User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cardiac injury caused by COVID-19 less common than thought
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
The study examined cardiac MRI scans in 31 patients before and after having COVID-19 infection and found no new evidence of myocardial injury in the post-COVID scans relative to the pre-COVID scans.
“To the best of our knowledge this is the first cardiac MRI study to assess myocardial injury pre- and post-COVID-19,” the authors stated.
They say that while this study cannot rule out the possibility of rare events of COVID-19–induced myocardial injury, “the complete absence of de novo late gadolinium enhancement lesions after COVID-19 in this cohort indicates that outside special circumstances, COVID-19–induced myocardial injury may be much less common than suggested by previous studies.”
The study was published online in JACC: Cardiovascular Imaging.
Coauthor Till F. Althoff, MD, Cardiovascular Institute, Clínic–University Hospital Barcelona, said in an interview that previous reports have found a high rate of cardiac lesions in patients undergoing imaging after having had COVID-19 infection.
“In some reports, this has been as high as 80% of patients even though they have not had severe COVID disease. These reports have been interpreted as showing the majority of patients have some COVID-induced cardiac damage, which is an alarming message,” he commented.
However, he pointed out that the patients in these reports did not undergo a cardiac MRI scan before they had COVID-19 so it wasn’t known whether these cardiac lesions were present before infection or not.
To try and gain more accurate information, the current study examined cardiac MRI scans in the same patients before and after they had COVID-19.
The researchers, from an arrhythmia unit, made use of the fact that all their patients have cardiac MRI data, so they used their large registry of patients in whom cardiac MRI had been performed, and cross referenced this to a health care database to identify those patients who had confirmed COVID-19 after they obtaining a cardiac scan at the arrhythmia unit. They then conducted another cardiac MRI scan in the 31 patients identified a median of 5 months after their COVID-19 infection.
“These 31 patients had a cardiac MRI scan pre-COVID and post COVID using exactly the same scanner with identical sequences, so the scans were absolutely comparable,” Dr. Althoff noted.
Of these 31 patients, 7 had been hospitalized at the time of acute presentation with COVID-19, of whom 2 required intensive care. Most patients (29) had been symptomatic, but none reported cardiac symptoms.
Results showed that, on the post–COVID-19 scan, late gadolinium enhancement lesions indicative of residual myocardial injury were encountered in 15 of the 31 patients (48%), which the researchers said is in line with previous reports.
However, intraindividual comparison with the pre–COVID-19 cardiac MRI scans showed all these lesions were preexisting with identical localization, pattern, and transmural distribution, and thus not COVID-19 related.
Quantitative analyses, performed independently, detected no increase in the size of individual lesions nor in the global left ventricular late gadolinium enhancement extent.
Comparison of pre- and post COVID-19 imaging sequences did not show any differences in ventricular functional or structural parameters.
“While this study only has 31 patients, the fact that we are conducting intra-individual comparisons, which rules out bias, means that we don’t need a large number of patients for reliable results,” Dr. Althoff said.
“These types of lesions are normal to see. We know that individuals without cardiac disease have these types of lesions, and they are not necessarily an indication of any specific pathology. I was kind of surprised by the interpretation of previous data, which is why we did the current study,” he added.
Dr. Althoff acknowledged that some cardiac injury may have been seen if much larger numbers of patients had been included. “But I think we can say from this data that COVID-induced cardiac damage is much less of an issue than we may have previously thought,” he added.
He also noted that most of the patients in this study had mild COVID-19, so the results cannot be extrapolated to severe COVID-19 infection.
However, Dr. Althoff pointed out that all the patients already had atrial fibrillation, so would have been at higher risk of cardiac injury from COVID-19.
“These patients had preexisting cardiac risk factors, and thus they would have been more susceptible to both a more severe course of COVID and an increased risk of myocardial damage due to COVID. The fact that we don’t find any myocardial injury due to COVID in this group is even more reassuring. The general population will be at even lower risk,” he commented.
“I think we can say that, in COVID patients who do not have any cardiac symptoms, our study suggests that the incidence of cardiac injury is very low,” Dr. Althoff said.
“Even in patients with severe COVID and myocardial involvement reflected by increased troponin levels, I wouldn’t be sure that they have any residual cardiac injury. While it has been reported that cardiac lesions have been found in such patients, pre-COVID MRI scans were not available so we don’t know if they were there before,” he added.
“We do not know the true incidence of cardiac injury after COVID, but I think we can say from this data that it is definitely not anywhere near the 40%-50% or even greater that some of the previous reports have suggested,” he stated.
Dr. Althoff suggested that, based on these data, some of the recommendations based on previous reports such the need for follow-up cardiac scans and caution about partaking in sports again after COVID-19 infection, are probably not necessary.
“Our data suggest that these concerns are unfounded, and we need to step back a bit and stop alarming patients about the risk of cardiac damage after COVID,” he said. “Yes, if patients have cardiac symptoms during or after COVID infection they should get checked out, but I do not think we need to do a cardiac risk assessment in patients without cardiac symptoms in COVID.”
This work is supported in part by grants from Instituto de Salud Carlos III, the Spanish government, Madrid, and Fundació la Marató de TV3 in Catalonia. Dr. Althoff has received research grants for investigator-initiated trials from Biosense Webster.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR IMAGING
Children and COVID: New-case counts offer dueling narratives
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
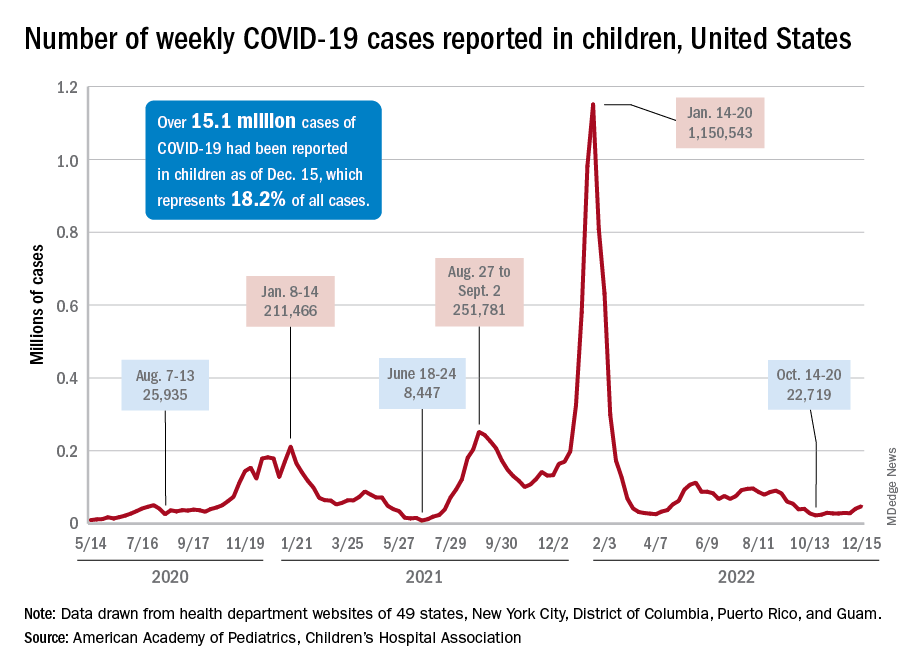
and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
High lipoprotein(a) levels plus hypertension add to CVD risk
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Dubious diagnosis: Is there a better way to define ‘prediabetes’?
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDF WORLD DIABETES CONGRESS 2022
Researchers probe ‘systematic error’ in gun injury data
These coding inaccuracies could distort our understanding of gun violence in the United States and make it seem like accidental shootings are more common than they really are, researchers reported in JAMA Network Open.
“The systematic error in intent classification is not widely known or acknowledged by researchers in this field,” Philip J. Cook, PhD, of Duke University, Durham, N.C., and Susan T. Parker, of the University of Michigan, Ann Arbor, wrote in an invited commentary about the new findings. “The bulk of all shootings, nonfatal and fatal together, are assaults, which is to say the result of one person intentionally shooting another. An accurate statistical portrait thus suggests that gun violence is predominantly a crime problem.”
In 2020, 79% of all homicides and 53% of all suicides involved firearms, the CDC reported. Gun violence is now the leading cause of death for children in the United States, government data show.
For the new study, Matthew Miller, MD, ScD, of Northeastern University and the Harvard Injury Control Research Center in Boston, and his colleagues examined how International Classification of Diseases (ICD) codes may misclassify the intent behind gunshot injuries.
Dr. Miller’s group looked at 1,227 incidents between 2008 and 2019 at three major trauma centers – Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston, and Harborview Medical Center in Seattle.
Of those shootings, 837 (68.2%) involved assaults, 168 (13.5%) were unintentional, 124 (9.9%) were deliberate self-harm, and 43 (3.4%) were instances of legal intervention, based on the researchers’ review of medical records.
ICD codes at discharge, however, labeled 581 cases (47.4%) as assaults and 432 (35.2%) as unintentional.
The researchers found that 234 of the 837 assaults (28%) and 9 of the 43 legal interventions (20.9%) were miscoded as unintentional. This problem occurred even when the “medical narrative explicitly indicated that the shooting was an act of interpersonal violence,” such as a drive-by shooting or an act of domestic violence, the researchers reported.
Hospital trauma registrars, who detail the circumstances surrounding injuries, were mostly in agreement with the researchers.
Medical coders “would likely have little trouble characterizing firearm injury intent accurately if incentives were created for them to do so,” the authors wrote.
Trends and interventions
Separately, researchers published studies showing that gun violence tends to affect various demographics differently, and that remediating abandoned houses could help reduce gun crime.
Lindsay Young, of the University of Cincinnati, and Henry Xiang, MD, PhD, director of the Center for Pediatric Trauma Research at Nationwide Children’s Hospital in Columbus, Ohio, analyzed rates of firearm deaths from 1981 to 2020.
They found that the rate of firearm-related homicide was five times higher among males than females, and the rate of suicide involving firearms was nearly seven times higher for men, they reported in PLOS ONE.
Black men were the group most affected by homicide, whereas White men were most affected by suicide, they found.
To see if fixing abandoned properties would improve health and reduce gun violence in low-income, Black neighborhoods in Philadelphia, Eugenia C. South, MD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a randomized trial.
They randomly assigned abandoned properties in some areas to undergo full remediation (installing working windows and doors, cleaning trash, and weeding); trash cleanup and weeding only; or no intervention.
“Abandoned houses that were remediated showed substantial drops in nearby weapons violations (−8.43%), gun assaults (−13.12%), and to a lesser extent shootings (−6.96%),” the researchers reported.
The intervention targets effects of segregation that have resulted from “historical and ongoing government and private-sector policies” that lead to disinvestment in Black, urban communities, they wrote. Abandoned houses can be used to store firearms and for other illegal activity. They also can engender feelings of fear, neglect, and stress in communities, the researchers noted.
Dr. Miller’s study was funded by the National Collaborative on Gun Violence Research; coauthors disclosed corporate, government, and university grants. The full list of disclosures can be found with the original article. Editorialists Dr. Cook and Dr. Parker report no relevant financial relationships. Dr. South’s study was funded by the National Institutes of Health. Dr. South and some coauthors disclosed government grants.
A version of this article first appeared on Medscape.com.
These coding inaccuracies could distort our understanding of gun violence in the United States and make it seem like accidental shootings are more common than they really are, researchers reported in JAMA Network Open.
“The systematic error in intent classification is not widely known or acknowledged by researchers in this field,” Philip J. Cook, PhD, of Duke University, Durham, N.C., and Susan T. Parker, of the University of Michigan, Ann Arbor, wrote in an invited commentary about the new findings. “The bulk of all shootings, nonfatal and fatal together, are assaults, which is to say the result of one person intentionally shooting another. An accurate statistical portrait thus suggests that gun violence is predominantly a crime problem.”
In 2020, 79% of all homicides and 53% of all suicides involved firearms, the CDC reported. Gun violence is now the leading cause of death for children in the United States, government data show.
For the new study, Matthew Miller, MD, ScD, of Northeastern University and the Harvard Injury Control Research Center in Boston, and his colleagues examined how International Classification of Diseases (ICD) codes may misclassify the intent behind gunshot injuries.
Dr. Miller’s group looked at 1,227 incidents between 2008 and 2019 at three major trauma centers – Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston, and Harborview Medical Center in Seattle.
Of those shootings, 837 (68.2%) involved assaults, 168 (13.5%) were unintentional, 124 (9.9%) were deliberate self-harm, and 43 (3.4%) were instances of legal intervention, based on the researchers’ review of medical records.
ICD codes at discharge, however, labeled 581 cases (47.4%) as assaults and 432 (35.2%) as unintentional.
The researchers found that 234 of the 837 assaults (28%) and 9 of the 43 legal interventions (20.9%) were miscoded as unintentional. This problem occurred even when the “medical narrative explicitly indicated that the shooting was an act of interpersonal violence,” such as a drive-by shooting or an act of domestic violence, the researchers reported.
Hospital trauma registrars, who detail the circumstances surrounding injuries, were mostly in agreement with the researchers.
Medical coders “would likely have little trouble characterizing firearm injury intent accurately if incentives were created for them to do so,” the authors wrote.
Trends and interventions
Separately, researchers published studies showing that gun violence tends to affect various demographics differently, and that remediating abandoned houses could help reduce gun crime.
Lindsay Young, of the University of Cincinnati, and Henry Xiang, MD, PhD, director of the Center for Pediatric Trauma Research at Nationwide Children’s Hospital in Columbus, Ohio, analyzed rates of firearm deaths from 1981 to 2020.
They found that the rate of firearm-related homicide was five times higher among males than females, and the rate of suicide involving firearms was nearly seven times higher for men, they reported in PLOS ONE.
Black men were the group most affected by homicide, whereas White men were most affected by suicide, they found.
To see if fixing abandoned properties would improve health and reduce gun violence in low-income, Black neighborhoods in Philadelphia, Eugenia C. South, MD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a randomized trial.
They randomly assigned abandoned properties in some areas to undergo full remediation (installing working windows and doors, cleaning trash, and weeding); trash cleanup and weeding only; or no intervention.
“Abandoned houses that were remediated showed substantial drops in nearby weapons violations (−8.43%), gun assaults (−13.12%), and to a lesser extent shootings (−6.96%),” the researchers reported.
The intervention targets effects of segregation that have resulted from “historical and ongoing government and private-sector policies” that lead to disinvestment in Black, urban communities, they wrote. Abandoned houses can be used to store firearms and for other illegal activity. They also can engender feelings of fear, neglect, and stress in communities, the researchers noted.
Dr. Miller’s study was funded by the National Collaborative on Gun Violence Research; coauthors disclosed corporate, government, and university grants. The full list of disclosures can be found with the original article. Editorialists Dr. Cook and Dr. Parker report no relevant financial relationships. Dr. South’s study was funded by the National Institutes of Health. Dr. South and some coauthors disclosed government grants.
A version of this article first appeared on Medscape.com.
These coding inaccuracies could distort our understanding of gun violence in the United States and make it seem like accidental shootings are more common than they really are, researchers reported in JAMA Network Open.
“The systematic error in intent classification is not widely known or acknowledged by researchers in this field,” Philip J. Cook, PhD, of Duke University, Durham, N.C., and Susan T. Parker, of the University of Michigan, Ann Arbor, wrote in an invited commentary about the new findings. “The bulk of all shootings, nonfatal and fatal together, are assaults, which is to say the result of one person intentionally shooting another. An accurate statistical portrait thus suggests that gun violence is predominantly a crime problem.”
In 2020, 79% of all homicides and 53% of all suicides involved firearms, the CDC reported. Gun violence is now the leading cause of death for children in the United States, government data show.
For the new study, Matthew Miller, MD, ScD, of Northeastern University and the Harvard Injury Control Research Center in Boston, and his colleagues examined how International Classification of Diseases (ICD) codes may misclassify the intent behind gunshot injuries.
Dr. Miller’s group looked at 1,227 incidents between 2008 and 2019 at three major trauma centers – Brigham and Women’s Hospital and Massachusetts General Hospital, both in Boston, and Harborview Medical Center in Seattle.
Of those shootings, 837 (68.2%) involved assaults, 168 (13.5%) were unintentional, 124 (9.9%) were deliberate self-harm, and 43 (3.4%) were instances of legal intervention, based on the researchers’ review of medical records.
ICD codes at discharge, however, labeled 581 cases (47.4%) as assaults and 432 (35.2%) as unintentional.
The researchers found that 234 of the 837 assaults (28%) and 9 of the 43 legal interventions (20.9%) were miscoded as unintentional. This problem occurred even when the “medical narrative explicitly indicated that the shooting was an act of interpersonal violence,” such as a drive-by shooting or an act of domestic violence, the researchers reported.
Hospital trauma registrars, who detail the circumstances surrounding injuries, were mostly in agreement with the researchers.
Medical coders “would likely have little trouble characterizing firearm injury intent accurately if incentives were created for them to do so,” the authors wrote.
Trends and interventions
Separately, researchers published studies showing that gun violence tends to affect various demographics differently, and that remediating abandoned houses could help reduce gun crime.
Lindsay Young, of the University of Cincinnati, and Henry Xiang, MD, PhD, director of the Center for Pediatric Trauma Research at Nationwide Children’s Hospital in Columbus, Ohio, analyzed rates of firearm deaths from 1981 to 2020.
They found that the rate of firearm-related homicide was five times higher among males than females, and the rate of suicide involving firearms was nearly seven times higher for men, they reported in PLOS ONE.
Black men were the group most affected by homicide, whereas White men were most affected by suicide, they found.
To see if fixing abandoned properties would improve health and reduce gun violence in low-income, Black neighborhoods in Philadelphia, Eugenia C. South, MD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a randomized trial.
They randomly assigned abandoned properties in some areas to undergo full remediation (installing working windows and doors, cleaning trash, and weeding); trash cleanup and weeding only; or no intervention.
“Abandoned houses that were remediated showed substantial drops in nearby weapons violations (−8.43%), gun assaults (−13.12%), and to a lesser extent shootings (−6.96%),” the researchers reported.
The intervention targets effects of segregation that have resulted from “historical and ongoing government and private-sector policies” that lead to disinvestment in Black, urban communities, they wrote. Abandoned houses can be used to store firearms and for other illegal activity. They also can engender feelings of fear, neglect, and stress in communities, the researchers noted.
Dr. Miller’s study was funded by the National Collaborative on Gun Violence Research; coauthors disclosed corporate, government, and university grants. The full list of disclosures can be found with the original article. Editorialists Dr. Cook and Dr. Parker report no relevant financial relationships. Dr. South’s study was funded by the National Institutes of Health. Dr. South and some coauthors disclosed government grants.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
COVID booster shot poll: People ‘don’t think they need one’
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Hospitals with more diverse and uninsured patients more likely to provide delayed fracture care
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regardless of individual patient-level characteristics such as race, ethnicity, or insurance status, these patients were more likely to miss the recommended 24-hour benchmark for surgery.
“Institutions that treat a less diverse patient population appeared to be more resilient to the mix of insurance status in their patient population and were more likely to meet time-to-surgery benchmarks, regardless of patient insurance status or population-based insurance mix,” write study author Ida Leah Gitajn, MD, an orthopedic trauma surgeon at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues.
“While it is unsurprising that increased delays were associated with underfunded institutions, the association between institutional-level racial disparity and surgical delays implies structural health systems bias,” the authors wrote.
The study was published online in JAMA Network Open.
Site performance varied
Racial inequalities in health care utilization and outcomes have been documented in many medical specialties, including orthopedic trauma, the study authors write. However, previous studies evaluating racial disparities in fracture care have been limited to patient-level associations rather than hospital-level factors.
The investigators conducted a secondary analysis of prospectively collected multicenter data for 2,565 patients with hip and femur fractures enrolled in two randomized trials at 23 sites in the United States and Canada. The researchers assessed whether disparities in meeting 24-hour time-to-surgery benchmarks exist at the patient level or at the institutional level, evaluating the association of race, ethnicity, and insurance status.
The cohort study used data from the Program of Randomized Trials to Evaluate Preoperative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT), which enrolled patients from 2018-2021 and followed them for 1 year. All patients with hip and femur fractures enrolled in the PREP-IT program were included in the analysis, which was conducted from April to September of this year.
The cohort included 2,565 patients with an average age of about 65 years. About 82% of patients were White, 13.4% were Black, 3.2% were Asian, and 1.1% were classified as another race or ethnicity. Among the study population, 32.5% of participants were employed, and 92.2% had health insurance. Nearly 40% had a femur fracture with an average injury severity score of 10.4.
Overall, 596 patients (23.2%) didn’t meet the 24-hour time-to-operating-room benchmark. Patients who didn’t meet the 24-hour surgical window were more likely to be older, women, and have a femur fracture. They were less likely to be employed.
The 23 sites had variability in meeting the 24-hour benchmark, race and ethnicity distribution, and population-based health insurance. Institutions met benchmarks at frequencies ranging from 45.2% (for 196 of 433 procedures) to 97.4% (37 of 38 procedures). Minority race and ethnicity distribution ranged from 0% (in 99 procedures) to 58.2% (in 53 of 91 procedures). The proportion of uninsured patients ranged from 0% (in 64 procedures) to 34.2% (in 13 of 38 procedures).
At the patient level, there was no association between missing the 24-hour benchmark and race or ethnicity, and there was no independent association between hospital population racial composition and surgical delay. In an analysis that controlled for patient-level characteristics, there was no association between missing the 24-hour benchmark and patient-level insurance status.
There was an independent association, however, between the hospital population insurance coverage and hospital population racial composition as an interaction term, suggesting a moderating effect (P = .03), the study authors write.
At low rates of uninsured patients, the probability of missing the 24-hour benchmark was 12.5%-14.6% when racial composition varied from 0%-50% minority patients. In contrast, at higher rates of uninsured patients, the risk of missing the 24-hour window was higher among more diverse populations. For instance, at 30% uninsured, the risk of missing the benchmark was 0.5% when the racial composition was low and 17.6% at 50% minority patients.
Additional studies are needed to understand the findings and how health system programs or structures play a role, the authors write. For instance, well-funded health systems that care for a higher proportion of insured patients likely have quality improvement programs and other support structures, such as operating room access, that ensure appropriate time-to-surgery benchmarks for time-sensitive fractures, they say.
Addressing inequalities
Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery, New York, said, “Despite these disparities being reported and well documented in recent years, unfortunately, not enough has been done to address them or understand their fundamental root causes.”
Dr. Amen, who wasn’t involved with this study, has researched racial and ethnic disparities in hip fracture surgery care across the United States. He and his colleagues found disparities in delayed time-to-surgery, particularly for Black patients.
“We live in a country and society where we want and strive for equality of care for patients regardless of race, ethnicity, gender, sexual orientation, or background,” he said. “We have a moral imperative to address these disparities as health care providers, not only among ourselves, but also in conjunction with lawmakers, hospital administrators, and health policy specialists.”
Uma Srikumaran, MD, an associate professor of orthopedic surgery at Johns Hopkins University, Baltimore, wasn’t involved with this study but has researched racial disparities in the timing of radiographic assessment and surgical treatment of hip fractures.
“Though we understand that racial disparities are pervasive in health care, we have a great deal left to understand about the extent of those disparities and all the various factors that contribute to them,” Dr. Srikumaran told this news organization.
Dr. Srikumaran and colleagues have found that Black patients had longer wait times for evaluation and surgery than White patients.
“We all want to get to the solutions, but those can be difficult to execute without an intricate understanding of the problem,” he said. “We should encourage this type of research all throughout health care in general but also very locally, as solutions are not likely to be one-size-fits-all.”
Dr. Srikumaran pointed to the need to measure the problem in specific pathologies, populations, geographies, hospital types, and other factors.
“Studying the trends of this issue will help us determine whether our national or local initiatives are making a difference and which interventions are most effective for a particular hospital, geographic location, or particular pathology,” he said. “Accordingly, if a particular hospital or health system isn’t looking at differences in the delivery of care by race, they are missing an opportunity to ensure equity and raise overall quality.”
The study was supported by funding from the Patient Centered Outcomes Research Institute. Dr. Gitajn reported receiving personal fees for consulting and teaching work from Stryker outside the submitted work. Dr. Amen and Dr. Srikumaran reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
AI versus other interventions for colonoscopy: How do they compare?
AI-based tools appear to outperform other methods intended to increase ADRs, including distal attachment devices, dye-based/virtual chromoendoscopy, water-based techniques, and balloon-assisted devices, researchers found in a systematic review and meta-analysis.
“ADR is a very important quality metric. The higher the ADR, the less likely the chance of interval cancer,” first author Muhammad Aziz, MD, co-chief gastroenterology fellow at the University of Toledo (Ohio), told this news organization. Interval cancer refers to colorectal cancer that is diagnosed within 5 years of a patient’s undergoing a negative colonoscopy.
“Numerous interventions have been attempted and researched to see the impact on ADR,” he said. “The new kid on the block – AI-assisted colonoscopy – is a game-changer. I knew that AI was impactful in improving ADR, but I didn’t know it would be the best.”
The study was published online in the Journal of Clinical Gastroenterology.
Analyzing detection rates
Current guidelines set an ADR benchmark of 25% overall, with 30% for men and 20% for women undergoing screening colonoscopy. Every 1% increase in ADR results in a 3% reduction in colorectal cancer, Dr. Aziz and his co-authors write.
Several methods can improve ADR over standard colonoscopy. Computer-aided detection and AI methods, which have emerged in recent years, alert the endoscopist of potential lesions in real time with visual signals.
No direct comparative studies had been conducted, so to make an indirect comparison, Dr. Aziz and colleagues undertook a systematic review and network meta-analysis of 94 randomized controlled trials that included 61,172 patients and 20 different study interventions.
The research team assessed the impact of AI in comparison with other endoscopic methods, using relative risk for proportional outcomes and mean difference for continuous outcomes. About 63% of the colonoscopies were for screening and surveillance, and 37% were diagnostic. The effectiveness was ranked by P-score (the probability of being the best treatment).
Overall, AI had the highest P-score (0.96), signifying the best modality of all interventions for improving ADR, the study authors write. A sensitivity analysis using the fixed effects model did not significantly alter the effect measure.
The network meta-analysis showed significantly higher ADR for AI, compared with autofluorescence imaging (relative risk, 1.33), dye-based chromoendoscopy (RR, 1.22), Endocap (RR, 1.32), Endocuff (RR, 1.19), Endocuff Vision (RR, 1.26), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR,1.26), full-spectrum endoscopy (RR, 1.40), high-definition (HD) colonoscopy (RR, 1.41), linked color imaging (1.21), narrow-band imaging (RR, 1.33), water exchange (RR, 1.22), and water immersion (RR, 1.47).
Among 34 studies of colonoscopies for screening or surveillance only, the ADR was significantly improved for linked color imaging (RR, 1.18), I-Scan with contrast and surface enhancement (RR, 1.25), Endocuff (RR, 1.20), Endocuff Vision (RR, 1.13), and water exchange (RR, 1.24), compared with HD colonoscopy. Only one AI study was included in this analysis, because the others had significantly more patients who underwent colonoscopy for diagnostic indications. In this case, AI did not improve ADR, compared with HD colonoscopy (RR, 1.44).
In addition, a significantly improved polyp detection rate (PDR) was noted for AI, compared with autofluorescence imaging (RR, 1.28), Endocap (RR, 1.18), Endocuff Vision (RR, 1.21), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR, 1.21), full-spectrum endoscopy (RR, 1.39), HD colonoscopy (RR, 1.34), linked color imaging (RR, 1.19), and narrow-band imaging (RR, 1.21). Again, AI had the highest P-score (RR, 0.93).
Among 17 studies of colonoscopy for screening and surveillance, only one AI study was included for PDR. A significantly higher PDR was noted for AI, compared with HD colonoscopy (RR, 1.33). None of the other interventions improved PDR over HD colonoscopy.
No AI advantage for serrated polyps
Twenty-three studies evaluated detection for serrated polyps, including three AI studies. AI did not improve the serrated polyp detection rate (SPDR), compared with other interventions. However, several modalities did improve SPDR: G-EYE, compared with full-spectrum endoscopy (RR, 3.93), linked color imaging, compared with full-spectrum endoscopy (RR, 1.88), and HD colonoscopy (RR, 1.71), and Endocuff Vision, compared with HD colonoscopy (RR, 1.36). G-EYE had the highest P-score (0.93).
AI significantly improved adenomas per colonoscopy, compared with full-spectrum endoscopy (mean difference, 0.38), HD colonoscopy (MD, 0.18), and narrow-band imaging (MD, 0.13), the authors note. However, the number of adenomas detected per colonoscopy was significantly lower for AI, compared with Endocap (-0.13). Endocap had the highest P-score (0.92).
“The strengths of this study include the wide range of endoscopic add-ons included, the number of trials included, and the granularity of some of the reporting data,” Jeremy Glissen Brown, MD, a gastroenterologist and an assistant professor of medicine at Duke University, told this news organization.
Dr. Glissen Brown, who wasn’t involved with this study, researches AI tools for polyp detection. He and colleagues have found that AI decreases adenoma miss rates and increases the number of first-pass adenomas detected per colonoscopy.
“The limitations include significant heterogeneity among many of the comparisons, as well as a high risk of bias, as it is technically difficult to achieve blinding of provider participants in the device-based RCTs [randomized controlled trials] that this analysis was based on,” he said.
Additional considerations
Dr. Aziz and colleagues note the need for additional studies of AI-based detection, particularly for screening and surveillance. For widespread adoption into clinical practice, new systems must have higher specificity, sensitivity, accuracy, and efficiency, they write.
“AI technology needs further optimization, as there is still the aspect of having a lot of false positives – lesions detected but not necessarily adenomas that can turn into cancer,” Dr. Aziz said. “This decreases the efficiency of the colonoscopy and increases the anesthesia and sedation time. In addition, different AI systems have different diagnostic yield, as it all depends on the images that were fed to the system or algorithm.”
Dr. Glissen Brown also pointed to the low number of AI-based studies involving serrated polyp lesion detection. Future research could investigate whether computer-aided detection systems (CADe) decrease miss rates and increase detection rates for sessile serrated lesions, he said.
For practical clinical purposes, Dr. Glissen Brown highlighted the potential complementary nature of the various colonoscopy tools. When used together, for instance, AI and Endocuff may increase ADRs even further and decrease the number of missed polyps through different mechanisms, he said.
“It is also important in device research to interrogate the cost versus benefit of any intervention or combination of interventions,” he said. “I think with CADe this is still something that we are figuring out. We will need to find novel ways of making these technologies affordable, especially as the debate of which clinically meaningful outcomes we examine when it comes to AI continues to evolve.”
No funding source for the study was reported. Two authors have received grant support from or have consulted for several pharmaceutical and medical device companies. Dr. Glissen Brown has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AI-based tools appear to outperform other methods intended to increase ADRs, including distal attachment devices, dye-based/virtual chromoendoscopy, water-based techniques, and balloon-assisted devices, researchers found in a systematic review and meta-analysis.
“ADR is a very important quality metric. The higher the ADR, the less likely the chance of interval cancer,” first author Muhammad Aziz, MD, co-chief gastroenterology fellow at the University of Toledo (Ohio), told this news organization. Interval cancer refers to colorectal cancer that is diagnosed within 5 years of a patient’s undergoing a negative colonoscopy.
“Numerous interventions have been attempted and researched to see the impact on ADR,” he said. “The new kid on the block – AI-assisted colonoscopy – is a game-changer. I knew that AI was impactful in improving ADR, but I didn’t know it would be the best.”
The study was published online in the Journal of Clinical Gastroenterology.
Analyzing detection rates
Current guidelines set an ADR benchmark of 25% overall, with 30% for men and 20% for women undergoing screening colonoscopy. Every 1% increase in ADR results in a 3% reduction in colorectal cancer, Dr. Aziz and his co-authors write.
Several methods can improve ADR over standard colonoscopy. Computer-aided detection and AI methods, which have emerged in recent years, alert the endoscopist of potential lesions in real time with visual signals.
No direct comparative studies had been conducted, so to make an indirect comparison, Dr. Aziz and colleagues undertook a systematic review and network meta-analysis of 94 randomized controlled trials that included 61,172 patients and 20 different study interventions.
The research team assessed the impact of AI in comparison with other endoscopic methods, using relative risk for proportional outcomes and mean difference for continuous outcomes. About 63% of the colonoscopies were for screening and surveillance, and 37% were diagnostic. The effectiveness was ranked by P-score (the probability of being the best treatment).
Overall, AI had the highest P-score (0.96), signifying the best modality of all interventions for improving ADR, the study authors write. A sensitivity analysis using the fixed effects model did not significantly alter the effect measure.
The network meta-analysis showed significantly higher ADR for AI, compared with autofluorescence imaging (relative risk, 1.33), dye-based chromoendoscopy (RR, 1.22), Endocap (RR, 1.32), Endocuff (RR, 1.19), Endocuff Vision (RR, 1.26), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR,1.26), full-spectrum endoscopy (RR, 1.40), high-definition (HD) colonoscopy (RR, 1.41), linked color imaging (1.21), narrow-band imaging (RR, 1.33), water exchange (RR, 1.22), and water immersion (RR, 1.47).
Among 34 studies of colonoscopies for screening or surveillance only, the ADR was significantly improved for linked color imaging (RR, 1.18), I-Scan with contrast and surface enhancement (RR, 1.25), Endocuff (RR, 1.20), Endocuff Vision (RR, 1.13), and water exchange (RR, 1.24), compared with HD colonoscopy. Only one AI study was included in this analysis, because the others had significantly more patients who underwent colonoscopy for diagnostic indications. In this case, AI did not improve ADR, compared with HD colonoscopy (RR, 1.44).
In addition, a significantly improved polyp detection rate (PDR) was noted for AI, compared with autofluorescence imaging (RR, 1.28), Endocap (RR, 1.18), Endocuff Vision (RR, 1.21), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR, 1.21), full-spectrum endoscopy (RR, 1.39), HD colonoscopy (RR, 1.34), linked color imaging (RR, 1.19), and narrow-band imaging (RR, 1.21). Again, AI had the highest P-score (RR, 0.93).
Among 17 studies of colonoscopy for screening and surveillance, only one AI study was included for PDR. A significantly higher PDR was noted for AI, compared with HD colonoscopy (RR, 1.33). None of the other interventions improved PDR over HD colonoscopy.
No AI advantage for serrated polyps
Twenty-three studies evaluated detection for serrated polyps, including three AI studies. AI did not improve the serrated polyp detection rate (SPDR), compared with other interventions. However, several modalities did improve SPDR: G-EYE, compared with full-spectrum endoscopy (RR, 3.93), linked color imaging, compared with full-spectrum endoscopy (RR, 1.88), and HD colonoscopy (RR, 1.71), and Endocuff Vision, compared with HD colonoscopy (RR, 1.36). G-EYE had the highest P-score (0.93).
AI significantly improved adenomas per colonoscopy, compared with full-spectrum endoscopy (mean difference, 0.38), HD colonoscopy (MD, 0.18), and narrow-band imaging (MD, 0.13), the authors note. However, the number of adenomas detected per colonoscopy was significantly lower for AI, compared with Endocap (-0.13). Endocap had the highest P-score (0.92).
“The strengths of this study include the wide range of endoscopic add-ons included, the number of trials included, and the granularity of some of the reporting data,” Jeremy Glissen Brown, MD, a gastroenterologist and an assistant professor of medicine at Duke University, told this news organization.
Dr. Glissen Brown, who wasn’t involved with this study, researches AI tools for polyp detection. He and colleagues have found that AI decreases adenoma miss rates and increases the number of first-pass adenomas detected per colonoscopy.
“The limitations include significant heterogeneity among many of the comparisons, as well as a high risk of bias, as it is technically difficult to achieve blinding of provider participants in the device-based RCTs [randomized controlled trials] that this analysis was based on,” he said.
Additional considerations
Dr. Aziz and colleagues note the need for additional studies of AI-based detection, particularly for screening and surveillance. For widespread adoption into clinical practice, new systems must have higher specificity, sensitivity, accuracy, and efficiency, they write.
“AI technology needs further optimization, as there is still the aspect of having a lot of false positives – lesions detected but not necessarily adenomas that can turn into cancer,” Dr. Aziz said. “This decreases the efficiency of the colonoscopy and increases the anesthesia and sedation time. In addition, different AI systems have different diagnostic yield, as it all depends on the images that were fed to the system or algorithm.”
Dr. Glissen Brown also pointed to the low number of AI-based studies involving serrated polyp lesion detection. Future research could investigate whether computer-aided detection systems (CADe) decrease miss rates and increase detection rates for sessile serrated lesions, he said.
For practical clinical purposes, Dr. Glissen Brown highlighted the potential complementary nature of the various colonoscopy tools. When used together, for instance, AI and Endocuff may increase ADRs even further and decrease the number of missed polyps through different mechanisms, he said.
“It is also important in device research to interrogate the cost versus benefit of any intervention or combination of interventions,” he said. “I think with CADe this is still something that we are figuring out. We will need to find novel ways of making these technologies affordable, especially as the debate of which clinically meaningful outcomes we examine when it comes to AI continues to evolve.”
No funding source for the study was reported. Two authors have received grant support from or have consulted for several pharmaceutical and medical device companies. Dr. Glissen Brown has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AI-based tools appear to outperform other methods intended to increase ADRs, including distal attachment devices, dye-based/virtual chromoendoscopy, water-based techniques, and balloon-assisted devices, researchers found in a systematic review and meta-analysis.
“ADR is a very important quality metric. The higher the ADR, the less likely the chance of interval cancer,” first author Muhammad Aziz, MD, co-chief gastroenterology fellow at the University of Toledo (Ohio), told this news organization. Interval cancer refers to colorectal cancer that is diagnosed within 5 years of a patient’s undergoing a negative colonoscopy.
“Numerous interventions have been attempted and researched to see the impact on ADR,” he said. “The new kid on the block – AI-assisted colonoscopy – is a game-changer. I knew that AI was impactful in improving ADR, but I didn’t know it would be the best.”
The study was published online in the Journal of Clinical Gastroenterology.
Analyzing detection rates
Current guidelines set an ADR benchmark of 25% overall, with 30% for men and 20% for women undergoing screening colonoscopy. Every 1% increase in ADR results in a 3% reduction in colorectal cancer, Dr. Aziz and his co-authors write.
Several methods can improve ADR over standard colonoscopy. Computer-aided detection and AI methods, which have emerged in recent years, alert the endoscopist of potential lesions in real time with visual signals.
No direct comparative studies had been conducted, so to make an indirect comparison, Dr. Aziz and colleagues undertook a systematic review and network meta-analysis of 94 randomized controlled trials that included 61,172 patients and 20 different study interventions.
The research team assessed the impact of AI in comparison with other endoscopic methods, using relative risk for proportional outcomes and mean difference for continuous outcomes. About 63% of the colonoscopies were for screening and surveillance, and 37% were diagnostic. The effectiveness was ranked by P-score (the probability of being the best treatment).
Overall, AI had the highest P-score (0.96), signifying the best modality of all interventions for improving ADR, the study authors write. A sensitivity analysis using the fixed effects model did not significantly alter the effect measure.
The network meta-analysis showed significantly higher ADR for AI, compared with autofluorescence imaging (relative risk, 1.33), dye-based chromoendoscopy (RR, 1.22), Endocap (RR, 1.32), Endocuff (RR, 1.19), Endocuff Vision (RR, 1.26), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR,1.26), full-spectrum endoscopy (RR, 1.40), high-definition (HD) colonoscopy (RR, 1.41), linked color imaging (1.21), narrow-band imaging (RR, 1.33), water exchange (RR, 1.22), and water immersion (RR, 1.47).
Among 34 studies of colonoscopies for screening or surveillance only, the ADR was significantly improved for linked color imaging (RR, 1.18), I-Scan with contrast and surface enhancement (RR, 1.25), Endocuff (RR, 1.20), Endocuff Vision (RR, 1.13), and water exchange (RR, 1.24), compared with HD colonoscopy. Only one AI study was included in this analysis, because the others had significantly more patients who underwent colonoscopy for diagnostic indications. In this case, AI did not improve ADR, compared with HD colonoscopy (RR, 1.44).
In addition, a significantly improved polyp detection rate (PDR) was noted for AI, compared with autofluorescence imaging (RR, 1.28), Endocap (RR, 1.18), Endocuff Vision (RR, 1.21), EndoRings (RR, 1.30), flexible spectral imaging color enhancement (RR, 1.21), full-spectrum endoscopy (RR, 1.39), HD colonoscopy (RR, 1.34), linked color imaging (RR, 1.19), and narrow-band imaging (RR, 1.21). Again, AI had the highest P-score (RR, 0.93).
Among 17 studies of colonoscopy for screening and surveillance, only one AI study was included for PDR. A significantly higher PDR was noted for AI, compared with HD colonoscopy (RR, 1.33). None of the other interventions improved PDR over HD colonoscopy.
No AI advantage for serrated polyps
Twenty-three studies evaluated detection for serrated polyps, including three AI studies. AI did not improve the serrated polyp detection rate (SPDR), compared with other interventions. However, several modalities did improve SPDR: G-EYE, compared with full-spectrum endoscopy (RR, 3.93), linked color imaging, compared with full-spectrum endoscopy (RR, 1.88), and HD colonoscopy (RR, 1.71), and Endocuff Vision, compared with HD colonoscopy (RR, 1.36). G-EYE had the highest P-score (0.93).
AI significantly improved adenomas per colonoscopy, compared with full-spectrum endoscopy (mean difference, 0.38), HD colonoscopy (MD, 0.18), and narrow-band imaging (MD, 0.13), the authors note. However, the number of adenomas detected per colonoscopy was significantly lower for AI, compared with Endocap (-0.13). Endocap had the highest P-score (0.92).
“The strengths of this study include the wide range of endoscopic add-ons included, the number of trials included, and the granularity of some of the reporting data,” Jeremy Glissen Brown, MD, a gastroenterologist and an assistant professor of medicine at Duke University, told this news organization.
Dr. Glissen Brown, who wasn’t involved with this study, researches AI tools for polyp detection. He and colleagues have found that AI decreases adenoma miss rates and increases the number of first-pass adenomas detected per colonoscopy.
“The limitations include significant heterogeneity among many of the comparisons, as well as a high risk of bias, as it is technically difficult to achieve blinding of provider participants in the device-based RCTs [randomized controlled trials] that this analysis was based on,” he said.
Additional considerations
Dr. Aziz and colleagues note the need for additional studies of AI-based detection, particularly for screening and surveillance. For widespread adoption into clinical practice, new systems must have higher specificity, sensitivity, accuracy, and efficiency, they write.
“AI technology needs further optimization, as there is still the aspect of having a lot of false positives – lesions detected but not necessarily adenomas that can turn into cancer,” Dr. Aziz said. “This decreases the efficiency of the colonoscopy and increases the anesthesia and sedation time. In addition, different AI systems have different diagnostic yield, as it all depends on the images that were fed to the system or algorithm.”
Dr. Glissen Brown also pointed to the low number of AI-based studies involving serrated polyp lesion detection. Future research could investigate whether computer-aided detection systems (CADe) decrease miss rates and increase detection rates for sessile serrated lesions, he said.
For practical clinical purposes, Dr. Glissen Brown highlighted the potential complementary nature of the various colonoscopy tools. When used together, for instance, AI and Endocuff may increase ADRs even further and decrease the number of missed polyps through different mechanisms, he said.
“It is also important in device research to interrogate the cost versus benefit of any intervention or combination of interventions,” he said. “I think with CADe this is still something that we are figuring out. We will need to find novel ways of making these technologies affordable, especially as the debate of which clinically meaningful outcomes we examine when it comes to AI continues to evolve.”
No funding source for the study was reported. Two authors have received grant support from or have consulted for several pharmaceutical and medical device companies. Dr. Glissen Brown has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
The 'Plaque Hypothesis': Focus on vulnerable lesions to cut events
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
New AHA statement on managing ACS in older adults
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.

