User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lp(a) tied to more early CV events than familial hypercholesterolemia
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
‘Modest’ benefit for lecanemab in Alzheimer’s disease, but adverse events are common
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
In the CLARITY AD trial, adverse events (AEs) were common compared with placebo, including amyloid-related edema and effusions; and a recent news report linked a second death to the drug.
Moving forward, “longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease,” wrote Christopher H. van Dyck, MD, Yale University, New Haven, Conn., and colleagues.
The full trial findings were presented at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, with simultaneous publication on Nov. 29 in the New England Journal of Medicine.
Complications in the field
The phase 3 trial of lecanemab has been closely watched in AD circles, especially considering positive early data released in September and reported by this news organization at that time.
The Food and Drug Administration is expected to make a decision about possible approval of the drug in January 2023. Only one other antiamyloid treatment, the highly controversial and expensive aducanumab (Aduhelm), is currently approved by the FDA.
For the new 18-month, randomized, double-blind CLARITY AD trial, researchers enrolled 1,795 patients aged 50-90 years (average age, 71 years) with early AD. All were randomly assigned to receive either a placebo (n = 898) or intravenous lecanemab, a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively targets amyloid beta (A-beta) protofibrils, at 10 mg/kg of body weight every 2 weeks (n = 897).
The study ran from 2019 to 2021. The participants (52% women, 20% non-White) were recruited in North America, Europe, and Asia. Safety data included all participants, and the modified intention-to-treat group included 1,734 participants, with 859 receiving lecanemab and 875 receiving placebo.
The primary endpoint was the Clinical Dementia Rating–Sum of Boxes (CDR-SB). Scores from 0.5 to 6 are signs of early AD, according to the study. The mean baseline score for both groups was 3.2. The adjusted mean change at 18 months was 1.21 for lecanemab versus 1.66 for placebo (difference, –0.45; 95% confidence interval [CI], –0.67 to –0.23; P < .001).
As Dr. van Dyck noted in his presentation at the CTAD meting, this represents a 27% slowing of the decline in the lecanemab group.
The published findings do not speculate about how this difference would affect the day-to-day life of participants who took the drug, although it does refer to “modestly less decline” of cognition/function in the lecanemab group.
Other measurements that suggest cognitive improvements in the lecanemab group versus placebo include the Alzheimer’s Disease Assessment Scale–Cognitive Subscale score (mean difference, –1.44; 95% CI, –2.27 to –0.61), the Alzheimer’s Disease Composite Score (mean difference, –0.05; 95% CI, –.074 to –.027,), and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment score (mean difference, 2.0; 95% CI, 1.2-2.8; all, P < .001).
Overall, Dr. van Dyck said, “Lecanemab met the primary and secondary endpoints versus placebo at 18 months, with highly significant differences starting at 6 months.”
In a substudy of 698 participants, results showed that amyloid burden fell at a higher rate in the lecanemab group than in the placebo group (difference, –59.1 centiloids; 95% CI, –62.6 to –55.6).
“Lecanemab has high selectivity for soluble aggregated species of A-beta as compared with monomeric amyloid, with moderate selectivity for fibrillar amyloid; this profile is considered to target the most toxic pathologic amyloid species,” the researchers wrote.
Concerning AE data
With respect to AEs, deaths occurred in both groups (0.7% in those who took lecanemab and 0.8% in those who took the placebo). The researchers did not attribute any deaths to the drug. However, according to a report in the journal Science published Nov. 27, a 65-year-old woman who was taking the drug as part of a clinical trial “recently died from a massive brain hemorrhage that some researchers link to the drug.”
The woman, the second person “whose death was linked to lecanemab,” died after suffering a stroke. Researchers summarized a case report as saying that the drug “contributed to her brain hemorrhage after biweekly infusions of lecanemab inflamed and weakened the blood vessels.”
Eisai, which sponsored the new trial, told Science that “all the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall or from any specific cause.”
In a CTAD presentation, study coauthor Marwan Sabbagh, MD, Barrow Neurological Institute, Phoenix, said two hemorrhage-related deaths occurred in an open-label extension. One was in the context of a tissue plasminogen activator treatment for a stroke, which fits with the description of the case in the Science report. “Causality with lecanemab is a little difficult ...,” he said. “Patients on anticoagulation might need further consideration.”
In the CLARITY AD Trial, serious AEs occurred in 14% of the lecanemab group, leading to discontinuation 6.9% of the time, and in 11.3% of the placebo group, leading to discontinuation 2.9% of the time, the investigators reported.
They added that, in the lecanemab group, the most common AEs, defined as affecting more than 10% of participants, were infusion-related reactions (26.4% vs. 7.4% for placebo); amyloid-related imaging abnormalities with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (17.3% vs. 9%, respectively); amyloid-related imaging abnormalities with edema or effusions (12.6% vs. 1.7%); headache (11.1% vs. 8.1%); and falls (10.4% vs. 9.6%).
In addition, macrohemorrhage was reported in 0.6% of the lecanemab group and 0.1% of the placebo group.
Cautious optimism
In separate interviews, two Alzheimer’s specialists who weren’t involved in the study praised the trial and described the findings as “exciting.” But they also highlighted its limitations.
Alvaro Pascual-Leone, MD, PhD, professor of neurology at Harvard Medical School and chief medical officer of Linus Health, said the study represents impressive progress after 60-plus trials examining anti-amyloid monoclonal antibodies. “This is the first trial that shows a clinical benefit that can be measured,” he said.
However, it’s unclear whether the changes “are really going to make a difference in people’s lives,” he said. The drug is likely to be expensive, owing to the large investment needed for research, he added, and patients will have to undergo costly testing, such as PET scans and spinal taps.
Still, “this could be a valuable adjunct to the armamentarium we have,” which includes interventions such as lifestyle changes, he said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, noted that the trial reached its primary and secondary endpoints and that the drug had what he called a “modest” effect on cognition.
However, the drugmaker will need to explore the adverse effects, he said, especially among patients with atrial fibrillation who take anticoagulants. And, he said, medicine is still far from the ultimate goal – fully reversing cognitive decline.
Michael Weiner, MD, president of the CTAD22 Scientific Committee, noted in a press release that there is “growing evidence” that some antiamyloid therapies, “especially lecanemab and donanemab” have shown promising results.
“Unfortunately, these treatments are also associated with abnormal differences seen in imaging, including brain swelling and bleeding in the brain,” said Dr. Weiner, professor of radiology, medicine, and neurology at the University of California, San Francisco.
“There is considerable controversy concerning the significance and impact of these findings, including whether or not governments and medical insurance will provide financial coverage for such treatments,” he added.
Rave reviews from the Alzheimer’s Association
In a statement, the Alzheimer’s Association raved about lecanemab and declared that the FDA should approve lecanemab on an accelerated basis. The study “confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease ...” the association said, adding that “it could mean many months more of recognizing their spouse, children and grandchildren.”
The association, which is a staunch supporter of aducanumab, called on the Centers for Medicare & Medicaid Services to cover the drug if the FDA approves it. The association’s statement did not address the drug’s potential high cost, the adverse effects, or the two reported deaths.
The trial was supported by Eisai (regulatory sponsor) with partial funding from Biogen. Dr. van Dyck reports having received research grants from Biogen, Eisai, Biohaven, Cerevel Therapeutics, Eli Lilly, Genentech, Janssen, Novartis, and UCB. He has been a consultant to Cerevel, Eisai, Ono Pharmaceutical, and Roche. Relevant financial relationships for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
AT CTAD 2022
U.S. flu activity already at mid-season levels
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.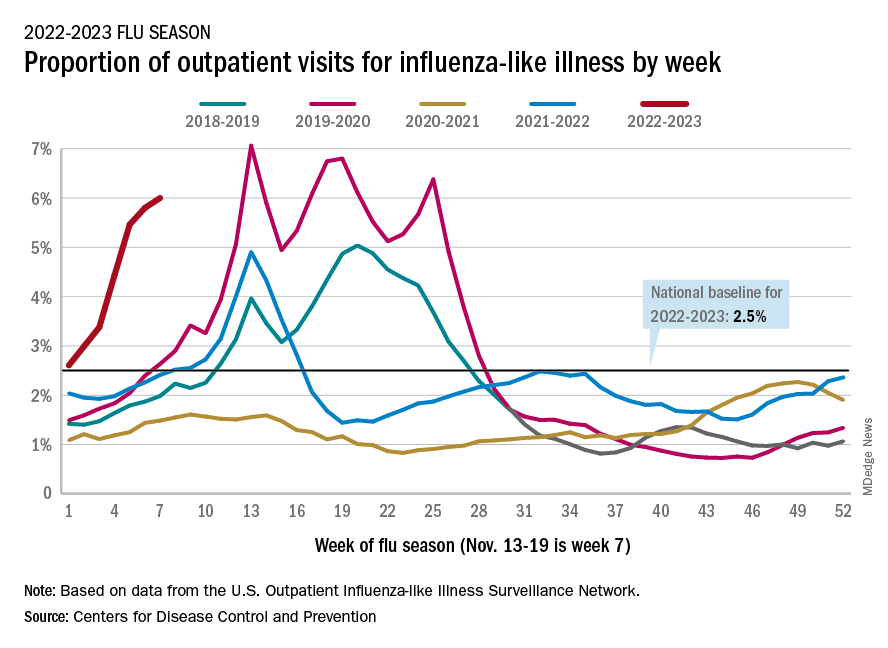
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
Yellow Nodule on the Scalp
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4
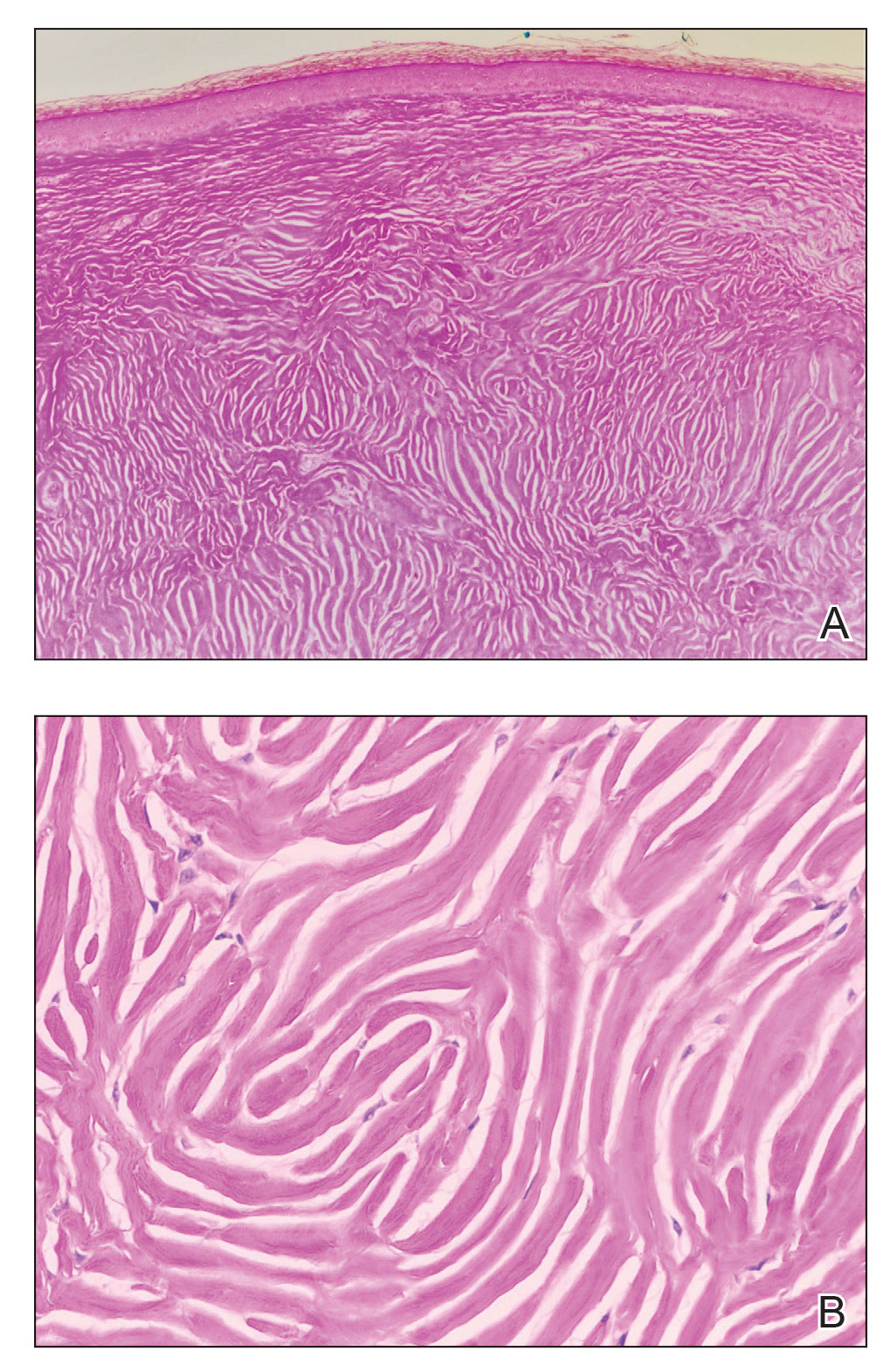
The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
A 45-year-old woman was referred to dermatology by a primary care physician for evaluation of a raised skin lesion on the scalp. She was otherwise healthy. The lesion had been present for many years but recently grew in size. The patient reported that the lesion was subject to recurrent physical trauma and she wanted it removed. Physical examination revealed a 6×6-mm, domeshaped, yellow nodule on the left inferior parietal scalp. There were no similar lesions located elsewhere on the body. A shave removal was performed and sent for histopathologic evaluation.

Advancing health equity in neurology is essential to patient care
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
FROM ANA 2022
Is it long COVID, or dementia, or both?
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
Just 8 minutes of exercise a day is all you need
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
FROM EUROPEAN HEART JOURNAL
Vitamin D fails to stave off statin-related muscle symptoms
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
More vaccinated people dying of COVID as fewer get booster shots
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.
“We can no longer say this is a pandemic of the unvaccinated,” Kaiser Family Foundation Vice President Cynthia Cox, who conducted the analysis, told The Washington Post.
People who had been vaccinated or boosted made up 58% of COVID-19 deaths in August, the analysis showed. The rate has been on the rise: 23% of coronavirus deaths were among vaccinated people in September 2021, and the vaccinated made up 42% of deaths in January and February 2022, the Post reported.
Research continues to show that people who are vaccinated or boosted have a lower risk of death. The rise in deaths among the vaccinated is the result of three factors, Ms. Cox said.
- A large majority of people in the United States have been vaccinated (267 million people, the said).
- People who are at the greatest risk of dying from COVID-19 are more likely to be vaccinated and boosted, such as the elderly.
- Vaccines lose their effectiveness over time; the virus changes to avoid vaccines; and people need to choose to get boosters to continue to be protected.
The case for the effectiveness of vaccines and boosters versus skipping the shots remains strong. People age 6 months and older who are unvaccinated are six times more likely to die of COVID-19, compared to those who got the primary series of shots, the Post reported. Survival rates were even better with additional booster shots, particularly among older people.
“I feel very confident that if people continue to get vaccinated at good numbers, if people get boosted, we can absolutely have a very safe and healthy holiday season,” Ashish Jha, White House coronavirus czar, said on Nov. 22.
The number of Americans who have gotten the most recent booster has been increasing ahead of the holidays. CDC data show that 12% of the U.S. population age 5 and older has received a booster.
A new study by a team of researchers from Harvard University and Yale University estimates that 94% of the U.S. population has been infected with COVID-19 at least once, leaving just 1 in 20 people who have never had the virus.
“Despite these high exposure numbers, there is still substantial population susceptibility to infection with an Omicron variant,” the authors wrote.
They said that if all states achieved the vaccination levels of Vermont, where 55% of people had at least one booster and 22% got a second one, there would be “an appreciable improvement in population immunity, with greater relative impact for protection against infection versus severe disease. This additional protection results from both the recovery of immunity lost due to waning and the increased effectiveness of the bivalent booster against Omicron infections.”
A version of this article first appeared on WebMD.com.
Don’t call me ‘Dr.,’ say some physicians – but most prefer the title
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN