User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Why this round of COVID-19 feels worse
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Merck’s new COVID-19 pill: ‘Game changer’ or just one more tool?
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Mentoring is key to growing women’s leadership in medicine
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
FROM THE ADVANCE PHM GENDER EQUITY CONFERENCE
Hospitals must identify and empower women leaders
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
FROM THE ADVANCE PHM GENDER EQUITY CONFERENCE
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
Is AFib a stroke cause or innocent bystander? The debate continues
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
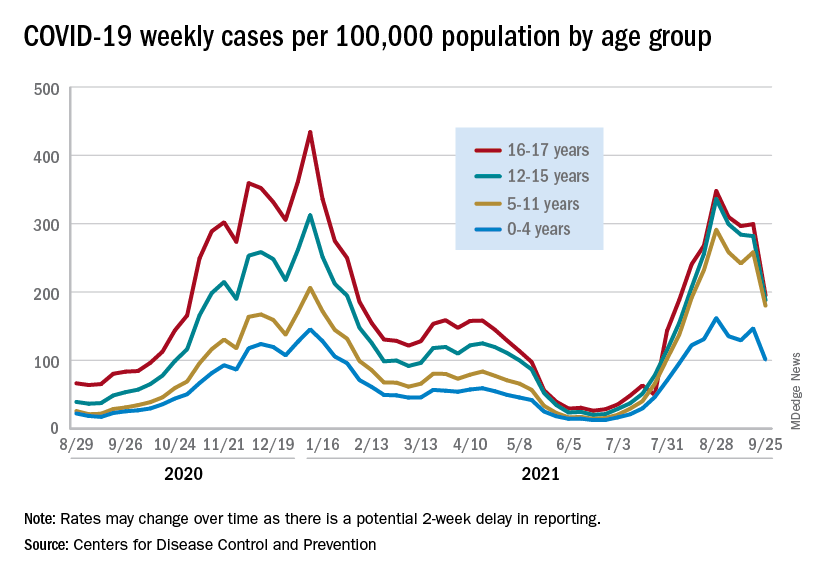
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
Vaccine holdouts embrace COVID antibody treatment, mystifying doctors
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Tolerance in medicine
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.







