User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
H5N1 Avian Influenza Spreads Across North America
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
National Noncompete Ban Unlikely to Survive Under Trump, Experts Say
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Most US Adults Plan to Skip Annual COVID Vaccines
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Pertussis Cases Spike in November
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
BCG Vaccine May Protect Against Long COVID Symptoms
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Flu Vaccine Guards Household Contacts of Infected People
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
AMR Could Surpass Cancer as Leading Cause of Death by 2050
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Watch That Attitude: Is There Ageism in Healthcare?
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
Varicella Outbreaks: 2022-2024
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.
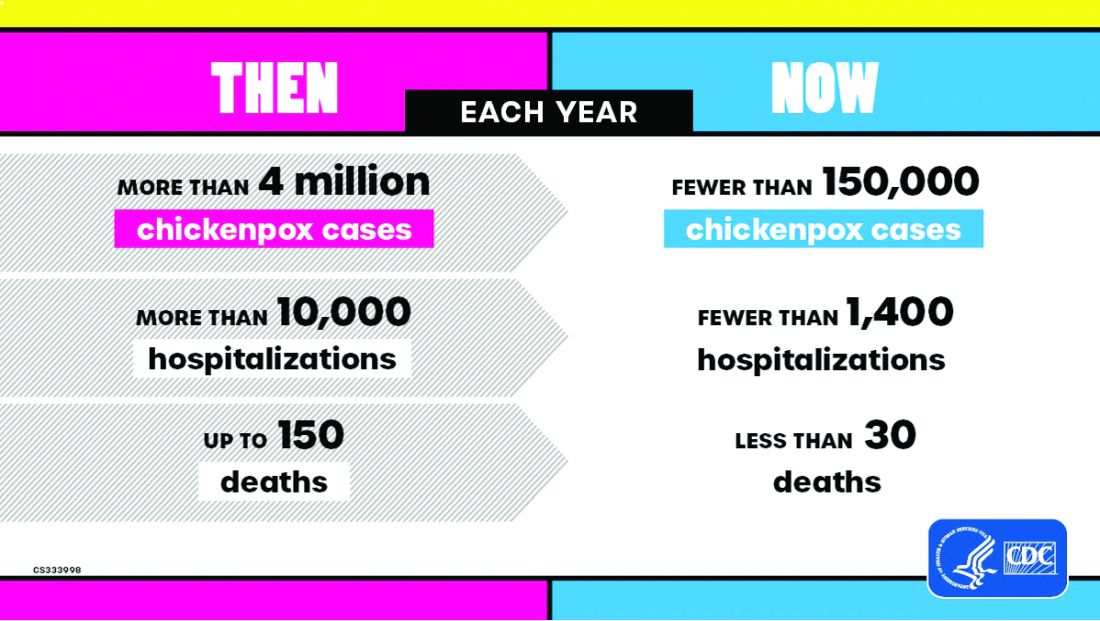
As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.