User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
CDC recommends hep B vaccination for most adults
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
First COVID-19 human challenge study provides insights
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Ivermectin doesn’t help treat COVID-19, large study finds
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Fingers take the fight to COVID-19
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pneumococcal pneumonia outcomes worse than those of Legionnaires disease
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
You’re not on a ‘best doctor’ list – does it matter?
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Polio: Five African countries vaccinating 23 million children
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Children and COVID: The long goodbye continues
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.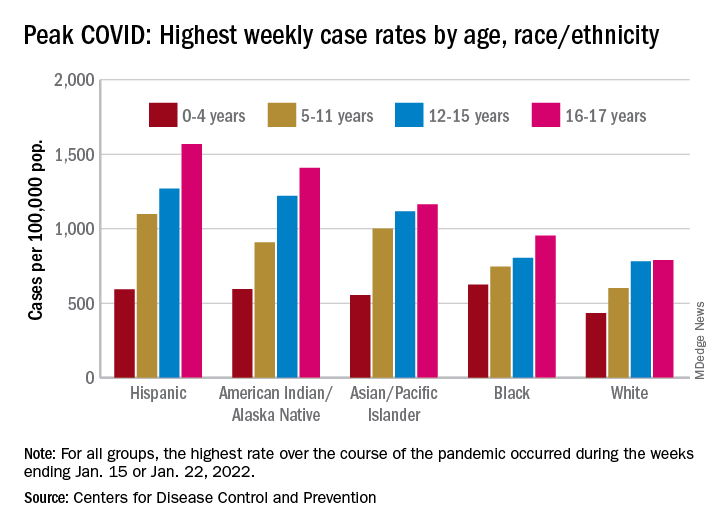
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
Surgery in CJD patients a potential risk factor for transmission
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN






