User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS
Resident physician work-hour regulations associated with improved physician safety and health
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
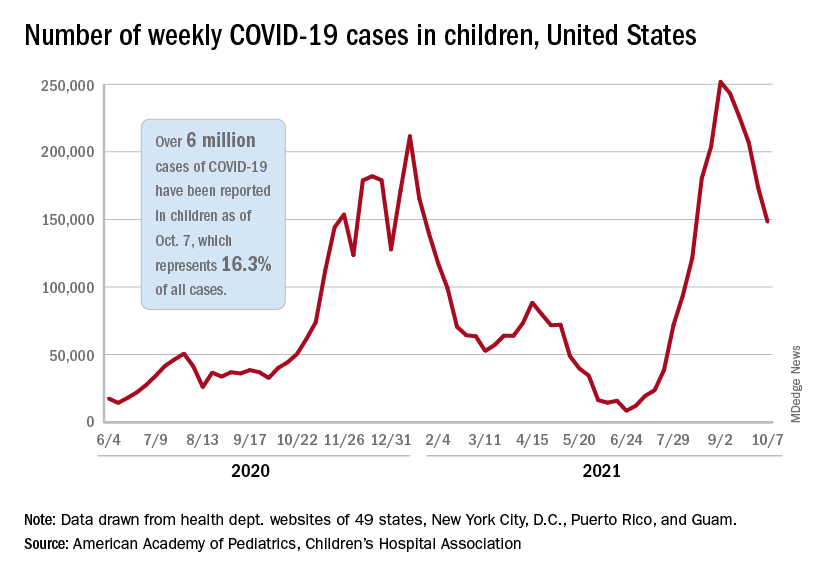
The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
Autopsy findings reveal venous thromboembolism in patients with COVID-19
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Rivaroxaban’s single daily dose may lead to higher bleeding risk than other DOACs
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
The results, which were published in the Annals of Internal Medicine, could help guide DOAC selection for high-risk groups with a prior history of peptic ulcer disease or major GI bleeding, said lead study authors Arnar Bragi Ingason, MD and Einar S. Björnsson, MD, PhD, in an email.
DOACs treat conditions such as atrial fibrillation, venous thromboembolism, and ischemic stroke and are known to cause GI bleeding. Previous studies have suggested that rivaroxaban poses a higher GI-bleeding risk than other DOACs.
These studies, which used large administrative databases, “had an inherent risk of selection bias due to insurance status, age, and comorbidities due to their origin from insurance/administrative databases. In addition, they lacked phenotypic details on GI bleeding events,” said Dr. Björnsson and Dr. Ingason, who are both of Landspitali University Hospital, Reykjavik, Iceland,
Daily dosage may exacerbate risk
Rivaroxaban is administered as a single daily dose, compared with apixaban’s and dabigatran’s twice-daily regimens. “We hypothesized that this may lead to a greater variance in drug plasma concentration, making these patients more susceptible to GI bleeding,” the lead authors said.
Using data from the Icelandic Medicine Registry, a national database of outpatient prescription information, they compared rates of GI bleeding among new users of apixaban, dabigatran, and rivaroxaban from 2014 to 2019. Overall, 5,868 patients receiving one of the DOACs took part in the study. Among these participants, 3,217 received rivaroxaban, 2,157 received apixaban, and 494 received dabigatran. The researchers used inverse probability weighting, Kaplan–Meier survival estimates, and Cox regression to compare GI bleeding.
Compared with dabigatran, rivaroxaban was associated with a 63%-104% higher overall risk for GI bleeding and 39%-95% higher risk for major GI bleeding. Rivaroxaban also had a 40%-42% higher overall risk for GI bleeding and 49%-50% higher risk for major GI bleeding, compared with apixaban.
The investigators were surprised by the low rate of upper GI bleeding for dabigatran, compared with the other two drugs. “However, these results must be interpreted in the context that the dabigatran group was relatively small,” said Dr. Björnsson and Dr. Ingason via email.
Overall, the study cohort was small, compared with previous registry studies.
Investigators also did not account for account for socioeconomic status or lifestyle factors, such as alcohol consumption or smoking. “However, because the cost of all DOACs is similar in Iceland, selection bias due to socioeconomic status is unlikely,” the investigators reported in their paper. “We are currently working on comparing the rates of thromboembolisms and overall major bleeding events between the drugs,” the lead authors said.
Clinicians should consider location of bleeding
Though retrospective, the study by Ingason et. al. “is likely as close as is feasible to a randomized trial as is possible,” said Don C. Rockey, MD, a professor of medicine at the Medical University of South Carolina, Charleston, in an interview.
“From the clinician’s perspective, it is important to take away that there may be differences among the DOACs in terms of where in the GI tract the bleeding occurs,” said Dr. Rockey. In the study, the greatest differences appeared to be in the upper GI tract, with rivaroxaban outpacing apixaban and dabigatran. In patients who are at risk for upper GI bleeding, it may be reasonable to consider use of dabigatran or apixaban, he suggested.
“A limitation of the study is that it is likely underpowered overall,” said Dr. Rockey. It also wasn’t clear how many deaths occurred either directly from GI bleeding or as a complication of GI bleeding, he said.The study also didn’t differentiate major bleeding among DOACs specifically in the upper or lower GI tract, Dr. Rockey added.
Other studies yield similar results
Dr. Ingason and Dr. Björnsson said their work complements previous studies, and Neena S. Abraham, MD, MSc , who has conducted a similar investigation to the new study, agreed with that statement.
Data from the last 4 years overwhelmingly show that rivaroxaban is most likely to cause GI bleeding, said Dr. Abraham, professor of medicine and a consultant with Mayo Clinic’s division of gastroenterology and hepatology, in an interview.
A comparative safety study Dr. Abraham coauthored in 2017 of rivaroxaban, apixaban, and dabigatran in a much larger U.S. cohort of 372,380 patients revealed that rivaroxaban had the worst GI bleeding profile. Apixaban was 66% safer than rivaroxaban and 64% safer than dabigatran to prevent gastrointestinal bleeding.
“I believe our group was the first to conduct this study and show clinically significant differences in GI safety of the available direct oral anticoagulants,” she said. Other investigators have since published similar results, and the topic of the new study needs no further investigation, according to Dr. Abraham.
“It is time for physicians to choose a better choice when prescribing a direct oral anticoagulant to their atrial fibrillation patients, and that choice is not rivaroxaban,” she said.
The Icelandic Centre for Research and the Landspítali University Hospital Research Fund provided funds for this study. Dr. Ingason, Dr. Björnsson, Dr. Rockey, and Dr. Abraham reported no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
New reports help nail down myocarditis risk with COVID-19 vaccine
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
Major insurers running billions of dollars behind on payments to hospitals and doctors
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Case reports underscore risk of cerebral edema, AFCE in children with COVID-19
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
FROM CNS 2021




