User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Medicare Part D Prescription Claims for Brodalumab: Analysis of Annual Trends for 2017-2019
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
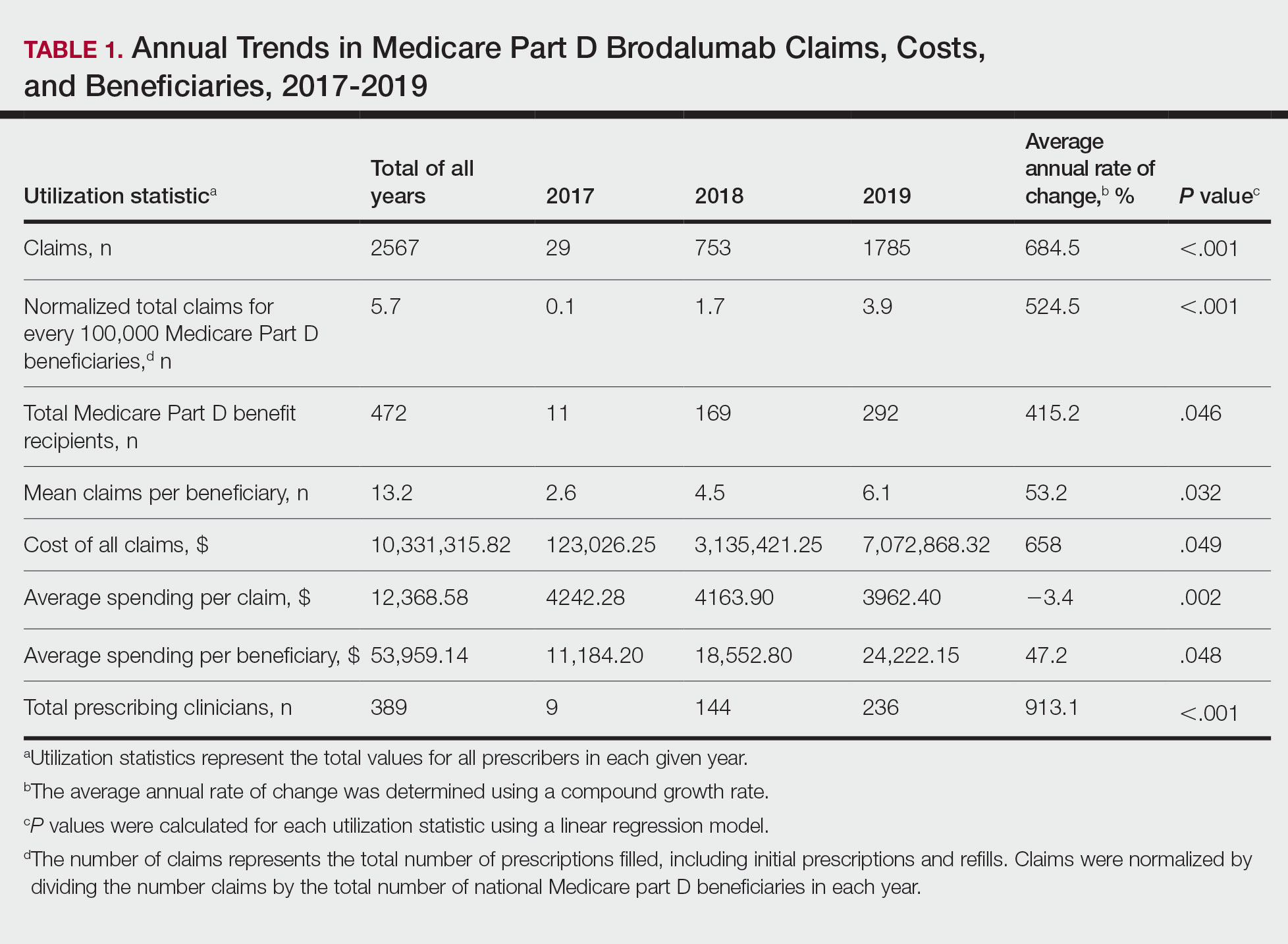
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).
In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).
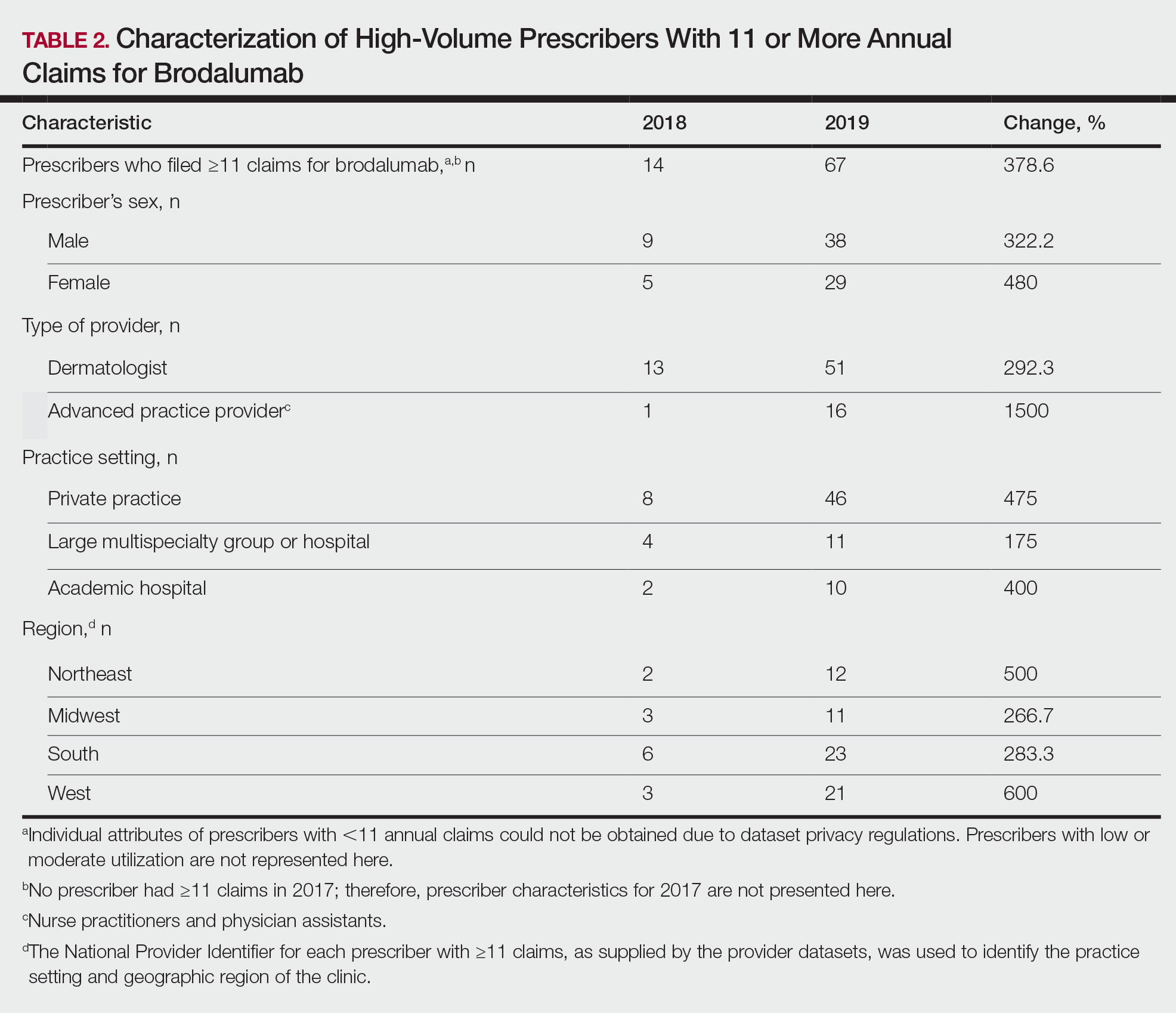
There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
Practice Points
- Brodalumab is associated with a boxed warning due to increased suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
- Brodalumab is underutilized compared to the other US Food and Drug Administration–approved IL-17 inhibitors used to treat psoriasis.
- Most experts agree that there is no increased risk for suicide associated with brodalumab. However, it remains imperative that prescribers assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services prior to initiating and during treatment with brodalumab.
Glucocorticoid-Induced Bone Loss: Dietary Supplementation Recommendations to Reduce the Risk for Osteoporosis and Osteoporotic Fractures
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
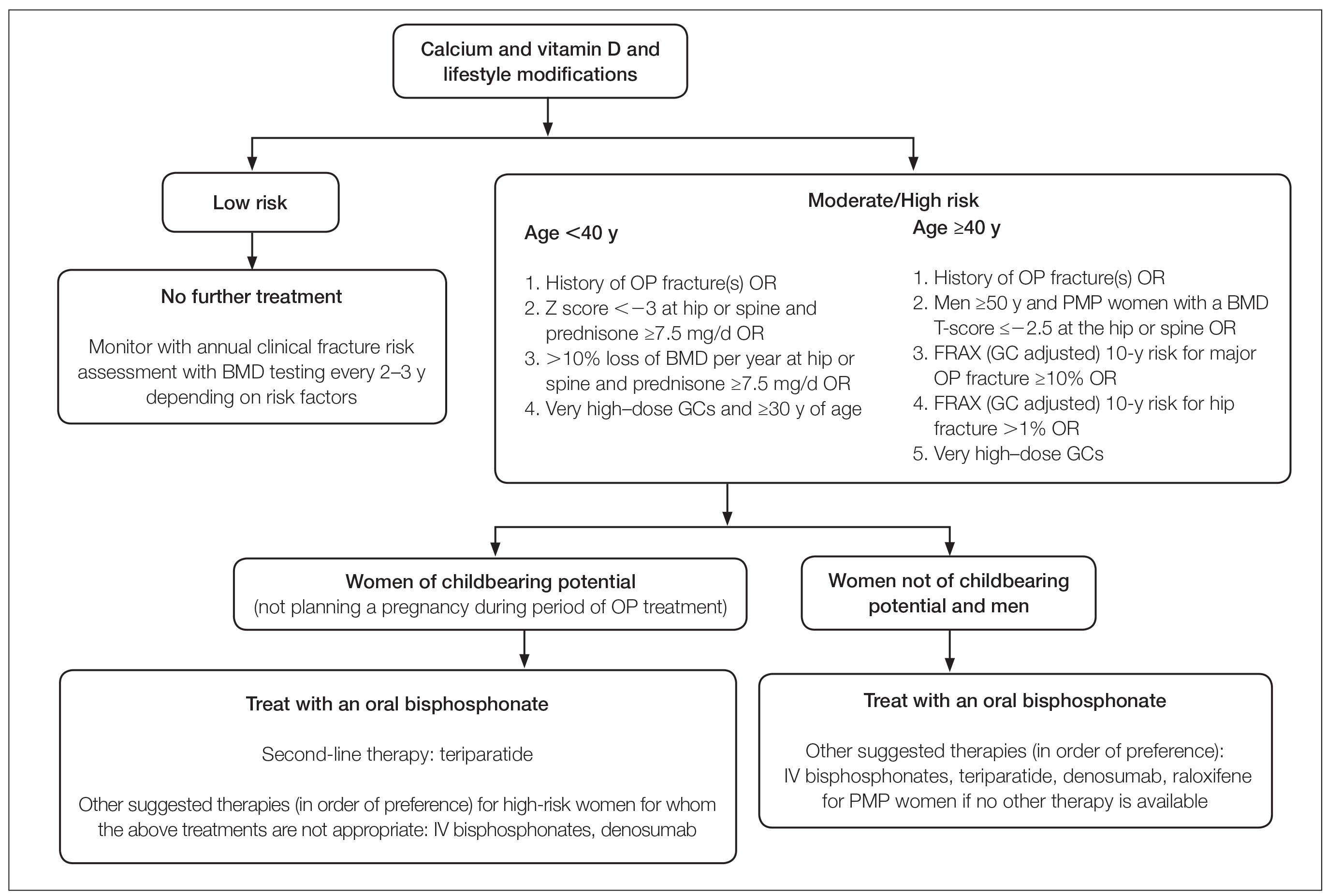
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6
Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Practice Points
- Many long-term glucocorticoid (GC) users never receive therapy to prevent bone loss, and others are only started on therapy once they have sustained an insufficiency fracture.
- Oral GCs should be used at the lowest effective daily dose for the shortest duration possible.
- Patients using topical and intralesional corticosteroids are unlikely to develop GC-induced osteoporosis.
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
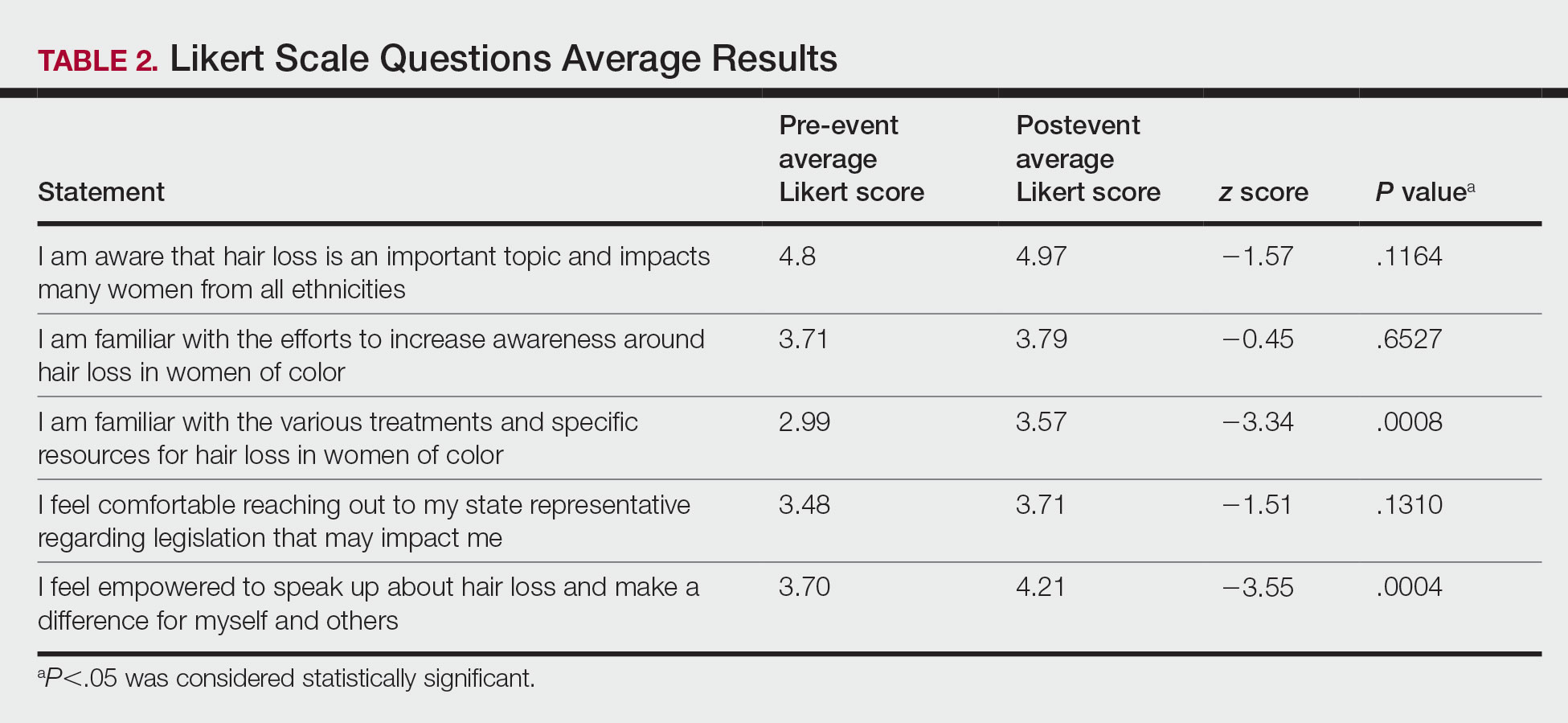
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Learning Experiences in LGBT Health During Dermatology Residency
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.
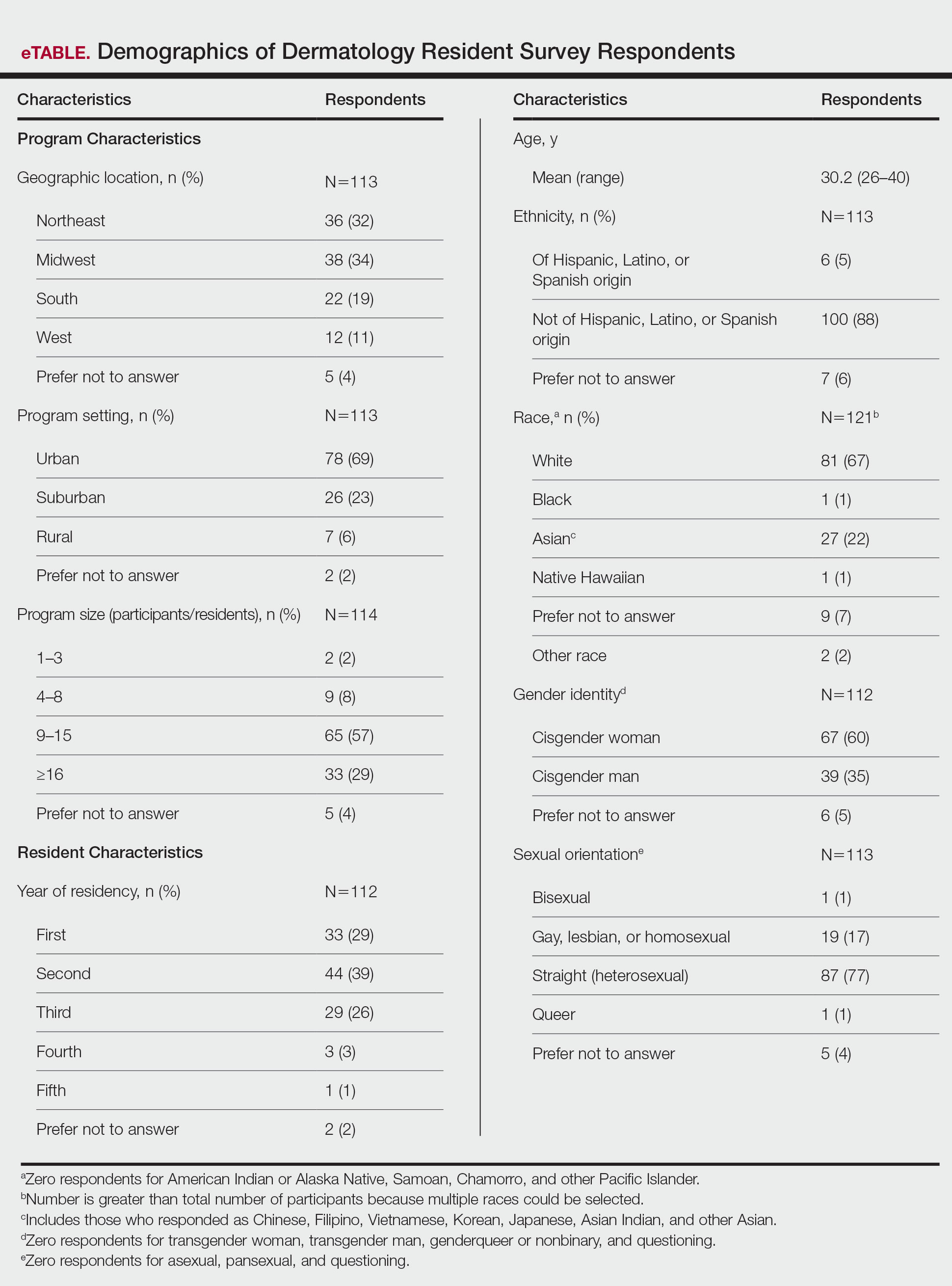
LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.
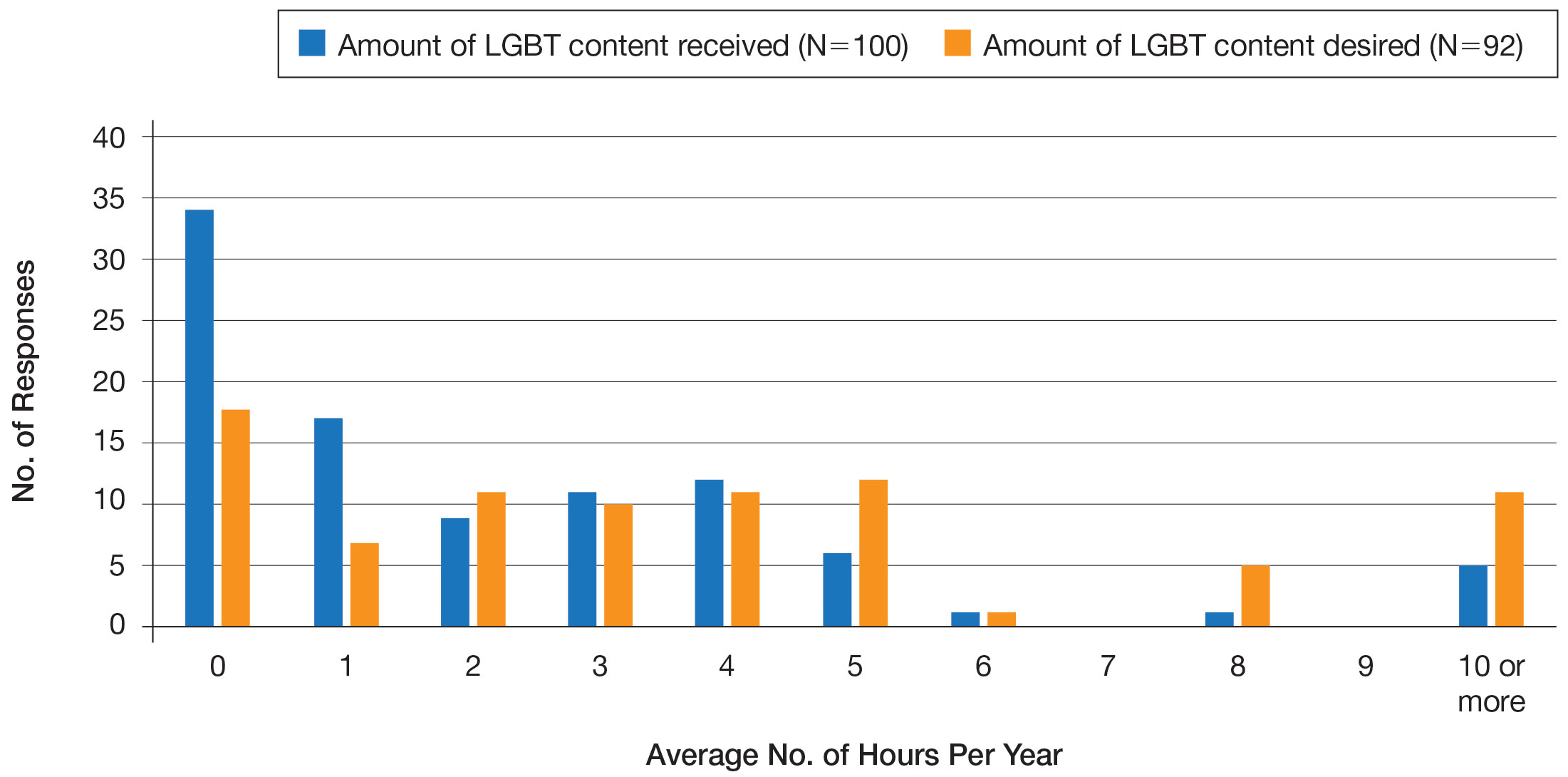
Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.
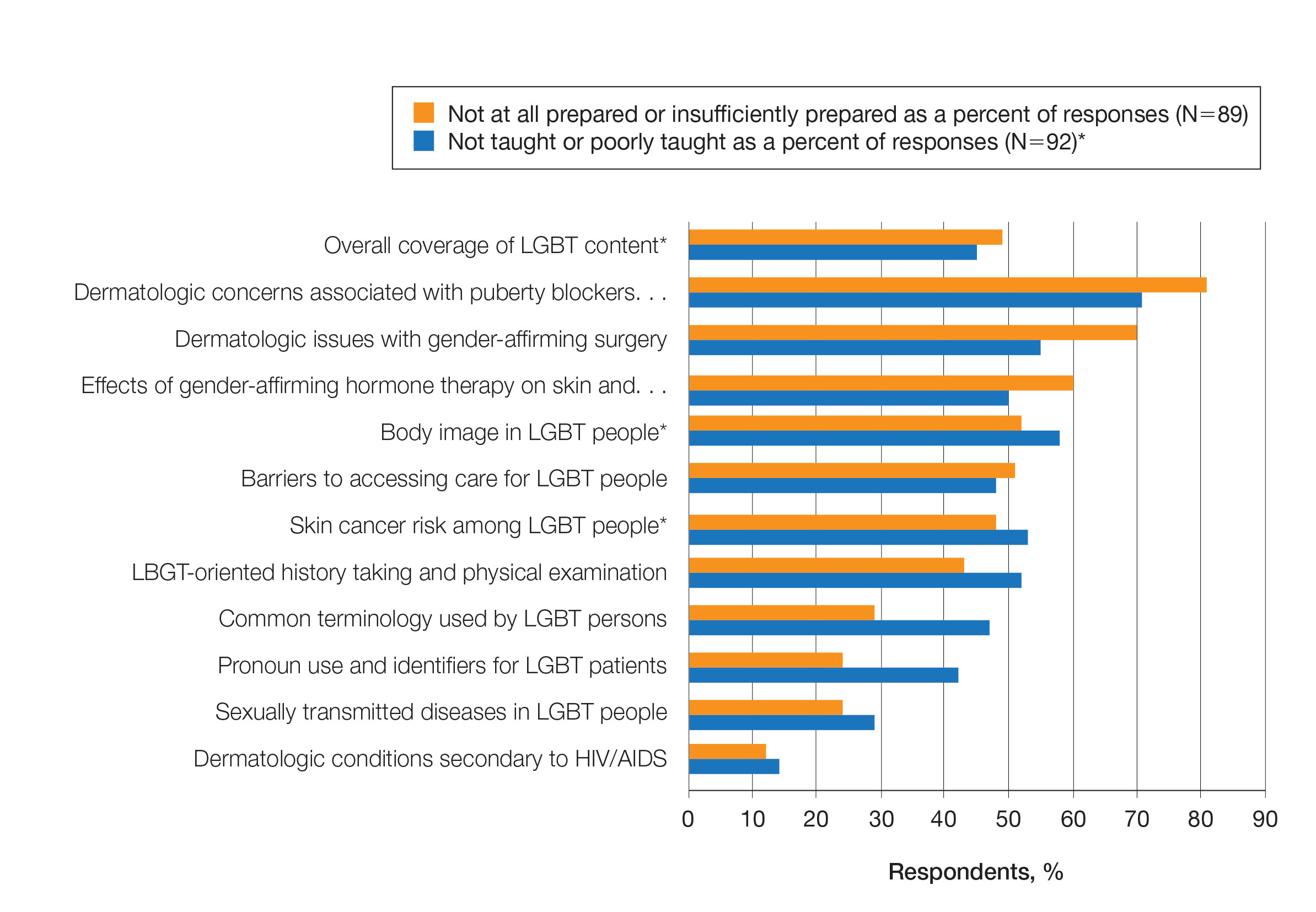
Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Practice Points
- Dermatologists have the potential to greatly impact lesbian, gay, bisexual, transgender (LGBT) health since many health concerns in this population are cutaneous.
- Improving LGBT health education and training in dermatology residency likely will increase dermatology residents' comfort level in treating this population.
Cutaneous Eruption in an Immunocompromised Patient
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.
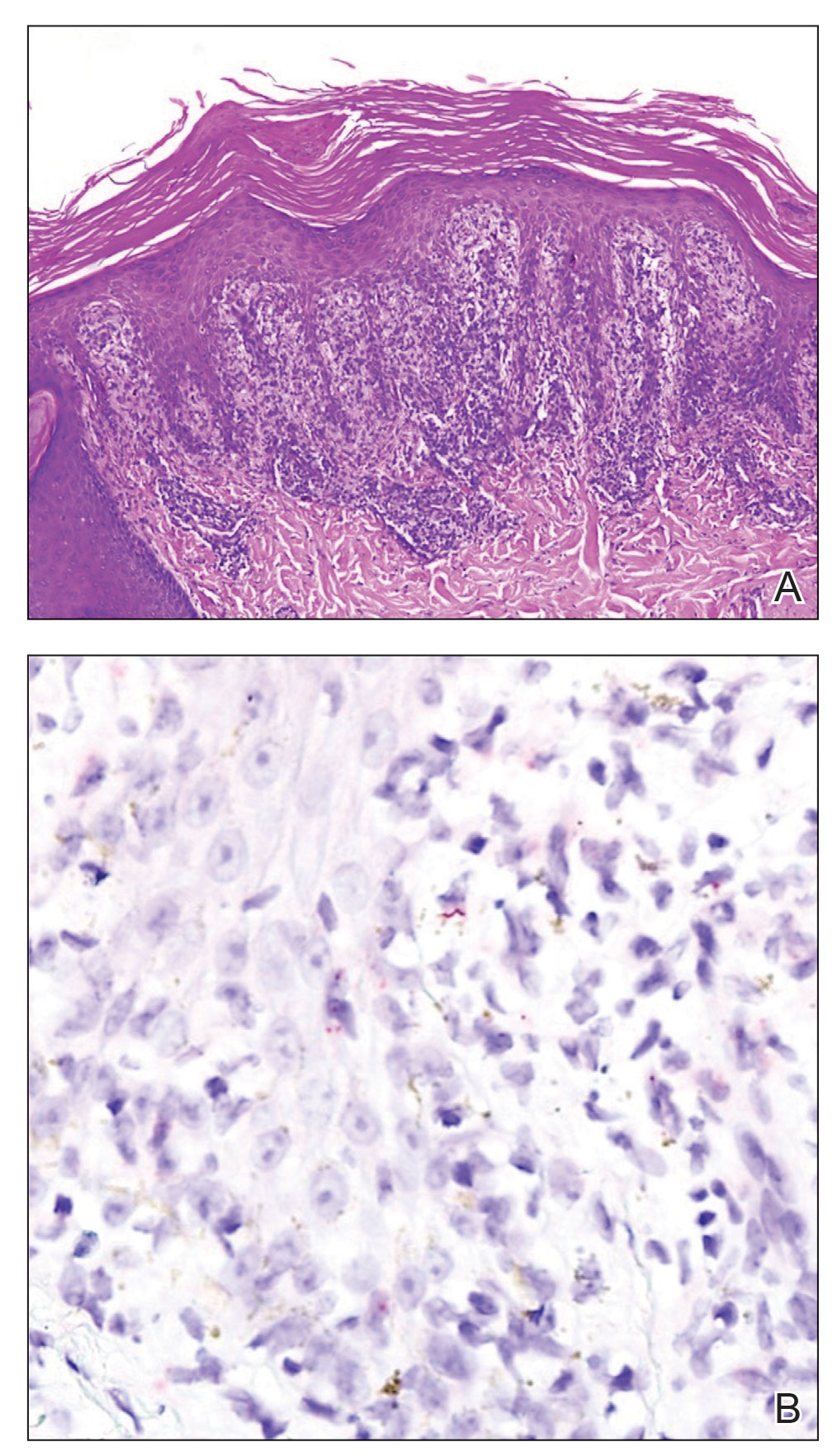
Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
A 29-year-old Black man with long-standing untreated HIV presented with mildly pruritic, scaly plaques on the palms and soles of 2 weeks’ duration. His medical history was notable for primary syphilis treated approximately 1 year prior. A review of symptoms was positive for blurry vision and floaters but negative for constitutional symptoms. Physical examination revealed well-defined scaly plaques over the palms, soles, and elbows with subungual hyperkeratosis. Patches of nonscarring alopecia over the scalp and split papules at the oral commissures also were noted. There were no palpable lymph nodes or genital involvement. Eye examination showed conjunctival injection and 20 cells per field in the vitreous humor. Laboratory evaluation revealed an HIV viral load of 31,623 copies/mL and a CD4 count of 47 cells/μL (reference range, 362–1531 cells/μL). A shave biopsy of the left elbow was performed for histopathologic evaluation.
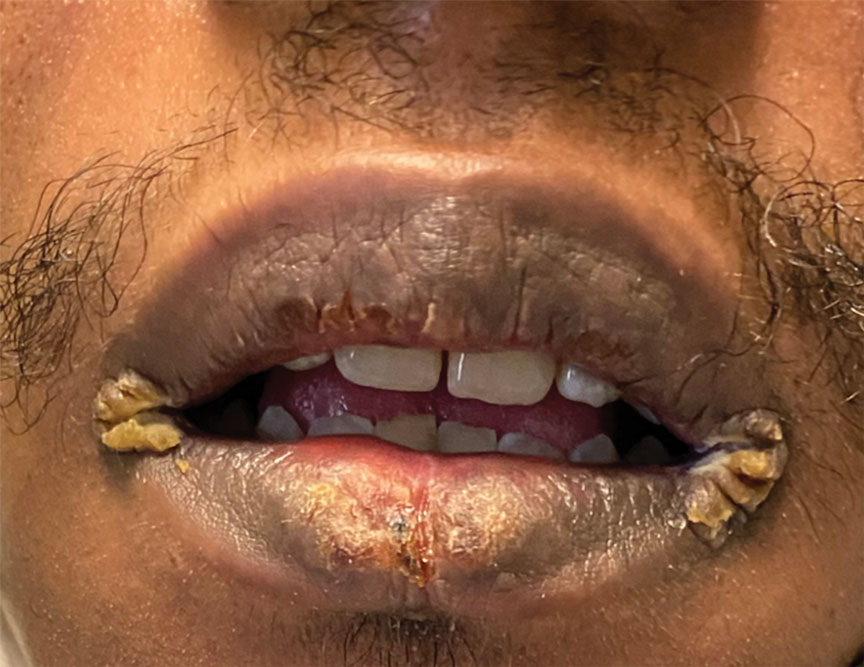
Dual-Physician Marriages: Understanding the Challenges and Rewards
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Resident Pearl
- As more physicians marry other physicians, there is an increasing need to understand the challenges and rewards of these relationships.
HIV Pre-exposure Prophylaxis (PrEP): A Survey of Dermatologists’ Knowledge and Practice Patterns
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.
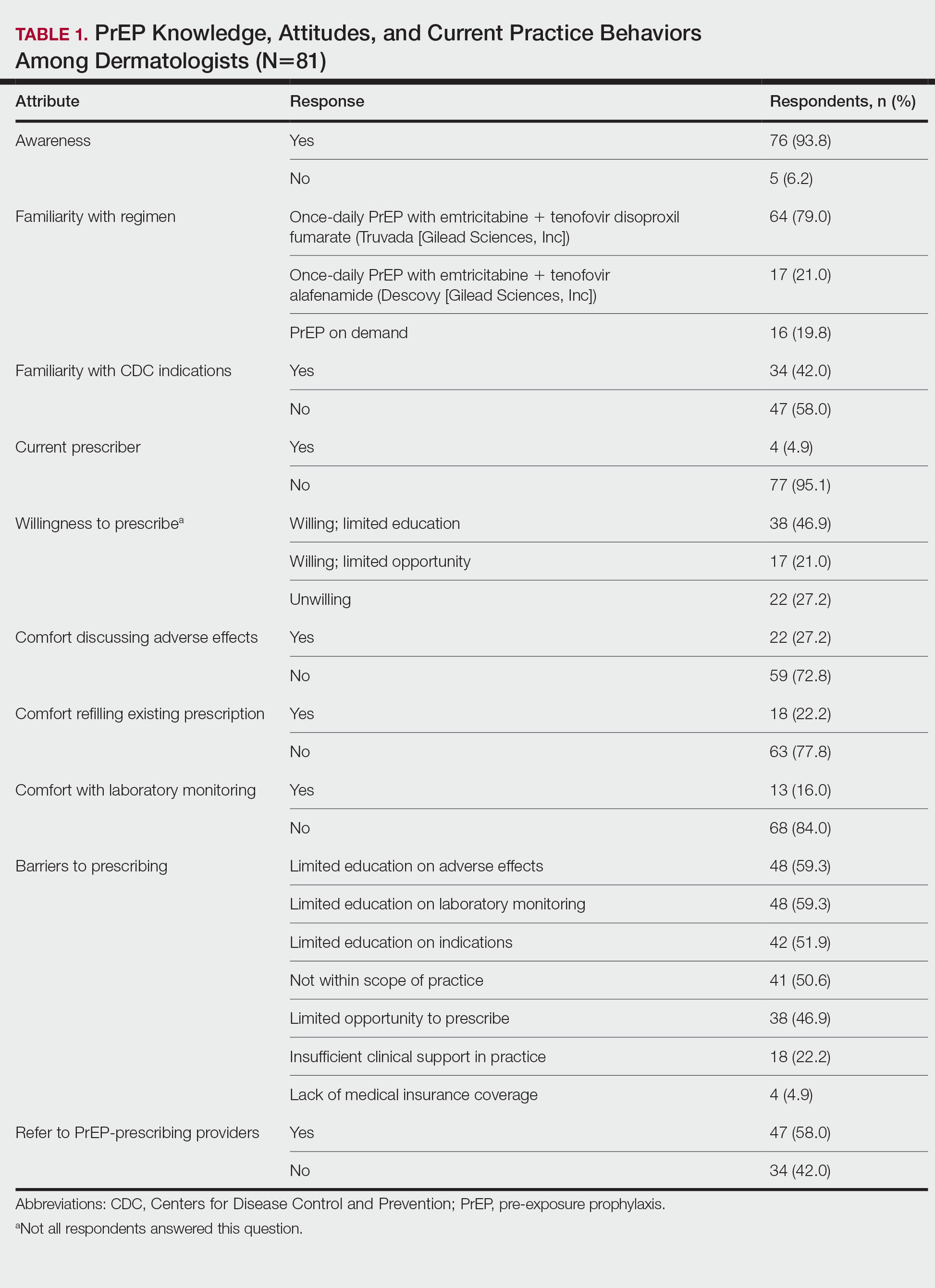
Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).
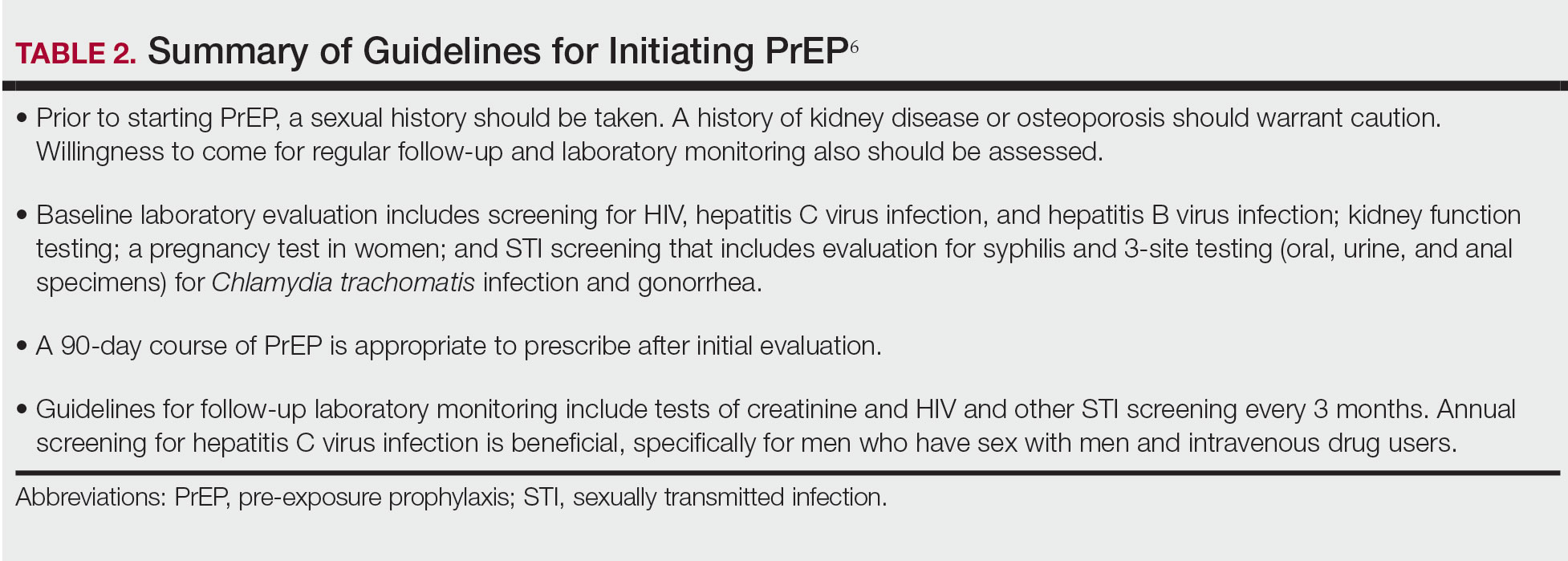
In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
Practice Points
- Sexually transmitted infections (STIs) often have skin manifestations, with patients presenting to dermatologists.
- Pre-exposure prophylaxis (PrEP) uses antiretrovirals taken prophylactically to prevent transmission of and infection with HIV. Dermatologists are aware of PrEP, but several barriers prevent them from being prescribers.
- Patients with a history of an STI should be considered for PrEP.
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.
Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1
Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.