User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
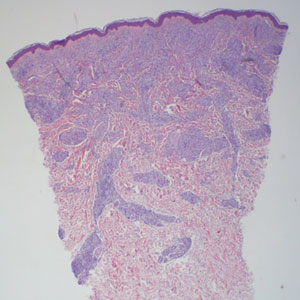
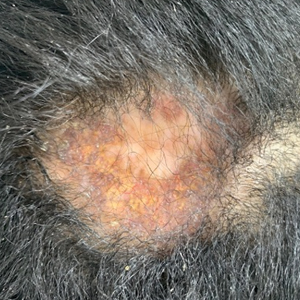
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48
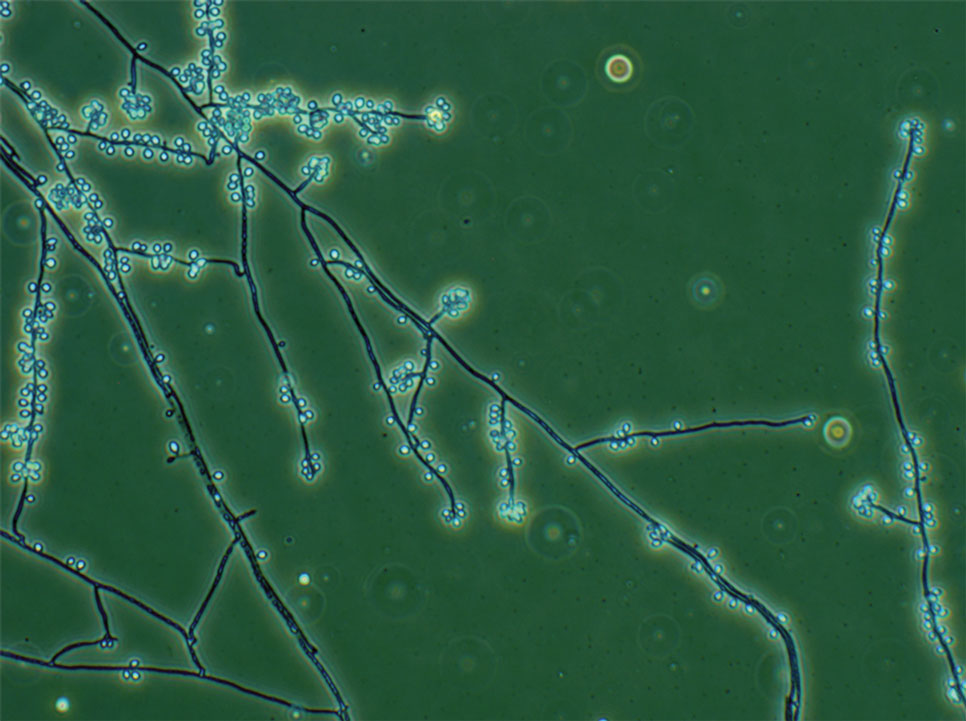
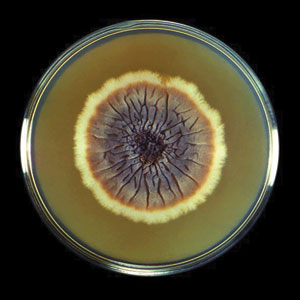
Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.
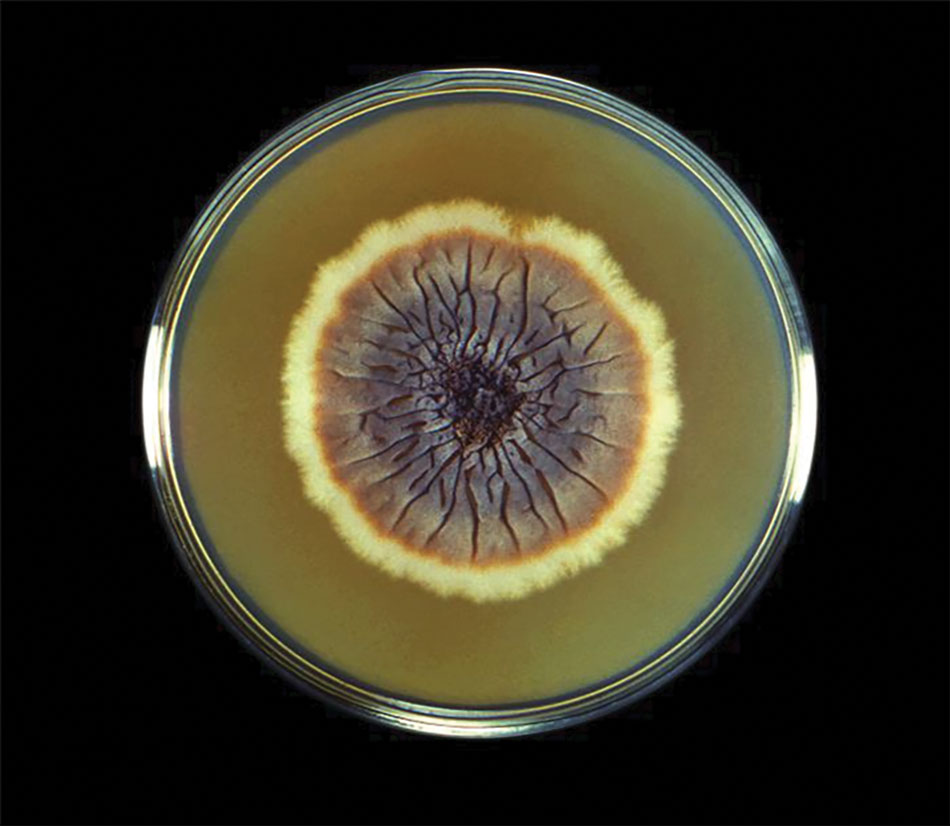
A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Practice Points
- Sporotrichosis is an implantation mycosis that is considered a neglected tropical disease warranting global advocacy to prevent infections and improve patient outcomes.
- Common diagnostic methods such as microscopy may have a low sensitivity for confirming sporotrichosis. Culture from lesional tissue or pus is considered the gold standard for diagnosis.
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Practice Points
- Toluene-2,5-diamine sulfate (PTDS) is a widely used alternative to para-phenylenediamine (PPD) that is itself a potent and likely underreported allergen.
- As high cross-reactivity has been reported, consider testing for both PTDS and PPD and possible delayed patch test reading.
- Allergic contact dermatitis to PTDS may manifest with erythema, edema, and/or pruritus, similar to PPD.
- Prevention entails avoidance of PTDS/PPD if sensitized, use of proper hand protection, and recommendation of alternative products.
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).
Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Practice Points
- Synthetic hair extensions are made from materials that can expose patients to high levels of carcinogens beginning in early childhood.
- The apple cider vinegar rinse method is an anecdotal remedy lacking data validating its ability to mitigate adverse reactions and complications associated with synthetic hair extensions, including carcinogenic exposure to materials they comprise.
- Dermatologists should inform patients of the potential exposure risks when using synthetic hair extensions to help patients make informed decisions regarding future styling habits and hair care choices.
Update on Management of Atopic Dermatitis in Young Children
Update on Management of Atopic Dermatitis in Young Children
Atopic dermatitis (AD) is a chronic inflammatory skin condition associated with skin barrier impairment and immune system dysregulation.1 Development of AD in young children can present challenges in determining appropriate treatment regimens. Natural remedies for AD often are promoted on social media over traditional treatments, including topical corticosteroids (TCSs), which can contribute to corticophobia.2 Dermatologists play a critical role not only in optimizing topical therapy but also addressing patient interest in natural approaches to AD, including diet-related questions. This article outlines the role of diet and probiotics in pediatric AD and reviews the topical treatments currently approved for this patient population.
Diet and Probiotics
With a growing focus on natural therapies for AD, dietary interventions have come to the forefront. A prevalent theme among patients and their families is addressing gut health and allergic triggers. Broad elimination diets have not shown clinical benefit in patients with AD regardless of age,3 and in children, they may result in nutritional deficiencies, poor growth, and increased risk for IgE-mediated food allergies.4 If a true food allergy is identified based on positive IgE and an acute clinical reaction, elimination of the allergen may provide some benefit.5
The link between gut microbiota and skin health has driven an interest in the role of probiotics in the treatment of pediatric AD. A meta-analysis of 20 articles concluded that, whether administered to infants or breastfeeding mothers, use of probiotics overall led to a significant reduction in AD risk in infants (P=.001). Lactobacillus and mixed strains were effective.6 While broad elimination diets are not used to treat AD, probiotic supplementation can be considered for prevention of AD.
Topical Corticosteroids
Topical corticosteroids are the cornerstone of AD treatment; however, corticophobia among patients is on the rise, leading to poor adherence and suboptimal control of AD.7 Mild cutaneous adverse effects (AEs) including skin atrophy, striae, and telangiectasias may occur. Rarely, systemic AEs occur due to absorption of TCSs into the bloodstream, mainly with application of potent steroids over large body surface areas or under occlusion.8 When the optimal potency of a TCS is chosen and used appropriately, incidence of AEs from TCS use is very low.9
Counseling parents about risk factors that can lead to AEs during treatment with TCSs and formulating regimens that minimize these risks while maintaining efficacy increases adherence and outcomes. Pulse maintenance dosing of TCSs typically involves application 1 to 2 times weekly to areas of the skin that are prone to frequent outbreaks. Pulse maintenance dosing can reduce the incidence of AD flares while also decreasing the total amount of topical medication needed as compared to the reactive approach alone, thereby reducing risk for AEs.8
Steroid-Sparing Topical Treatments
Although TCSs are considered first-line agents, recently there has been an advent of steroid-sparing topical agents approved by the US Food and Drug Administration (FDA) for pediatric patients with AD, including topical calcineurin inhibitors (TCIs), phosphodiesterase 4 inhibitors, a Janus kinase inhibitor, and aryl hydrocarbon receptor agonists. Offering steroid-sparing agents in these patients can help ease parental anxiety regarding TCS overuse.
Topical Calcineurin Inhibitors—Pimecrolimus cream 1% and tacrolimus ointment 0.03% are approved for patients aged 2 years and older and have anti-inflammatory and antipruritic effects equivalent to low-potency TCS. Tacrolimus ointment 0.1% is approved for patients aged 16 years and older with similar efficacy to a midpotency TCSs. Pimecrolimus cream 1% and tacrolimus ointment 0.03% often are used off-label in children younger than 2 years, as supported by clinical trials showing their safety and efficacy.10
Topical calcineurin inhibitors can replace or supplement TCSs, making TCIs a desirable option for avoidance of steroid-related AEs. The addition of a TCI to spot treatment or a pulse regimen in a young patient can reassure them and their caregivers that the provider is proactively reducing the risk of TCS overuse. The largest barrier to TCI use is the FDA’s black box warning based on the oral formulation of tacrolimus, citing a potential increased risk for lymphoma and skin cancer; however, there is no evidence for substantial systemic absorption of topical pimecrolimus or tacrolimus.11 Large task-force reviews have found no association between TCI use and development of malignancy.12,13 Based on the current data, counseling patients and their caregivers that this risk primarily is theoretical may help them more confidently integrate TCIs into their treatment regimen. Burning and tingling may occur in a minority of pediatric patients using TCIs for AD. Applying the medication to open wounds or inflamed skin increases the risk for stinging, but pretreatment with a short course of TCSs before transitioning to a TCI may boost tolerance.14
Phosphodiesterase 4 Inhibitors—Crisaborole ointment 2%, a phosphodiesterase 4 inhibitor, is approved for children aged 3 months and older with mild to moderate AD. Its use has been more limited than TCSs and TCIs, as local irritation including stinging and burning can occur in up to 50% of patients.15 One study comparing crisaborole 2% with tacrolimus 0.03% revealed greater improvement with tacrolimus.16 A second phosphodiesterase 4 inhibitor approved for once-daily use in children aged 6 years and older with mild to moderate AD is roflumilast cream 0.15%. Roflumilast reduces eczema severity and pruritus, with AEs also limited to application-site stinging and burning.17
Janus Kinase Inhibitor—Ruxolitinib cream 1.5%, a Janus kinase inhibitor, has been approved by the FDA since 2023 for twice-daily use in children aged 12 years and older with AD. Similar to TCIs, ruxolitinib cream carries a black box warning. Short-term safety data on ruxolitinib cream have revealed low levels of ruxolitinib concentration in plasma18; however, long-term studies on topical Janus kinase inhibitors for AD in pediatric and adult populations are lacking. To reduce the risk for systemic absorption, recommendations include limiting usage to 60 g per week and limiting treatment to less than 20% of the body surface area.19 Ruxolitinib has efficacy similar to or possibly superior to triamcinolone 0.1%.20 Ruxolitinib is emerging as a promising nonsteroidal option that potentially is highly efficacious and well tolerated without cutaneous AEs.
Aryl Hydrocarbon Receptor Agonist—Tapinarof cream 1% is an aryl hydrocarbon receptor agonist that has been approved by the FDA since 2024 for children aged 2 years and older as a once-daily treatment for moderate to severe AD. Adverse events include folliculitis, nasopharyngitis, and headache, which are mostly mild or moderate.21
Final Thoughts
Topical management of pediatric AD includes traditional therapy with TCSs and newer steroid-sparing agents, which can help address corticophobia. Anticipatory guidance regarding the safety and long-term effects of individual therapies is critical to ensuring patient adherence to treatment regimens. Probiotics may help prevent pediatric AD, but future studies are needed to determine their role in treatment.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Voillot P, Riche B, Portafax M, et al. Social media platforms listening study on atopic dermatitis: quantitative and qualitative findings. J Med Internet Res. 2022;24:E31140.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258-264.
- Rustad AM, Nickles MA, Bilimoria SN, et al. The role of diet modification in atopic dermatitis: navigating the complexity. Am J Clin Dermatol. 2022;23:27-36.
- Khan A, Adalsteinsson J, Whitaker-Worth DL. Atopic dermatitis and nutrition. Clin Dermatol. 2022;40:135-144.
- Chen L, Ni Y, Wu X, et al. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: a systematic review and meta-analysis. J Dermatolog Treat. 2022;33:2931-2939.
- Herzum A, Occella C, Gariazzo L, et al. Corticophobia among parents of children with atopic dermatitis: assessing major and minor risk factors for high TOPICOP scores. J Clin Med. 2023;12:6813.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
- Reitamo S, Rustin M, Ruzicka T, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol. 2002;109:547-555.
- Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28:52-56.
- Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the AAD Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Fonacier L, Spergel J, Charlesworth EN, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115:1249-1253.
- Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495-504.
- Lin CPL, Gordon S, Her MJ, et al. A retrospective study: application site pain with the use of crisaborole, a topical phosphodiesterase 4 inhibitor. J Am Acad Dermatol. 2019;80:1451-1453.
- Ryan Wolf J, Chen A, Wieser J, et al. Improved patient- and caregiver-reported outcomes distinguish tacrolimus 0.03% from crisaborole in children with atopic dermatitis. J Eur Acad Dermatol Venereol. 2024;38:1364-1372.
- Simpson EL, Eichenfield LF, Alonso-Llamazares J, et al. Roflumilast cream, 0.15%, for atopic dermatitis in adults and children: INTEGUMENT-1 and INTEGUMENT-2 randomized clinical trials. JAMA Dermatol. 2024;160:1161-1170.
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016.
- Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of carefor the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89:E1-E20.
- Sadeghi S, Mohandesi NA. Efficacy and safety of topical JAK inhibitors in the treatment of atopic dermatitis in paediatrics and adults: a systematic review. Exp Dermatol. 2023;32:599-610.
- Silverberg JI, Eichenfield LF, Hebert AA, et al. Tapinarof cream 1% once daily: significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J Am Acad Dermatol. 2024;91:457-465.
Atopic dermatitis (AD) is a chronic inflammatory skin condition associated with skin barrier impairment and immune system dysregulation.1 Development of AD in young children can present challenges in determining appropriate treatment regimens. Natural remedies for AD often are promoted on social media over traditional treatments, including topical corticosteroids (TCSs), which can contribute to corticophobia.2 Dermatologists play a critical role not only in optimizing topical therapy but also addressing patient interest in natural approaches to AD, including diet-related questions. This article outlines the role of diet and probiotics in pediatric AD and reviews the topical treatments currently approved for this patient population.
Diet and Probiotics
With a growing focus on natural therapies for AD, dietary interventions have come to the forefront. A prevalent theme among patients and their families is addressing gut health and allergic triggers. Broad elimination diets have not shown clinical benefit in patients with AD regardless of age,3 and in children, they may result in nutritional deficiencies, poor growth, and increased risk for IgE-mediated food allergies.4 If a true food allergy is identified based on positive IgE and an acute clinical reaction, elimination of the allergen may provide some benefit.5
The link between gut microbiota and skin health has driven an interest in the role of probiotics in the treatment of pediatric AD. A meta-analysis of 20 articles concluded that, whether administered to infants or breastfeeding mothers, use of probiotics overall led to a significant reduction in AD risk in infants (P=.001). Lactobacillus and mixed strains were effective.6 While broad elimination diets are not used to treat AD, probiotic supplementation can be considered for prevention of AD.
Topical Corticosteroids
Topical corticosteroids are the cornerstone of AD treatment; however, corticophobia among patients is on the rise, leading to poor adherence and suboptimal control of AD.7 Mild cutaneous adverse effects (AEs) including skin atrophy, striae, and telangiectasias may occur. Rarely, systemic AEs occur due to absorption of TCSs into the bloodstream, mainly with application of potent steroids over large body surface areas or under occlusion.8 When the optimal potency of a TCS is chosen and used appropriately, incidence of AEs from TCS use is very low.9
Counseling parents about risk factors that can lead to AEs during treatment with TCSs and formulating regimens that minimize these risks while maintaining efficacy increases adherence and outcomes. Pulse maintenance dosing of TCSs typically involves application 1 to 2 times weekly to areas of the skin that are prone to frequent outbreaks. Pulse maintenance dosing can reduce the incidence of AD flares while also decreasing the total amount of topical medication needed as compared to the reactive approach alone, thereby reducing risk for AEs.8
Steroid-Sparing Topical Treatments
Although TCSs are considered first-line agents, recently there has been an advent of steroid-sparing topical agents approved by the US Food and Drug Administration (FDA) for pediatric patients with AD, including topical calcineurin inhibitors (TCIs), phosphodiesterase 4 inhibitors, a Janus kinase inhibitor, and aryl hydrocarbon receptor agonists. Offering steroid-sparing agents in these patients can help ease parental anxiety regarding TCS overuse.
Topical Calcineurin Inhibitors—Pimecrolimus cream 1% and tacrolimus ointment 0.03% are approved for patients aged 2 years and older and have anti-inflammatory and antipruritic effects equivalent to low-potency TCS. Tacrolimus ointment 0.1% is approved for patients aged 16 years and older with similar efficacy to a midpotency TCSs. Pimecrolimus cream 1% and tacrolimus ointment 0.03% often are used off-label in children younger than 2 years, as supported by clinical trials showing their safety and efficacy.10
Topical calcineurin inhibitors can replace or supplement TCSs, making TCIs a desirable option for avoidance of steroid-related AEs. The addition of a TCI to spot treatment or a pulse regimen in a young patient can reassure them and their caregivers that the provider is proactively reducing the risk of TCS overuse. The largest barrier to TCI use is the FDA’s black box warning based on the oral formulation of tacrolimus, citing a potential increased risk for lymphoma and skin cancer; however, there is no evidence for substantial systemic absorption of topical pimecrolimus or tacrolimus.11 Large task-force reviews have found no association between TCI use and development of malignancy.12,13 Based on the current data, counseling patients and their caregivers that this risk primarily is theoretical may help them more confidently integrate TCIs into their treatment regimen. Burning and tingling may occur in a minority of pediatric patients using TCIs for AD. Applying the medication to open wounds or inflamed skin increases the risk for stinging, but pretreatment with a short course of TCSs before transitioning to a TCI may boost tolerance.14
Phosphodiesterase 4 Inhibitors—Crisaborole ointment 2%, a phosphodiesterase 4 inhibitor, is approved for children aged 3 months and older with mild to moderate AD. Its use has been more limited than TCSs and TCIs, as local irritation including stinging and burning can occur in up to 50% of patients.15 One study comparing crisaborole 2% with tacrolimus 0.03% revealed greater improvement with tacrolimus.16 A second phosphodiesterase 4 inhibitor approved for once-daily use in children aged 6 years and older with mild to moderate AD is roflumilast cream 0.15%. Roflumilast reduces eczema severity and pruritus, with AEs also limited to application-site stinging and burning.17
Janus Kinase Inhibitor—Ruxolitinib cream 1.5%, a Janus kinase inhibitor, has been approved by the FDA since 2023 for twice-daily use in children aged 12 years and older with AD. Similar to TCIs, ruxolitinib cream carries a black box warning. Short-term safety data on ruxolitinib cream have revealed low levels of ruxolitinib concentration in plasma18; however, long-term studies on topical Janus kinase inhibitors for AD in pediatric and adult populations are lacking. To reduce the risk for systemic absorption, recommendations include limiting usage to 60 g per week and limiting treatment to less than 20% of the body surface area.19 Ruxolitinib has efficacy similar to or possibly superior to triamcinolone 0.1%.20 Ruxolitinib is emerging as a promising nonsteroidal option that potentially is highly efficacious and well tolerated without cutaneous AEs.
Aryl Hydrocarbon Receptor Agonist—Tapinarof cream 1% is an aryl hydrocarbon receptor agonist that has been approved by the FDA since 2024 for children aged 2 years and older as a once-daily treatment for moderate to severe AD. Adverse events include folliculitis, nasopharyngitis, and headache, which are mostly mild or moderate.21
Final Thoughts
Topical management of pediatric AD includes traditional therapy with TCSs and newer steroid-sparing agents, which can help address corticophobia. Anticipatory guidance regarding the safety and long-term effects of individual therapies is critical to ensuring patient adherence to treatment regimens. Probiotics may help prevent pediatric AD, but future studies are needed to determine their role in treatment.
Atopic dermatitis (AD) is a chronic inflammatory skin condition associated with skin barrier impairment and immune system dysregulation.1 Development of AD in young children can present challenges in determining appropriate treatment regimens. Natural remedies for AD often are promoted on social media over traditional treatments, including topical corticosteroids (TCSs), which can contribute to corticophobia.2 Dermatologists play a critical role not only in optimizing topical therapy but also addressing patient interest in natural approaches to AD, including diet-related questions. This article outlines the role of diet and probiotics in pediatric AD and reviews the topical treatments currently approved for this patient population.
Diet and Probiotics
With a growing focus on natural therapies for AD, dietary interventions have come to the forefront. A prevalent theme among patients and their families is addressing gut health and allergic triggers. Broad elimination diets have not shown clinical benefit in patients with AD regardless of age,3 and in children, they may result in nutritional deficiencies, poor growth, and increased risk for IgE-mediated food allergies.4 If a true food allergy is identified based on positive IgE and an acute clinical reaction, elimination of the allergen may provide some benefit.5
The link between gut microbiota and skin health has driven an interest in the role of probiotics in the treatment of pediatric AD. A meta-analysis of 20 articles concluded that, whether administered to infants or breastfeeding mothers, use of probiotics overall led to a significant reduction in AD risk in infants (P=.001). Lactobacillus and mixed strains were effective.6 While broad elimination diets are not used to treat AD, probiotic supplementation can be considered for prevention of AD.
Topical Corticosteroids
Topical corticosteroids are the cornerstone of AD treatment; however, corticophobia among patients is on the rise, leading to poor adherence and suboptimal control of AD.7 Mild cutaneous adverse effects (AEs) including skin atrophy, striae, and telangiectasias may occur. Rarely, systemic AEs occur due to absorption of TCSs into the bloodstream, mainly with application of potent steroids over large body surface areas or under occlusion.8 When the optimal potency of a TCS is chosen and used appropriately, incidence of AEs from TCS use is very low.9
Counseling parents about risk factors that can lead to AEs during treatment with TCSs and formulating regimens that minimize these risks while maintaining efficacy increases adherence and outcomes. Pulse maintenance dosing of TCSs typically involves application 1 to 2 times weekly to areas of the skin that are prone to frequent outbreaks. Pulse maintenance dosing can reduce the incidence of AD flares while also decreasing the total amount of topical medication needed as compared to the reactive approach alone, thereby reducing risk for AEs.8
Steroid-Sparing Topical Treatments
Although TCSs are considered first-line agents, recently there has been an advent of steroid-sparing topical agents approved by the US Food and Drug Administration (FDA) for pediatric patients with AD, including topical calcineurin inhibitors (TCIs), phosphodiesterase 4 inhibitors, a Janus kinase inhibitor, and aryl hydrocarbon receptor agonists. Offering steroid-sparing agents in these patients can help ease parental anxiety regarding TCS overuse.
Topical Calcineurin Inhibitors—Pimecrolimus cream 1% and tacrolimus ointment 0.03% are approved for patients aged 2 years and older and have anti-inflammatory and antipruritic effects equivalent to low-potency TCS. Tacrolimus ointment 0.1% is approved for patients aged 16 years and older with similar efficacy to a midpotency TCSs. Pimecrolimus cream 1% and tacrolimus ointment 0.03% often are used off-label in children younger than 2 years, as supported by clinical trials showing their safety and efficacy.10
Topical calcineurin inhibitors can replace or supplement TCSs, making TCIs a desirable option for avoidance of steroid-related AEs. The addition of a TCI to spot treatment or a pulse regimen in a young patient can reassure them and their caregivers that the provider is proactively reducing the risk of TCS overuse. The largest barrier to TCI use is the FDA’s black box warning based on the oral formulation of tacrolimus, citing a potential increased risk for lymphoma and skin cancer; however, there is no evidence for substantial systemic absorption of topical pimecrolimus or tacrolimus.11 Large task-force reviews have found no association between TCI use and development of malignancy.12,13 Based on the current data, counseling patients and their caregivers that this risk primarily is theoretical may help them more confidently integrate TCIs into their treatment regimen. Burning and tingling may occur in a minority of pediatric patients using TCIs for AD. Applying the medication to open wounds or inflamed skin increases the risk for stinging, but pretreatment with a short course of TCSs before transitioning to a TCI may boost tolerance.14
Phosphodiesterase 4 Inhibitors—Crisaborole ointment 2%, a phosphodiesterase 4 inhibitor, is approved for children aged 3 months and older with mild to moderate AD. Its use has been more limited than TCSs and TCIs, as local irritation including stinging and burning can occur in up to 50% of patients.15 One study comparing crisaborole 2% with tacrolimus 0.03% revealed greater improvement with tacrolimus.16 A second phosphodiesterase 4 inhibitor approved for once-daily use in children aged 6 years and older with mild to moderate AD is roflumilast cream 0.15%. Roflumilast reduces eczema severity and pruritus, with AEs also limited to application-site stinging and burning.17
Janus Kinase Inhibitor—Ruxolitinib cream 1.5%, a Janus kinase inhibitor, has been approved by the FDA since 2023 for twice-daily use in children aged 12 years and older with AD. Similar to TCIs, ruxolitinib cream carries a black box warning. Short-term safety data on ruxolitinib cream have revealed low levels of ruxolitinib concentration in plasma18; however, long-term studies on topical Janus kinase inhibitors for AD in pediatric and adult populations are lacking. To reduce the risk for systemic absorption, recommendations include limiting usage to 60 g per week and limiting treatment to less than 20% of the body surface area.19 Ruxolitinib has efficacy similar to or possibly superior to triamcinolone 0.1%.20 Ruxolitinib is emerging as a promising nonsteroidal option that potentially is highly efficacious and well tolerated without cutaneous AEs.
Aryl Hydrocarbon Receptor Agonist—Tapinarof cream 1% is an aryl hydrocarbon receptor agonist that has been approved by the FDA since 2024 for children aged 2 years and older as a once-daily treatment for moderate to severe AD. Adverse events include folliculitis, nasopharyngitis, and headache, which are mostly mild or moderate.21
Final Thoughts
Topical management of pediatric AD includes traditional therapy with TCSs and newer steroid-sparing agents, which can help address corticophobia. Anticipatory guidance regarding the safety and long-term effects of individual therapies is critical to ensuring patient adherence to treatment regimens. Probiotics may help prevent pediatric AD, but future studies are needed to determine their role in treatment.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Voillot P, Riche B, Portafax M, et al. Social media platforms listening study on atopic dermatitis: quantitative and qualitative findings. J Med Internet Res. 2022;24:E31140.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258-264.
- Rustad AM, Nickles MA, Bilimoria SN, et al. The role of diet modification in atopic dermatitis: navigating the complexity. Am J Clin Dermatol. 2022;23:27-36.
- Khan A, Adalsteinsson J, Whitaker-Worth DL. Atopic dermatitis and nutrition. Clin Dermatol. 2022;40:135-144.
- Chen L, Ni Y, Wu X, et al. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: a systematic review and meta-analysis. J Dermatolog Treat. 2022;33:2931-2939.
- Herzum A, Occella C, Gariazzo L, et al. Corticophobia among parents of children with atopic dermatitis: assessing major and minor risk factors for high TOPICOP scores. J Clin Med. 2023;12:6813.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
- Reitamo S, Rustin M, Ruzicka T, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol. 2002;109:547-555.
- Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28:52-56.
- Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the AAD Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Fonacier L, Spergel J, Charlesworth EN, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115:1249-1253.
- Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495-504.
- Lin CPL, Gordon S, Her MJ, et al. A retrospective study: application site pain with the use of crisaborole, a topical phosphodiesterase 4 inhibitor. J Am Acad Dermatol. 2019;80:1451-1453.
- Ryan Wolf J, Chen A, Wieser J, et al. Improved patient- and caregiver-reported outcomes distinguish tacrolimus 0.03% from crisaborole in children with atopic dermatitis. J Eur Acad Dermatol Venereol. 2024;38:1364-1372.
- Simpson EL, Eichenfield LF, Alonso-Llamazares J, et al. Roflumilast cream, 0.15%, for atopic dermatitis in adults and children: INTEGUMENT-1 and INTEGUMENT-2 randomized clinical trials. JAMA Dermatol. 2024;160:1161-1170.
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016.
- Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of carefor the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89:E1-E20.
- Sadeghi S, Mohandesi NA. Efficacy and safety of topical JAK inhibitors in the treatment of atopic dermatitis in paediatrics and adults: a systematic review. Exp Dermatol. 2023;32:599-610.
- Silverberg JI, Eichenfield LF, Hebert AA, et al. Tapinarof cream 1% once daily: significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J Am Acad Dermatol. 2024;91:457-465.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Voillot P, Riche B, Portafax M, et al. Social media platforms listening study on atopic dermatitis: quantitative and qualitative findings. J Med Internet Res. 2022;24:E31140.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258-264.
- Rustad AM, Nickles MA, Bilimoria SN, et al. The role of diet modification in atopic dermatitis: navigating the complexity. Am J Clin Dermatol. 2022;23:27-36.
- Khan A, Adalsteinsson J, Whitaker-Worth DL. Atopic dermatitis and nutrition. Clin Dermatol. 2022;40:135-144.
- Chen L, Ni Y, Wu X, et al. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: a systematic review and meta-analysis. J Dermatolog Treat. 2022;33:2931-2939.
- Herzum A, Occella C, Gariazzo L, et al. Corticophobia among parents of children with atopic dermatitis: assessing major and minor risk factors for high TOPICOP scores. J Clin Med. 2023;12:6813.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156:203-221.
- Reitamo S, Rustin M, Ruzicka T, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol. 2002;109:547-555.
- Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28:52-56.
- Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the AAD Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Fonacier L, Spergel J, Charlesworth EN, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115:1249-1253.
- Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495-504.
- Lin CPL, Gordon S, Her MJ, et al. A retrospective study: application site pain with the use of crisaborole, a topical phosphodiesterase 4 inhibitor. J Am Acad Dermatol. 2019;80:1451-1453.
- Ryan Wolf J, Chen A, Wieser J, et al. Improved patient- and caregiver-reported outcomes distinguish tacrolimus 0.03% from crisaborole in children with atopic dermatitis. J Eur Acad Dermatol Venereol. 2024;38:1364-1372.
- Simpson EL, Eichenfield LF, Alonso-Llamazares J, et al. Roflumilast cream, 0.15%, for atopic dermatitis in adults and children: INTEGUMENT-1 and INTEGUMENT-2 randomized clinical trials. JAMA Dermatol. 2024;160:1161-1170.
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016.
- Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of carefor the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89:E1-E20.
- Sadeghi S, Mohandesi NA. Efficacy and safety of topical JAK inhibitors in the treatment of atopic dermatitis in paediatrics and adults: a systematic review. Exp Dermatol. 2023;32:599-610.
- Silverberg JI, Eichenfield LF, Hebert AA, et al. Tapinarof cream 1% once daily: significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J Am Acad Dermatol. 2024;91:457-465.
Update on Management of Atopic Dermatitis in Young Children
Update on Management of Atopic Dermatitis in Young Children
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.
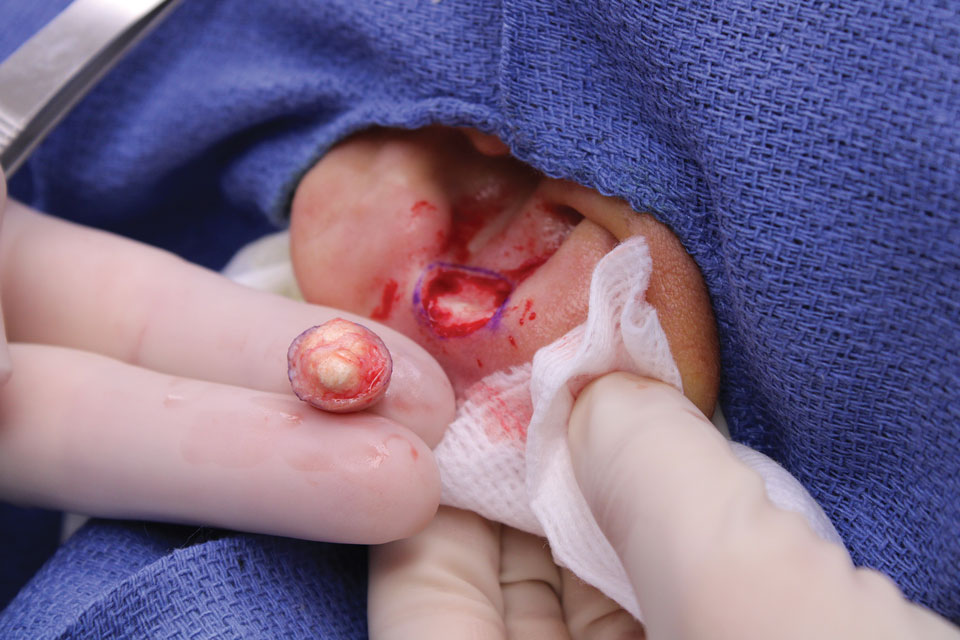
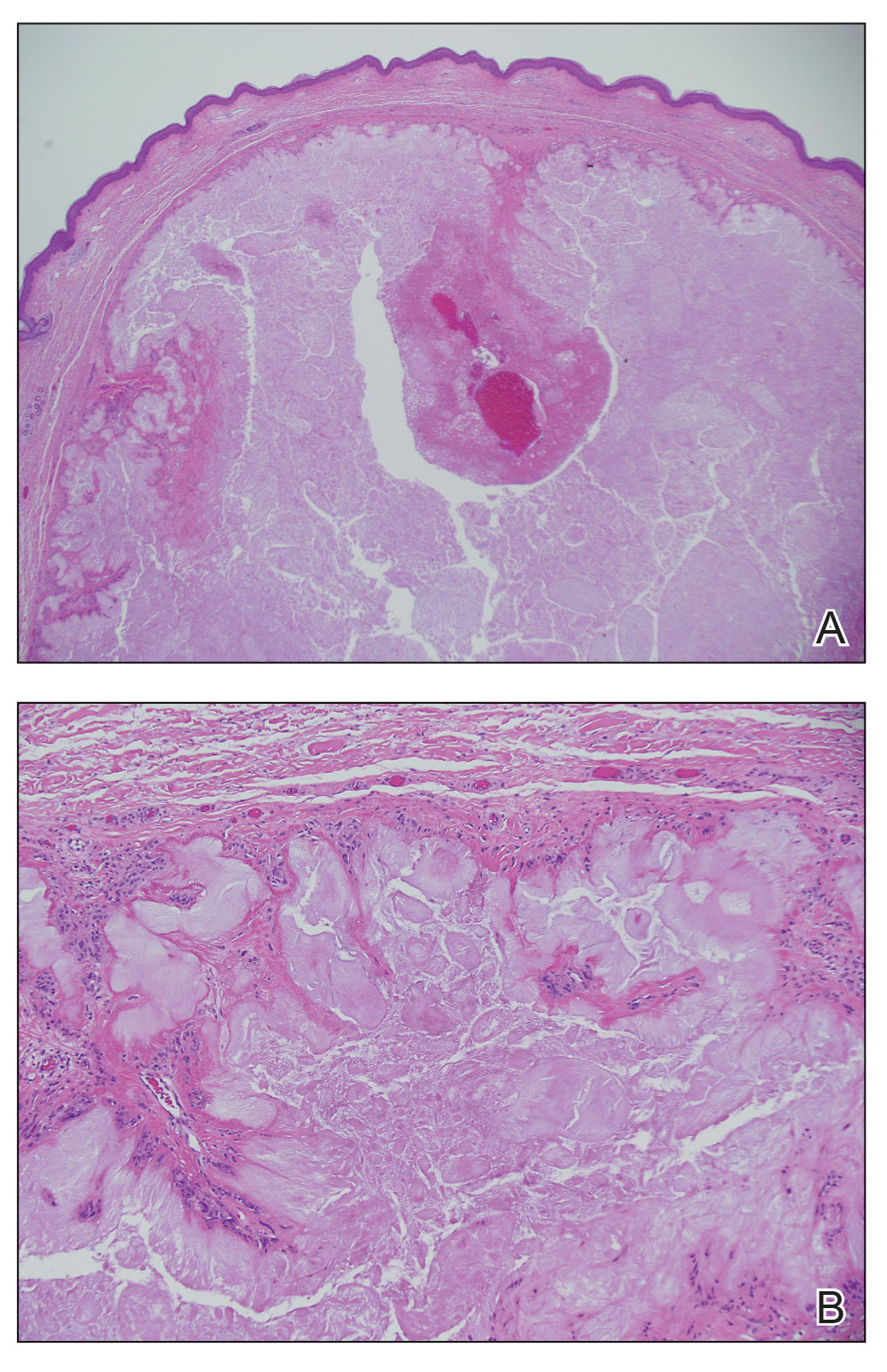
Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
A 46-year-old man with a history of hypertension, hyperlipidemia, and type 2 diabetes presented to the dermatology clinic with a painless nodule on the left ear of 2 years’ duration. The patient denied any bleeding, drainage, or prior trauma to the area. He noted that the lesion had grown slowly over time. Physical examination revealed a 1.5×1.5-cm, flesh-colored, subcutaneous nodule with overlying telangiectasias on the left antihelix.
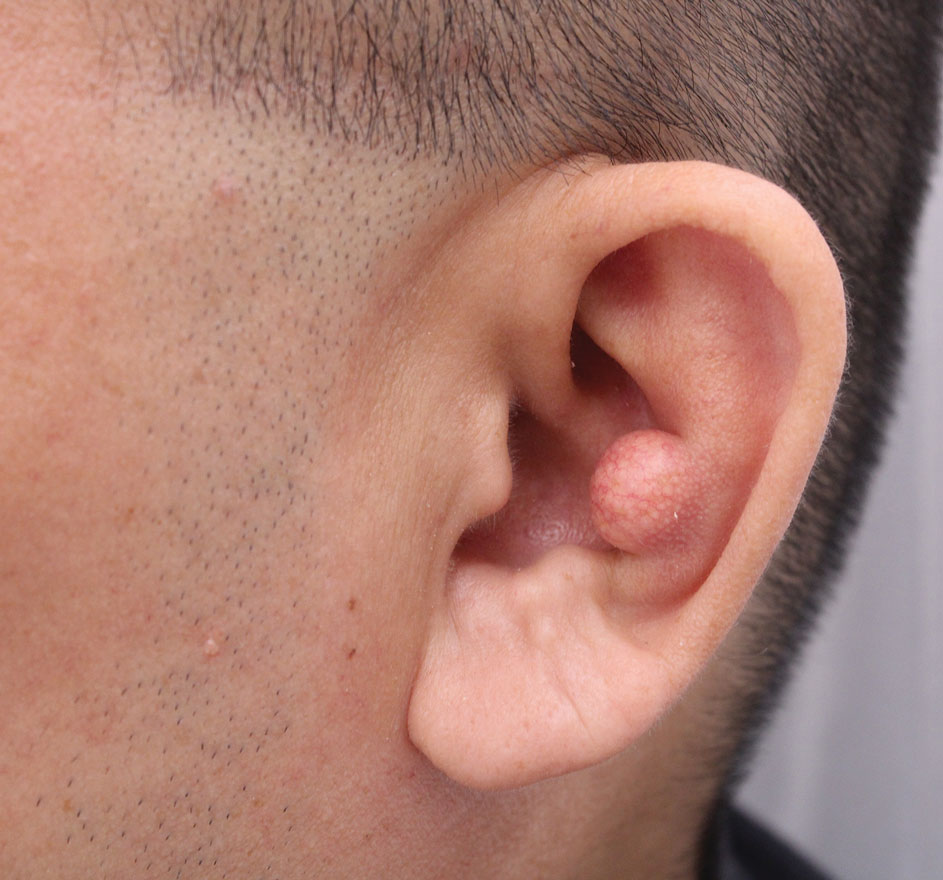
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.
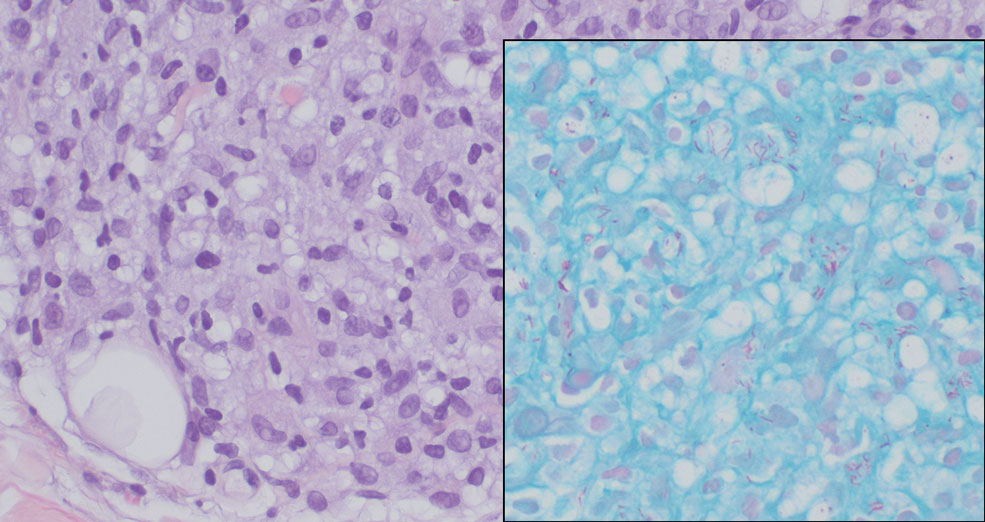
The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.
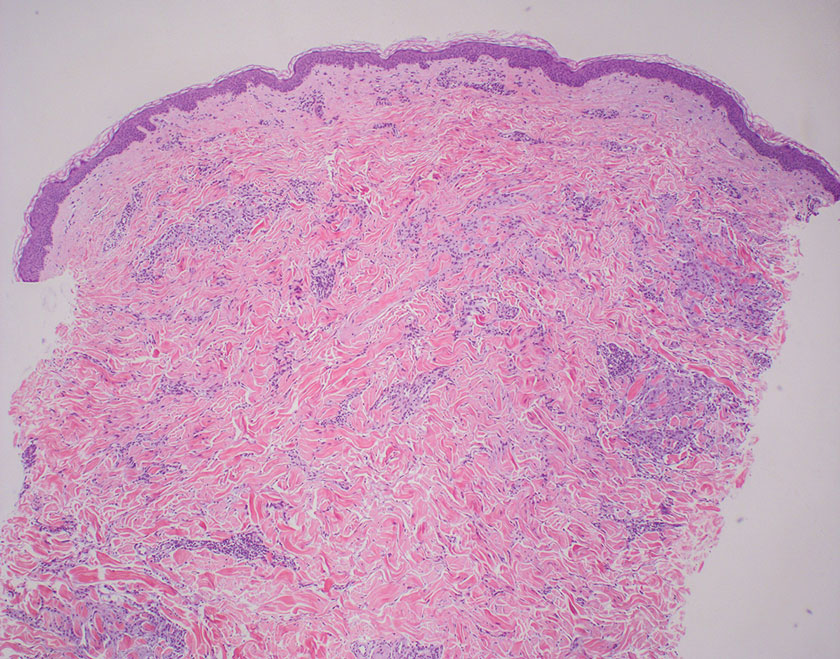
Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.
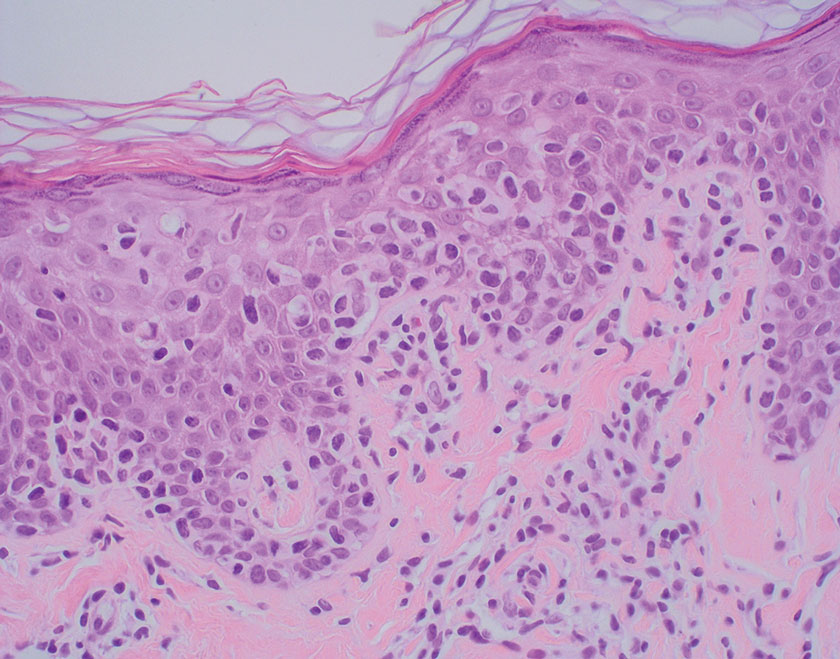
Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.
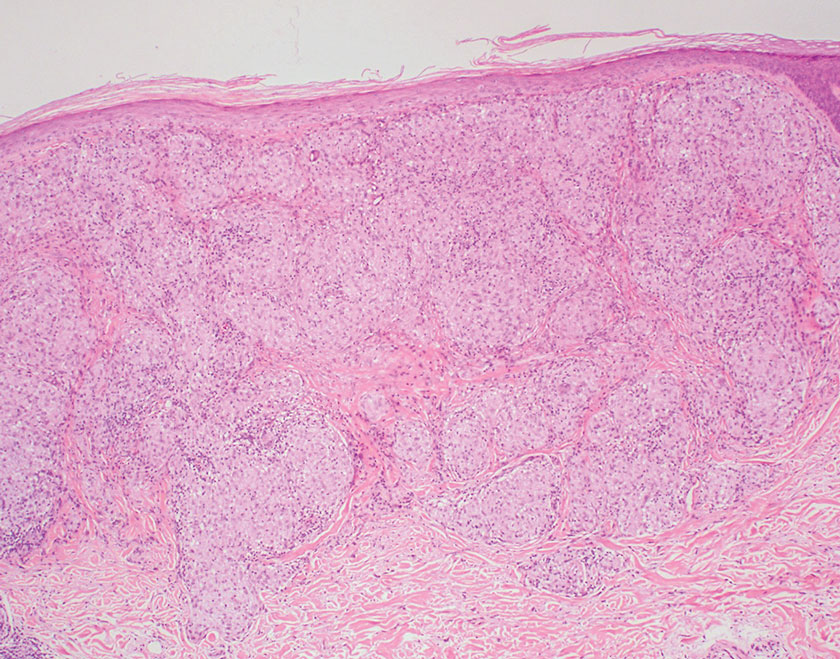
Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.
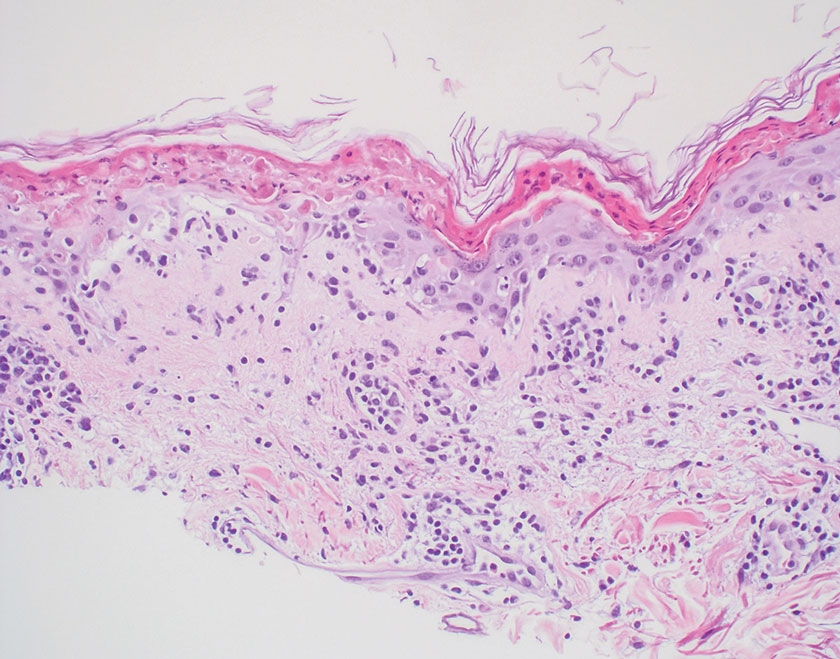
- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.

The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.

Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.

Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.

Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.

THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.

The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.

Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.

Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.

Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.

- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
A 44-year-old woman presented to the dermatology clinic with a widespread red, itchy, bumpy rash of 1 year’s duration. Physical examination revealed smooth, coalescing, erythematous and edematous plaques on the face (notably the forehead, malar cheeks, and nose), back, arms, and legs. Several plaques on the back had central hypopigmentation. The patient also reported numbness and weakness in the fingers and toes, and hypoesthesia within the lesions was noted. A biopsy of one of the lesions on the left ventral forearm was performed.
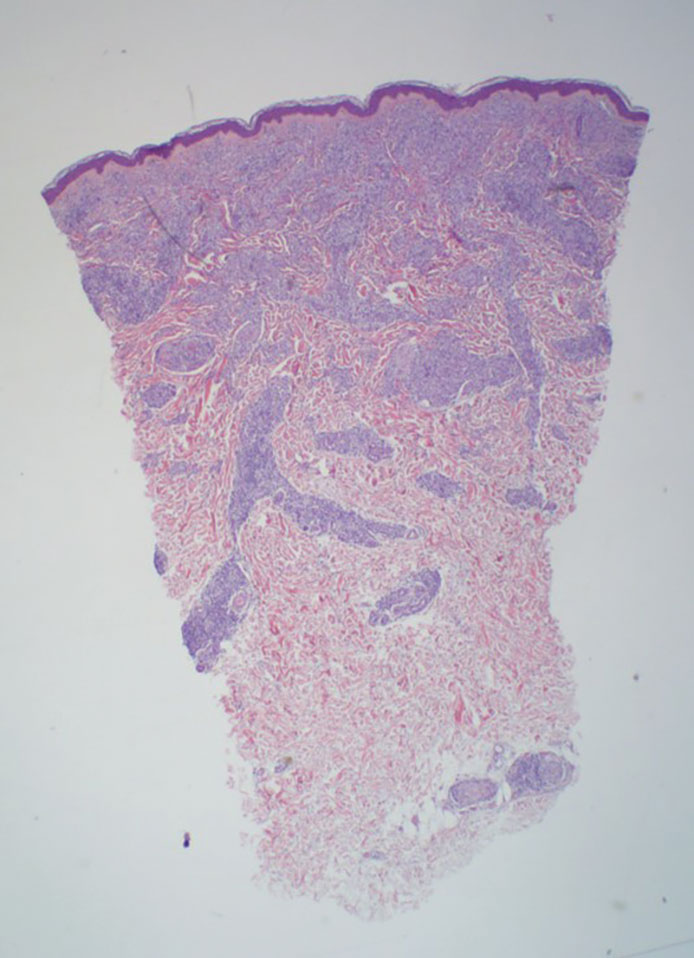
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
PRACTICE POINTS
- Tapinarof cream 1% can be absorbed systemically and cause acute generalized exanthematous pustulosis (AGEP).
- Clinical configuration and histology can be useful to distinguish AGEP from mimickers.
- Topical application of drugs in general, particularly over large body surface areas, may lead to systemic drug eruptions.
Longitudinal Erythronychia Manifesting With Pain and Cold Sensitivity
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.
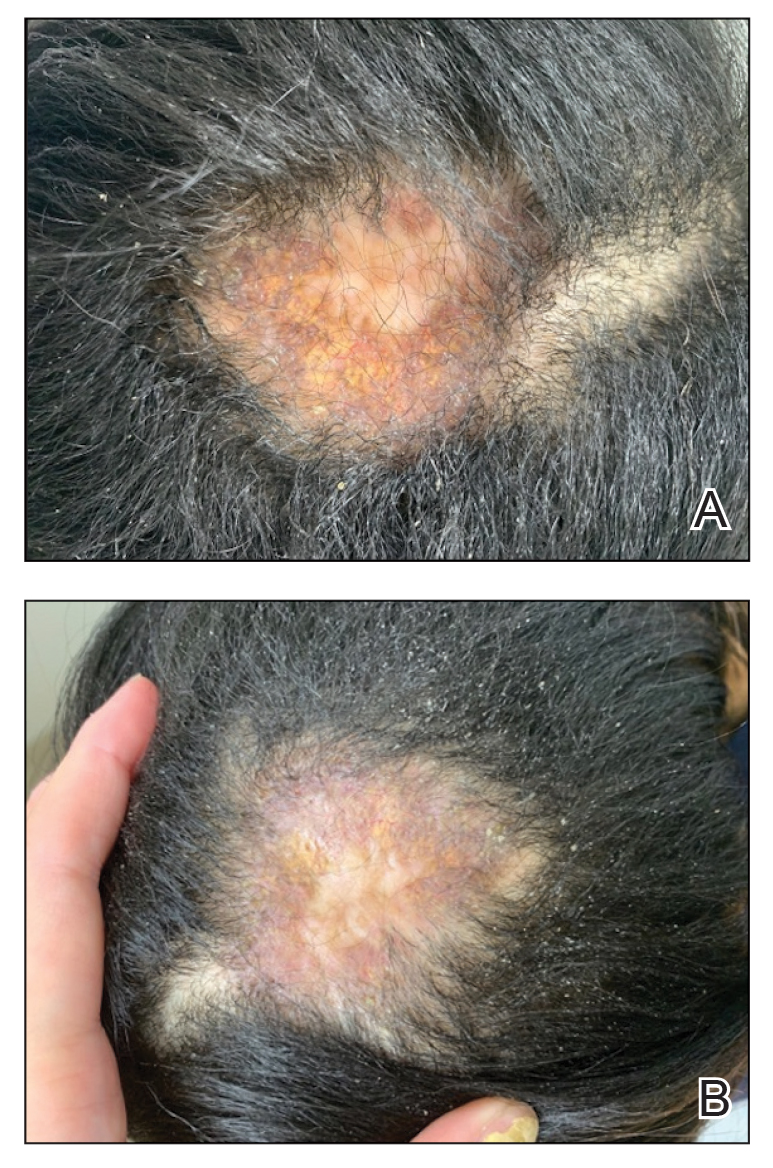
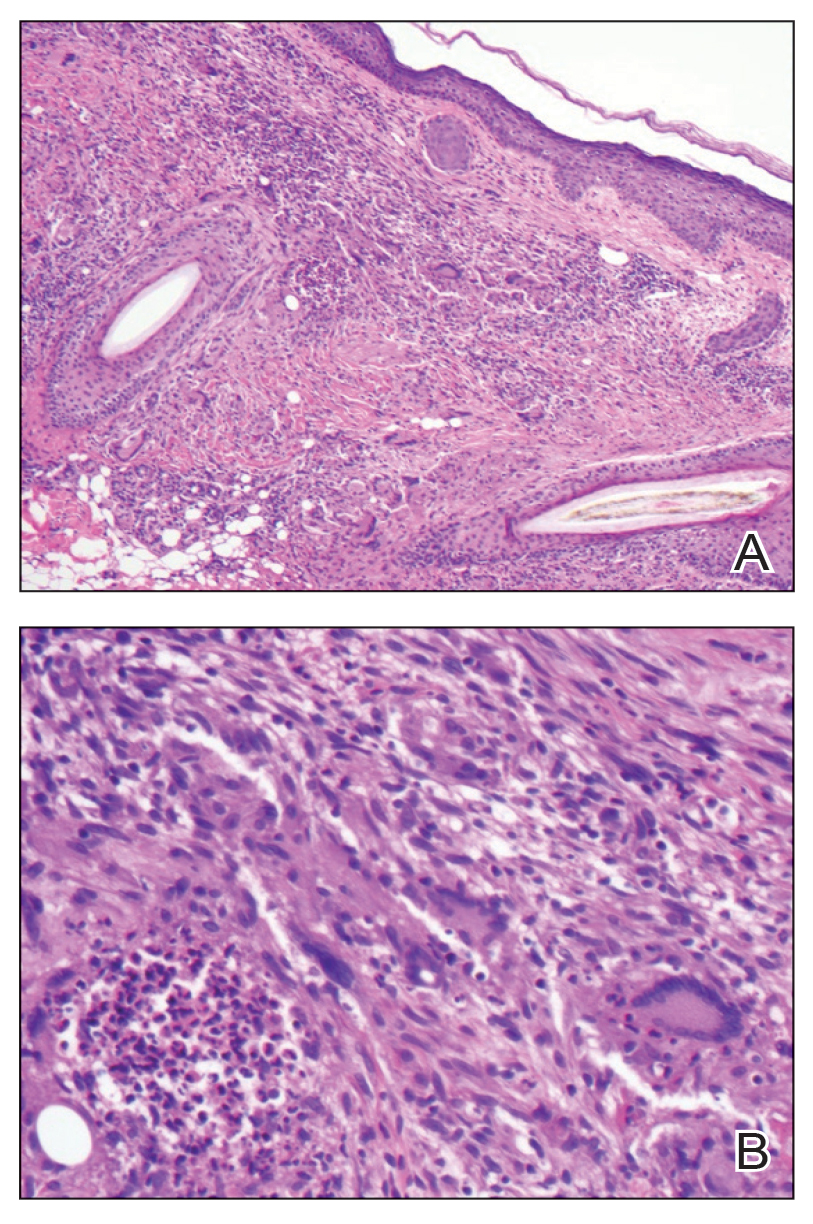
We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
PRACTICE POINTS
- In skin of color, necrobiotic xanthogranuloma can appear orange or brown compared to its yellow appearance in lighter skin types.
- When necrobiotic xanthogranuloma is suspected, a thorough malignancy workup should be conducted.