compulsive disorder under a humanitarian device exemption. Some medical ethicists, psychiatrists, and neurosurgeons have questioned the growing use of DBS for obsessive-compulsive disorder in the absence of evidence for its efficacy in a randomized controlled trial, calling this practice "misuse" of the device (Health Aff. 2011;30:302-11). One of these critics, Dr. Thomas E. Schlaepfer of Bonn, Germany, also is head of the group with perhaps the largest experience in using DBS to treat patients with depression.
Dr. Millet said he has received grant support from Medtronic, marketer of the Reclaim DBS therapy device. He also said he has been a consultant to, has been a speaker for, or has received research funding from nine other drug or device companies.
On Twitter @mitchelzoler
EXPERT OPINION FROM THE ECNP CONGRESS
First-episode schizophrenia patients often have hyperprolactinemia
BARCELONA – More than a third of treatment-naive patients with newly diagnosed schizophrenia had hyperprolactinemia, in an analysis of data from 73 such patients enrolled in a multicenter, international study.
"Hyperprolactinemia can be found independent of antipsychotic treatment in drug-naive, first-episode schizophrenia patients," Dr. Anita Riecher-Rössler said at the annual congress of the European College of Neuropsychopharmacology The frequent occurrence of hyperprolactinemia in these patients has clinical implications, she added.
First, physicians should measure prolactin levels in newly diagnosed schizophrenia patients to both check for hyperprolactinemia and to establish the patient’s baseline level before starting antipsychotic treatment. Patients should also get assessed for signs and symptoms of hyperprolactinemia, such as menstrual irregularities, loss of libido, and infertility, Dr. Riecher-Rössler said. Patients found to have hyperprolactinemia should undergo a thorough work-up to see if it has a somatic cause that requires treatment. Physicians should also avoid prescribing antipsychotic drugs that can raise prolactin levels to patients who already have an elevated level of the hormone. First-generation antipsychotic drugs, as well as certain second-generation agents such as amisulpride and risperidone are known inducers of hyperprolactinemia (Pharmacotherapy 2009;29:64-73).
"In patients with hyperprolactinemia, consider using a prolactin-sparing antipsychotic, or treat women with estradiol to normalize their prolactin level," said Dr. Riecher-Rössler, a professor of psychiatry and head of the Center for Gender Research and Early Detection at the University of Basel, Switzerland. Hyperprolactinemia poses a threat to young women of triggering early menopause with the resulting complications of loss of fertility and early-onset osteoporosis, she noted. But it also poses a threat to men, causing suppression of the testes and reduced sexuality. "Hyperprolactinemia is a reason to avoid using antipsychotic drugs that can cause it, especially in young women because of the risk of causing early menopause," she said, although drug-induced hyperprolactinemia is less of a threat in patients who start treatment with normal prolactin levels.
The finding also raises the possibility that hyperprolactinemia might play a role in causing schizophrenia, or that emerging schizophrenia or other stresses on the patient cause elevated prolactin levels,
If the patient has no other apparent cause of hyperprolactinemia "the idea is that it is stress induced, and stress is part of an emerging psychosis," she said in an interview. "If you treat the psychosis, you may also lower the prolactin."
Because elevated prolactin levels trigger release of prolactin-inhibiting factor, which is dopamine, another possibility is that high dopamine levels triggered by hyperprolactinemia might affect the brain and play a role in the pathogenesis of schizophrenia. These hypotheses need further study, Dr. Riecher-Rössler said.
The data she and her associates used came from the European First Episode of Schizophrenia Trial (EUFEST), which enrolled 498 patients with newly diagnosed schizophrenia at 50 centers in Europe and Israel (Lancet 2008;371:1085-97). From this group, 249 patients had reliable records on their medical treatment before entering the study and had blood samples available that had been drawn prior to the start of their treatment. The researchers identified 73 of these patients who had been completely free of any antipsychotic or other drug treatment that could have possibly caused elevated prolactin levels prior to their initial blood draw.
Analysis of the blood specimens showed that 19 of the 51 men (37%) and 11 of the 22 women (50%) had hyperprolactinemia, defined as a blood prolactin level greater than 38 U/L in men and greater than 53 U/L in women. The overall prevalence in all 73 patients was 41%.
All antipsychotics have adverse effects, and the risk that some might trigger hyperprolactinemia has to be taken into account along with all the other possible adverse effects when choosing drugs to prescribe to patients, she said.
"Do you prescribe olanzapine or quetiapine, which cause weight gain, or do you use amisulpride or risperidone, which raise prolactin?" Dr. Riecher-Rössler asked. "Which side effect is the patient prepared to cope with? I try to avoid using amisulpride in women who are young and still want to become pregnant or in young men so I don’t suppress their sexuality."
She and her associates reported the results in an article published earlier this year (Psychol Med. 2013 [doi:10.1017/S0033291713000226]).
EUFEST received support from Astra Zeneca, Pfizer, and Sanofi. Dr. Riecher-Rössler said she had no disclosures.
On Twitter @mitchelzoler
BARCELONA – More than a third of treatment-naive patients with newly diagnosed schizophrenia had hyperprolactinemia, in an analysis of data from 73 such patients enrolled in a multicenter, international study.
"Hyperprolactinemia can be found independent of antipsychotic treatment in drug-naive, first-episode schizophrenia patients," Dr. Anita Riecher-Rössler said at the annual congress of the European College of Neuropsychopharmacology The frequent occurrence of hyperprolactinemia in these patients has clinical implications, she added.
First, physicians should measure prolactin levels in newly diagnosed schizophrenia patients to both check for hyperprolactinemia and to establish the patient’s baseline level before starting antipsychotic treatment. Patients should also get assessed for signs and symptoms of hyperprolactinemia, such as menstrual irregularities, loss of libido, and infertility, Dr. Riecher-Rössler said. Patients found to have hyperprolactinemia should undergo a thorough work-up to see if it has a somatic cause that requires treatment. Physicians should also avoid prescribing antipsychotic drugs that can raise prolactin levels to patients who already have an elevated level of the hormone. First-generation antipsychotic drugs, as well as certain second-generation agents such as amisulpride and risperidone are known inducers of hyperprolactinemia (Pharmacotherapy 2009;29:64-73).
"In patients with hyperprolactinemia, consider using a prolactin-sparing antipsychotic, or treat women with estradiol to normalize their prolactin level," said Dr. Riecher-Rössler, a professor of psychiatry and head of the Center for Gender Research and Early Detection at the University of Basel, Switzerland. Hyperprolactinemia poses a threat to young women of triggering early menopause with the resulting complications of loss of fertility and early-onset osteoporosis, she noted. But it also poses a threat to men, causing suppression of the testes and reduced sexuality. "Hyperprolactinemia is a reason to avoid using antipsychotic drugs that can cause it, especially in young women because of the risk of causing early menopause," she said, although drug-induced hyperprolactinemia is less of a threat in patients who start treatment with normal prolactin levels.
The finding also raises the possibility that hyperprolactinemia might play a role in causing schizophrenia, or that emerging schizophrenia or other stresses on the patient cause elevated prolactin levels,
If the patient has no other apparent cause of hyperprolactinemia "the idea is that it is stress induced, and stress is part of an emerging psychosis," she said in an interview. "If you treat the psychosis, you may also lower the prolactin."
Because elevated prolactin levels trigger release of prolactin-inhibiting factor, which is dopamine, another possibility is that high dopamine levels triggered by hyperprolactinemia might affect the brain and play a role in the pathogenesis of schizophrenia. These hypotheses need further study, Dr. Riecher-Rössler said.
The data she and her associates used came from the European First Episode of Schizophrenia Trial (EUFEST), which enrolled 498 patients with newly diagnosed schizophrenia at 50 centers in Europe and Israel (Lancet 2008;371:1085-97). From this group, 249 patients had reliable records on their medical treatment before entering the study and had blood samples available that had been drawn prior to the start of their treatment. The researchers identified 73 of these patients who had been completely free of any antipsychotic or other drug treatment that could have possibly caused elevated prolactin levels prior to their initial blood draw.
Analysis of the blood specimens showed that 19 of the 51 men (37%) and 11 of the 22 women (50%) had hyperprolactinemia, defined as a blood prolactin level greater than 38 U/L in men and greater than 53 U/L in women. The overall prevalence in all 73 patients was 41%.
All antipsychotics have adverse effects, and the risk that some might trigger hyperprolactinemia has to be taken into account along with all the other possible adverse effects when choosing drugs to prescribe to patients, she said.
"Do you prescribe olanzapine or quetiapine, which cause weight gain, or do you use amisulpride or risperidone, which raise prolactin?" Dr. Riecher-Rössler asked. "Which side effect is the patient prepared to cope with? I try to avoid using amisulpride in women who are young and still want to become pregnant or in young men so I don’t suppress their sexuality."
She and her associates reported the results in an article published earlier this year (Psychol Med. 2013 [doi:10.1017/S0033291713000226]).
EUFEST received support from Astra Zeneca, Pfizer, and Sanofi. Dr. Riecher-Rössler said she had no disclosures.
On Twitter @mitchelzoler
BARCELONA – More than a third of treatment-naive patients with newly diagnosed schizophrenia had hyperprolactinemia, in an analysis of data from 73 such patients enrolled in a multicenter, international study.
"Hyperprolactinemia can be found independent of antipsychotic treatment in drug-naive, first-episode schizophrenia patients," Dr. Anita Riecher-Rössler said at the annual congress of the European College of Neuropsychopharmacology The frequent occurrence of hyperprolactinemia in these patients has clinical implications, she added.
First, physicians should measure prolactin levels in newly diagnosed schizophrenia patients to both check for hyperprolactinemia and to establish the patient’s baseline level before starting antipsychotic treatment. Patients should also get assessed for signs and symptoms of hyperprolactinemia, such as menstrual irregularities, loss of libido, and infertility, Dr. Riecher-Rössler said. Patients found to have hyperprolactinemia should undergo a thorough work-up to see if it has a somatic cause that requires treatment. Physicians should also avoid prescribing antipsychotic drugs that can raise prolactin levels to patients who already have an elevated level of the hormone. First-generation antipsychotic drugs, as well as certain second-generation agents such as amisulpride and risperidone are known inducers of hyperprolactinemia (Pharmacotherapy 2009;29:64-73).
"In patients with hyperprolactinemia, consider using a prolactin-sparing antipsychotic, or treat women with estradiol to normalize their prolactin level," said Dr. Riecher-Rössler, a professor of psychiatry and head of the Center for Gender Research and Early Detection at the University of Basel, Switzerland. Hyperprolactinemia poses a threat to young women of triggering early menopause with the resulting complications of loss of fertility and early-onset osteoporosis, she noted. But it also poses a threat to men, causing suppression of the testes and reduced sexuality. "Hyperprolactinemia is a reason to avoid using antipsychotic drugs that can cause it, especially in young women because of the risk of causing early menopause," she said, although drug-induced hyperprolactinemia is less of a threat in patients who start treatment with normal prolactin levels.
The finding also raises the possibility that hyperprolactinemia might play a role in causing schizophrenia, or that emerging schizophrenia or other stresses on the patient cause elevated prolactin levels,
If the patient has no other apparent cause of hyperprolactinemia "the idea is that it is stress induced, and stress is part of an emerging psychosis," she said in an interview. "If you treat the psychosis, you may also lower the prolactin."
Because elevated prolactin levels trigger release of prolactin-inhibiting factor, which is dopamine, another possibility is that high dopamine levels triggered by hyperprolactinemia might affect the brain and play a role in the pathogenesis of schizophrenia. These hypotheses need further study, Dr. Riecher-Rössler said.
The data she and her associates used came from the European First Episode of Schizophrenia Trial (EUFEST), which enrolled 498 patients with newly diagnosed schizophrenia at 50 centers in Europe and Israel (Lancet 2008;371:1085-97). From this group, 249 patients had reliable records on their medical treatment before entering the study and had blood samples available that had been drawn prior to the start of their treatment. The researchers identified 73 of these patients who had been completely free of any antipsychotic or other drug treatment that could have possibly caused elevated prolactin levels prior to their initial blood draw.
Analysis of the blood specimens showed that 19 of the 51 men (37%) and 11 of the 22 women (50%) had hyperprolactinemia, defined as a blood prolactin level greater than 38 U/L in men and greater than 53 U/L in women. The overall prevalence in all 73 patients was 41%.
All antipsychotics have adverse effects, and the risk that some might trigger hyperprolactinemia has to be taken into account along with all the other possible adverse effects when choosing drugs to prescribe to patients, she said.
"Do you prescribe olanzapine or quetiapine, which cause weight gain, or do you use amisulpride or risperidone, which raise prolactin?" Dr. Riecher-Rössler asked. "Which side effect is the patient prepared to cope with? I try to avoid using amisulpride in women who are young and still want to become pregnant or in young men so I don’t suppress their sexuality."
She and her associates reported the results in an article published earlier this year (Psychol Med. 2013 [doi:10.1017/S0033291713000226]).
EUFEST received support from Astra Zeneca, Pfizer, and Sanofi. Dr. Riecher-Rössler said she had no disclosures.
On Twitter @mitchelzoler
AT THE ECNP CONGRESS
Major finding: Hyperprolactinemia occurred in 50% of women and 37% of men with treatment-naive, first-episode schizophrenia.
Data source: EUFEST, a multinational study of 498 first-episode schizophrenia patients.
Disclosures: EUFEST received support from AstraZeneca, Pfizer, and Sanofi. Dr. Riecher-Rössler said she had no disclosures.
Lurasidone’s potential for bipolar depression draws praise
BARCELONA – Lurasidone, an atypical antipsychotic approved by the Food and Drug Administration last June for the treatment of depression in patients with bipolar I disorder, has the potential to become a first-line treatment for these patients as physicians grow more familiar and comfortable with the drug, Dr. Joseph R. Calabrese said at the annual Congress of the European College of Neuropsychopharmacology.
In addition to showing significant efficacy for reducing depression in bipolar I patients when used as either monotherapy or as adjunctive therapy in a pair of phase III trials, lurasidone (Latuda) "does not have the metabolic burden caused by some other drugs" used in these patients, and also showed no statistically significant increase in dropout rates compared with placebo. The discontinuation rate because of adverse effects was especially low when the drug was administered at a dosage of 20-60 mg/day. This safety finding is "remarkable," said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland.
The results from the two phase III trials provide "unequivocal evidence that the drug has short-term efficacy for patients with depressive episodes in bipolar I disorder. I think the evidence is very persuasive," Dr. Calabrese said in an interview. "I think this drug will be used adjunctively at first, but with more familiarity, physicians will start using it up front. The No. 1 attraction for first-line therapy is no metabolic burden; that’s a huge issue. And sedation is minimal." Dr. Calabrese stressed that the trial results showed no dropouts because of sedation, and 10%-15% of patients reported a sedation effect, compared with the 40% rate typically seen with lamotrigine (Lamictal).
One of the trials assessed lurasidone as an adjunctive treatment in 340 patients already on treatment with lithium or valproate. After 6 weeks, patients on lurasidone plus one of the other drugs had an average drop from baseline in their Montgomery-Åsberg Depression Rating Scale (MADRS) score of 17.1 points, compared with an average 13.5-point drop in the control patients who were on lithium or valproate plus placebo, a statistically significant difference. Fifty-seven percent of the 179 patients receiving lurasidone had at least a 50% drop in their MADRS score, compared with 42% of 161 patients in the control arm. Remission – achievement of a MADRS score of 12 or less – occurred in half the lurasidone patients and 35% of the controls.
Also notable was the impact the drug had on the 10 individual components of the MADRS score. Six of these 10 items showed statistically significant reductions with lurasidone treatment, including the two core measures of depression, apparent sadness and reported sadness.
The second trial tested lurasidone as monotherapy, at two different dosages, either 20-60 mg/day or 80-120 mg/day, and both were compared with placebo during 6 weeks of treatment, with a total of 485 patients randomized and completing 6 weeks on treatment. Lurasidone resulted in a similar pattern of efficacy, with 42% (lower dosage) and 40% of patients having their MADRS score drop to 12 points or less, compared with a 25% remission rate in the placebo group. Both dosages of lurasidone led to statistically significant reductions in 7 of the 10 MADRS component measures compared with the control arm.
The monotherapy study also showed that while the lower lurasidone dosage was just as effective as the higher dosage, it was more tolerable, with lower rates of akathisia and extrapyramidal symptoms. Lurasidone appears to produce low rates of sedation, appetite stimulation, and weight gain because of its negligible binding to the histamine1 receptor, Dr. Calabrese said. In the adjunctive study, lurasidone treatment resulted in virtually no change in weight or body mass index, and very small changes in cholesterol, triglycerides, and fasting glucose.
The two trials were sponsored by Sunovion, the company that markets lurasidone. Dr. Calabrese said he has been a consultant to and has received research support from Sunovion as well as from more than a dozen other drug companies.
On Twitter @mitchelzoler
BARCELONA – Lurasidone, an atypical antipsychotic approved by the Food and Drug Administration last June for the treatment of depression in patients with bipolar I disorder, has the potential to become a first-line treatment for these patients as physicians grow more familiar and comfortable with the drug, Dr. Joseph R. Calabrese said at the annual Congress of the European College of Neuropsychopharmacology.
In addition to showing significant efficacy for reducing depression in bipolar I patients when used as either monotherapy or as adjunctive therapy in a pair of phase III trials, lurasidone (Latuda) "does not have the metabolic burden caused by some other drugs" used in these patients, and also showed no statistically significant increase in dropout rates compared with placebo. The discontinuation rate because of adverse effects was especially low when the drug was administered at a dosage of 20-60 mg/day. This safety finding is "remarkable," said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland.
The results from the two phase III trials provide "unequivocal evidence that the drug has short-term efficacy for patients with depressive episodes in bipolar I disorder. I think the evidence is very persuasive," Dr. Calabrese said in an interview. "I think this drug will be used adjunctively at first, but with more familiarity, physicians will start using it up front. The No. 1 attraction for first-line therapy is no metabolic burden; that’s a huge issue. And sedation is minimal." Dr. Calabrese stressed that the trial results showed no dropouts because of sedation, and 10%-15% of patients reported a sedation effect, compared with the 40% rate typically seen with lamotrigine (Lamictal).
One of the trials assessed lurasidone as an adjunctive treatment in 340 patients already on treatment with lithium or valproate. After 6 weeks, patients on lurasidone plus one of the other drugs had an average drop from baseline in their Montgomery-Åsberg Depression Rating Scale (MADRS) score of 17.1 points, compared with an average 13.5-point drop in the control patients who were on lithium or valproate plus placebo, a statistically significant difference. Fifty-seven percent of the 179 patients receiving lurasidone had at least a 50% drop in their MADRS score, compared with 42% of 161 patients in the control arm. Remission – achievement of a MADRS score of 12 or less – occurred in half the lurasidone patients and 35% of the controls.
Also notable was the impact the drug had on the 10 individual components of the MADRS score. Six of these 10 items showed statistically significant reductions with lurasidone treatment, including the two core measures of depression, apparent sadness and reported sadness.
The second trial tested lurasidone as monotherapy, at two different dosages, either 20-60 mg/day or 80-120 mg/day, and both were compared with placebo during 6 weeks of treatment, with a total of 485 patients randomized and completing 6 weeks on treatment. Lurasidone resulted in a similar pattern of efficacy, with 42% (lower dosage) and 40% of patients having their MADRS score drop to 12 points or less, compared with a 25% remission rate in the placebo group. Both dosages of lurasidone led to statistically significant reductions in 7 of the 10 MADRS component measures compared with the control arm.
The monotherapy study also showed that while the lower lurasidone dosage was just as effective as the higher dosage, it was more tolerable, with lower rates of akathisia and extrapyramidal symptoms. Lurasidone appears to produce low rates of sedation, appetite stimulation, and weight gain because of its negligible binding to the histamine1 receptor, Dr. Calabrese said. In the adjunctive study, lurasidone treatment resulted in virtually no change in weight or body mass index, and very small changes in cholesterol, triglycerides, and fasting glucose.
The two trials were sponsored by Sunovion, the company that markets lurasidone. Dr. Calabrese said he has been a consultant to and has received research support from Sunovion as well as from more than a dozen other drug companies.
On Twitter @mitchelzoler
BARCELONA – Lurasidone, an atypical antipsychotic approved by the Food and Drug Administration last June for the treatment of depression in patients with bipolar I disorder, has the potential to become a first-line treatment for these patients as physicians grow more familiar and comfortable with the drug, Dr. Joseph R. Calabrese said at the annual Congress of the European College of Neuropsychopharmacology.
In addition to showing significant efficacy for reducing depression in bipolar I patients when used as either monotherapy or as adjunctive therapy in a pair of phase III trials, lurasidone (Latuda) "does not have the metabolic burden caused by some other drugs" used in these patients, and also showed no statistically significant increase in dropout rates compared with placebo. The discontinuation rate because of adverse effects was especially low when the drug was administered at a dosage of 20-60 mg/day. This safety finding is "remarkable," said Dr. Calabrese, professor of psychiatry and head of the mood disorders program at Case Western Reserve University in Cleveland.
The results from the two phase III trials provide "unequivocal evidence that the drug has short-term efficacy for patients with depressive episodes in bipolar I disorder. I think the evidence is very persuasive," Dr. Calabrese said in an interview. "I think this drug will be used adjunctively at first, but with more familiarity, physicians will start using it up front. The No. 1 attraction for first-line therapy is no metabolic burden; that’s a huge issue. And sedation is minimal." Dr. Calabrese stressed that the trial results showed no dropouts because of sedation, and 10%-15% of patients reported a sedation effect, compared with the 40% rate typically seen with lamotrigine (Lamictal).
One of the trials assessed lurasidone as an adjunctive treatment in 340 patients already on treatment with lithium or valproate. After 6 weeks, patients on lurasidone plus one of the other drugs had an average drop from baseline in their Montgomery-Åsberg Depression Rating Scale (MADRS) score of 17.1 points, compared with an average 13.5-point drop in the control patients who were on lithium or valproate plus placebo, a statistically significant difference. Fifty-seven percent of the 179 patients receiving lurasidone had at least a 50% drop in their MADRS score, compared with 42% of 161 patients in the control arm. Remission – achievement of a MADRS score of 12 or less – occurred in half the lurasidone patients and 35% of the controls.
Also notable was the impact the drug had on the 10 individual components of the MADRS score. Six of these 10 items showed statistically significant reductions with lurasidone treatment, including the two core measures of depression, apparent sadness and reported sadness.
The second trial tested lurasidone as monotherapy, at two different dosages, either 20-60 mg/day or 80-120 mg/day, and both were compared with placebo during 6 weeks of treatment, with a total of 485 patients randomized and completing 6 weeks on treatment. Lurasidone resulted in a similar pattern of efficacy, with 42% (lower dosage) and 40% of patients having their MADRS score drop to 12 points or less, compared with a 25% remission rate in the placebo group. Both dosages of lurasidone led to statistically significant reductions in 7 of the 10 MADRS component measures compared with the control arm.
The monotherapy study also showed that while the lower lurasidone dosage was just as effective as the higher dosage, it was more tolerable, with lower rates of akathisia and extrapyramidal symptoms. Lurasidone appears to produce low rates of sedation, appetite stimulation, and weight gain because of its negligible binding to the histamine1 receptor, Dr. Calabrese said. In the adjunctive study, lurasidone treatment resulted in virtually no change in weight or body mass index, and very small changes in cholesterol, triglycerides, and fasting glucose.
The two trials were sponsored by Sunovion, the company that markets lurasidone. Dr. Calabrese said he has been a consultant to and has received research support from Sunovion as well as from more than a dozen other drug companies.
On Twitter @mitchelzoler
AT THE ECNP CONGRESS
Major finding: Lurasidone monotherapy led to a 40%-42% remission rate, compared with a 25% rate among control patients on placebo.
Data source: Data came from one of two pivotal trials, which randomized 485 patients in the depressed phase of bipolar I disorder.
Disclosures: The two trials were sponsored by Sunovion, the company that markets lurasidone. Dr. Calabrese said he has been a consultant to and has received research support from Sunovion as well as from more than a dozen other drug companies.