User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
PRACTICE POINTS
- Dermatology application costs increased from 2021 to 2024, largely due to expenses related to away rotations and, in some cases, a return to in-person interviews.
- Away rotations play a critical role in the dermatology match; however, they also contribute substantially to financial burden.
- The cost-saving impact of virtual interviews during the COVID-19 pandemic highlights a meaningful opportunity for future cost reduction.
- Further interventions are needed to meaningfully reduce financial burden and promote equity.
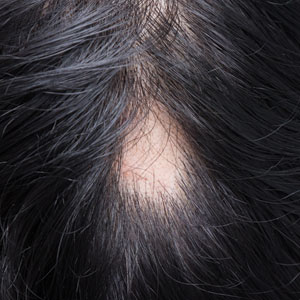
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
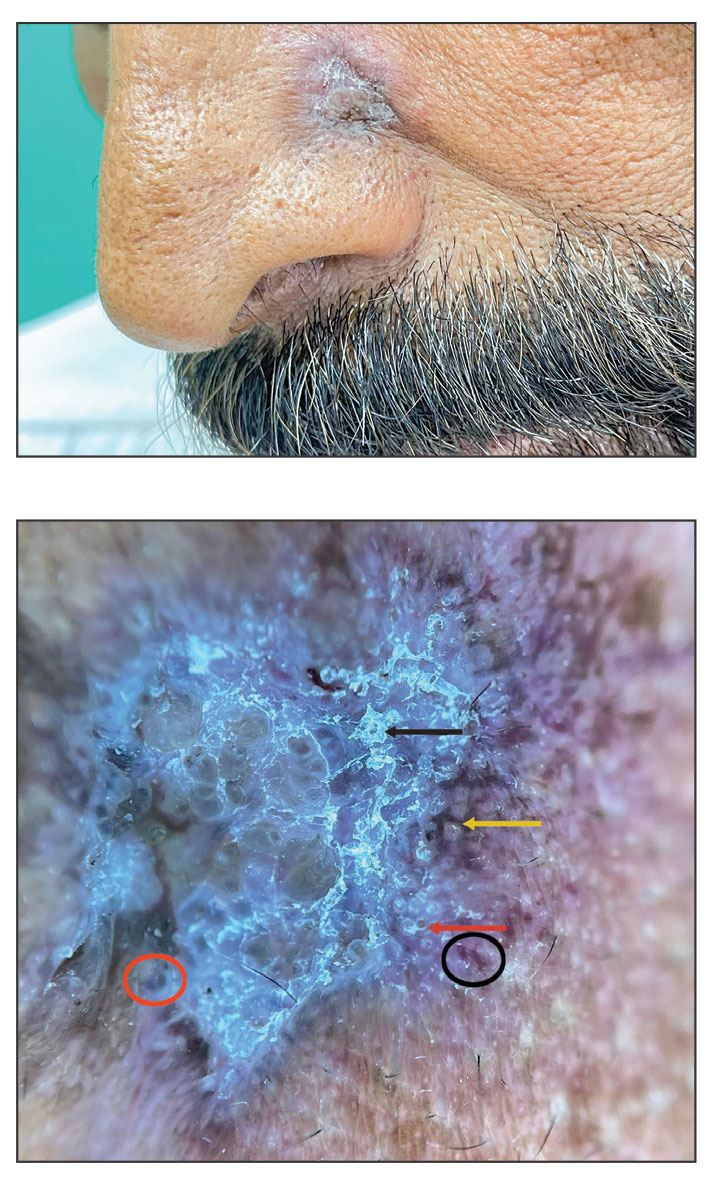
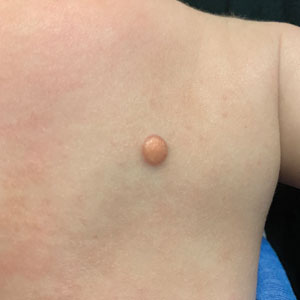
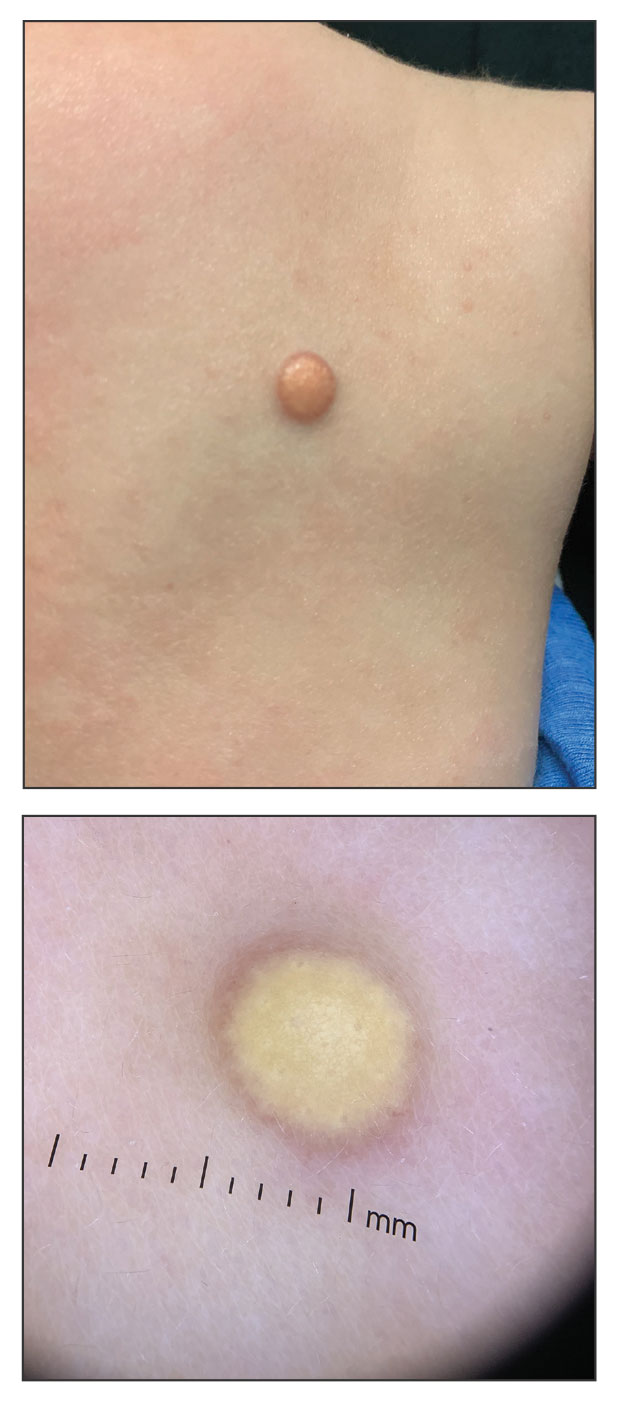
Solitary Yellow Papule on the Upper Back in an Infant
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
A 6-month-old male infant with a history of cradle cap and an infantile hemangioma on the left shoulder presented to the dermatology clinic for evaluation of a slow-growing yellow papule on the upper back of 3 months’ duration. The lesion initially was noted 2 months prior to the current presentation by the patient’s pediatrician, who recommended follow-up with dermatology after an unsuccessful attempt at incision and drainage. Physical examination revealed a 7-mm, yellow, dome-shaped papule with a red collarette on the right upper back. No axillary freckling, ocular findings, or other skin findings were found. The patient was born at term with no complications, and his mother reported that he was otherwise healthy. There were no developmental concerns or known allergies, and his family history was negative for any similar lesions. Dermoscopic examination of the lesion revealed a well-circumscribed, circular, yellow-orange papule with an erythematous border and setting-sun appearance.
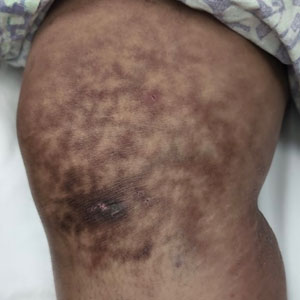
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
A 25-year-old woman with an unremarkable medical history presented to the dermatology clinic for evaluation of a persistent rash on the right knee and distal thigh of several months’ duration. The patient noted that the rash had been asymptomatic, and she denied any history of trauma to the area. She reported that she worked as a teacher and had repeatedly stayed up late using her laptop for months. Rather than use a desk, she often would work sitting with her laptop in her lap.
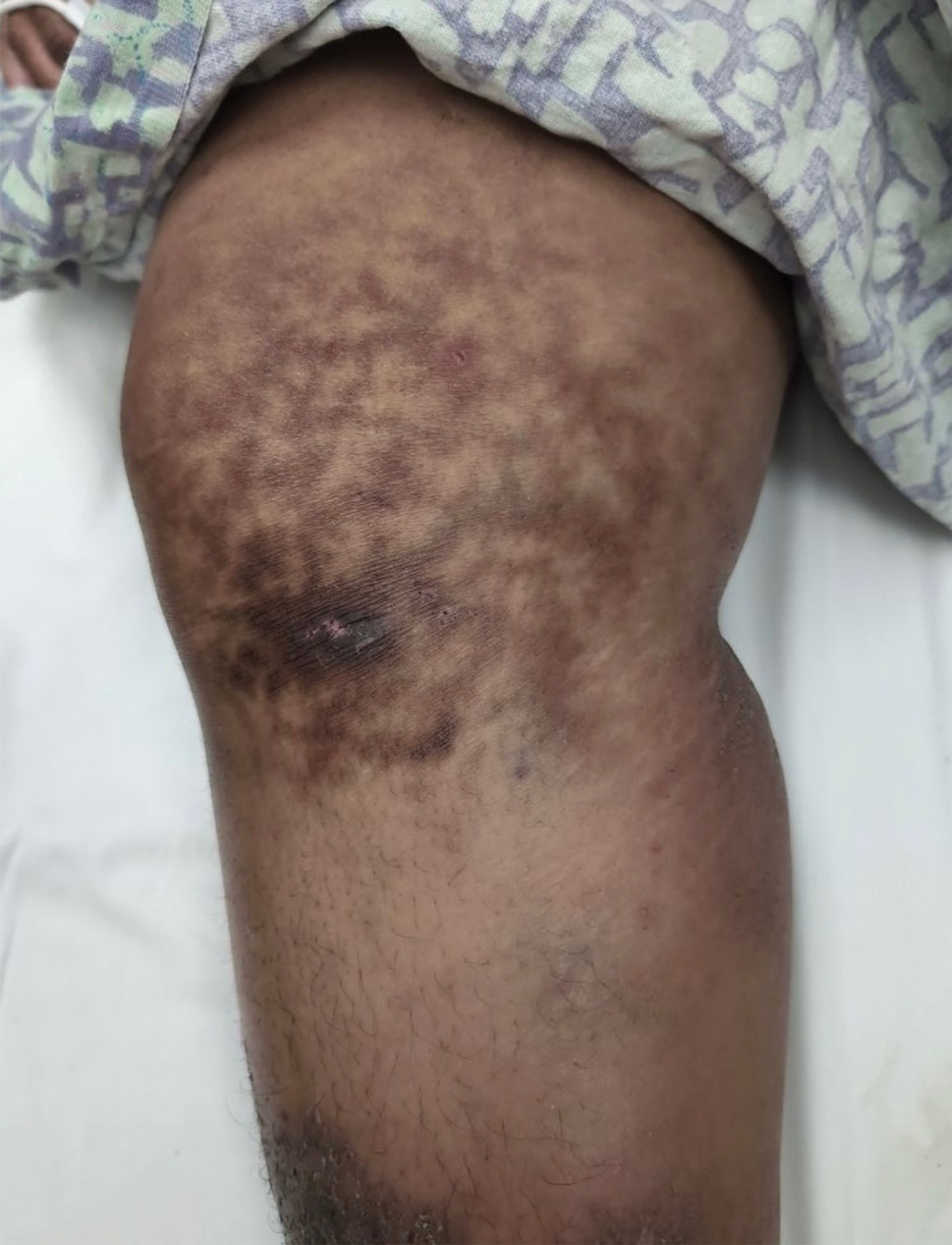
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).
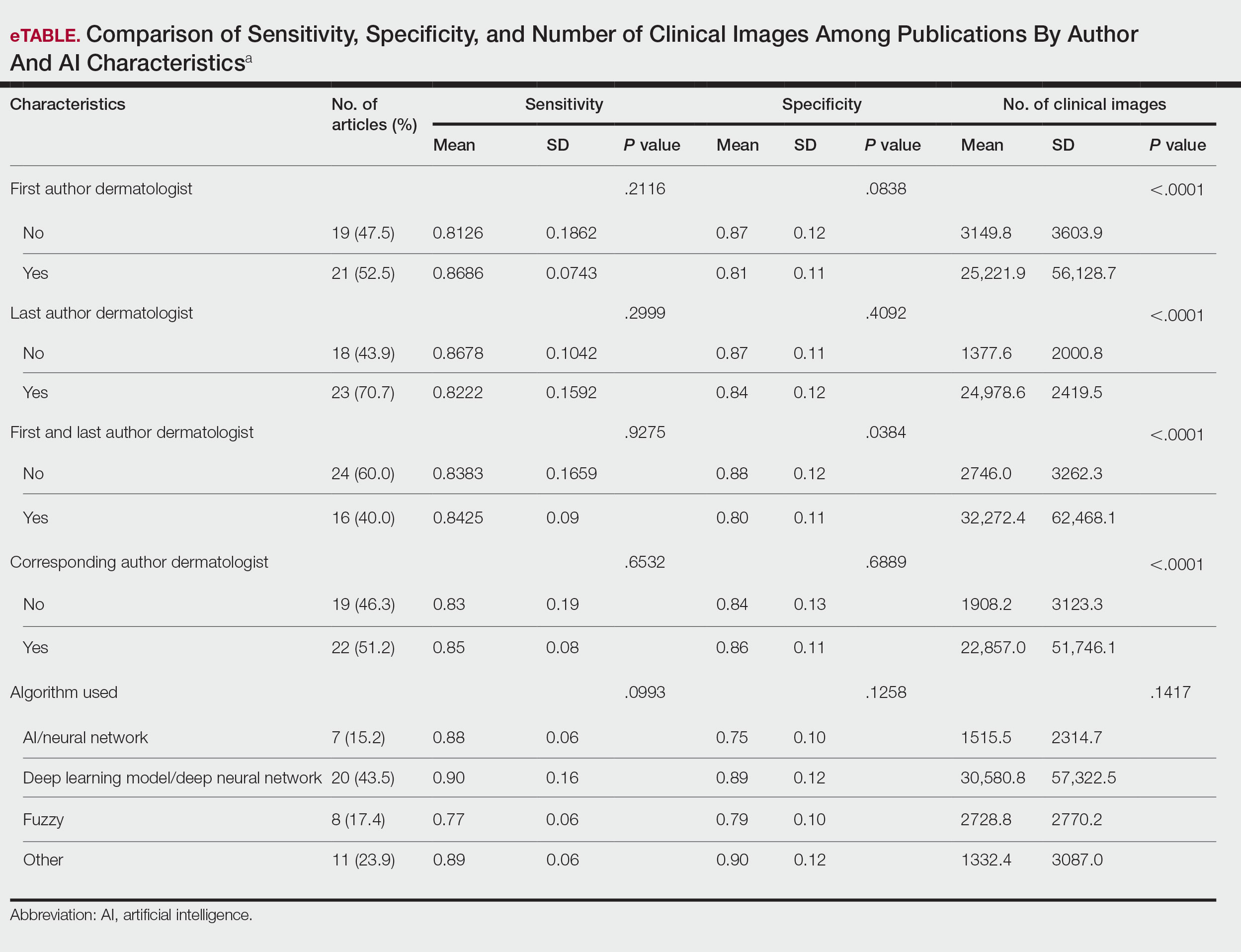
Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Practice Points
- Artificial intelligence (AI) technology is emerging as a valuable tool in diagnosing and classifying dermatologic conditions.
- Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists.
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.
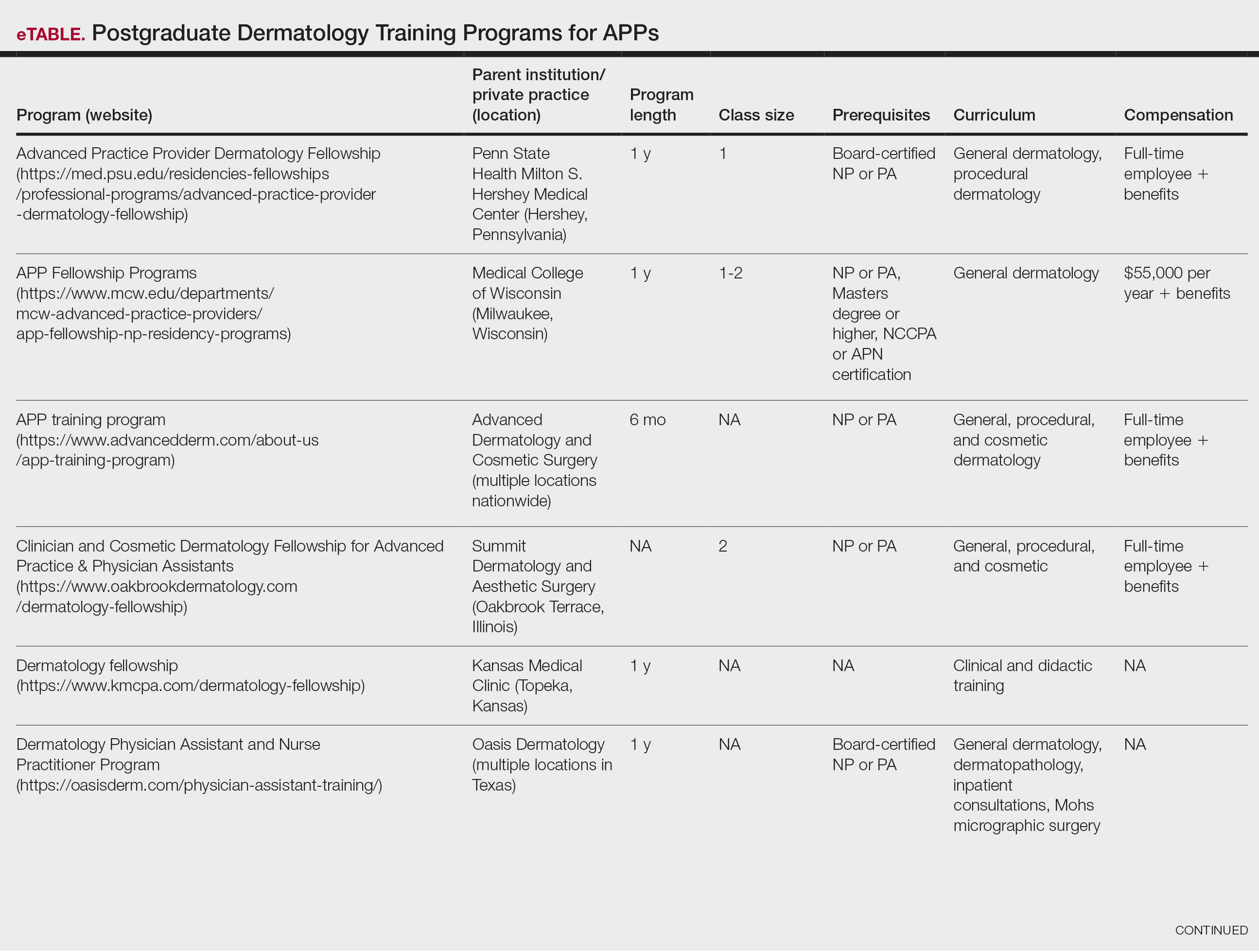
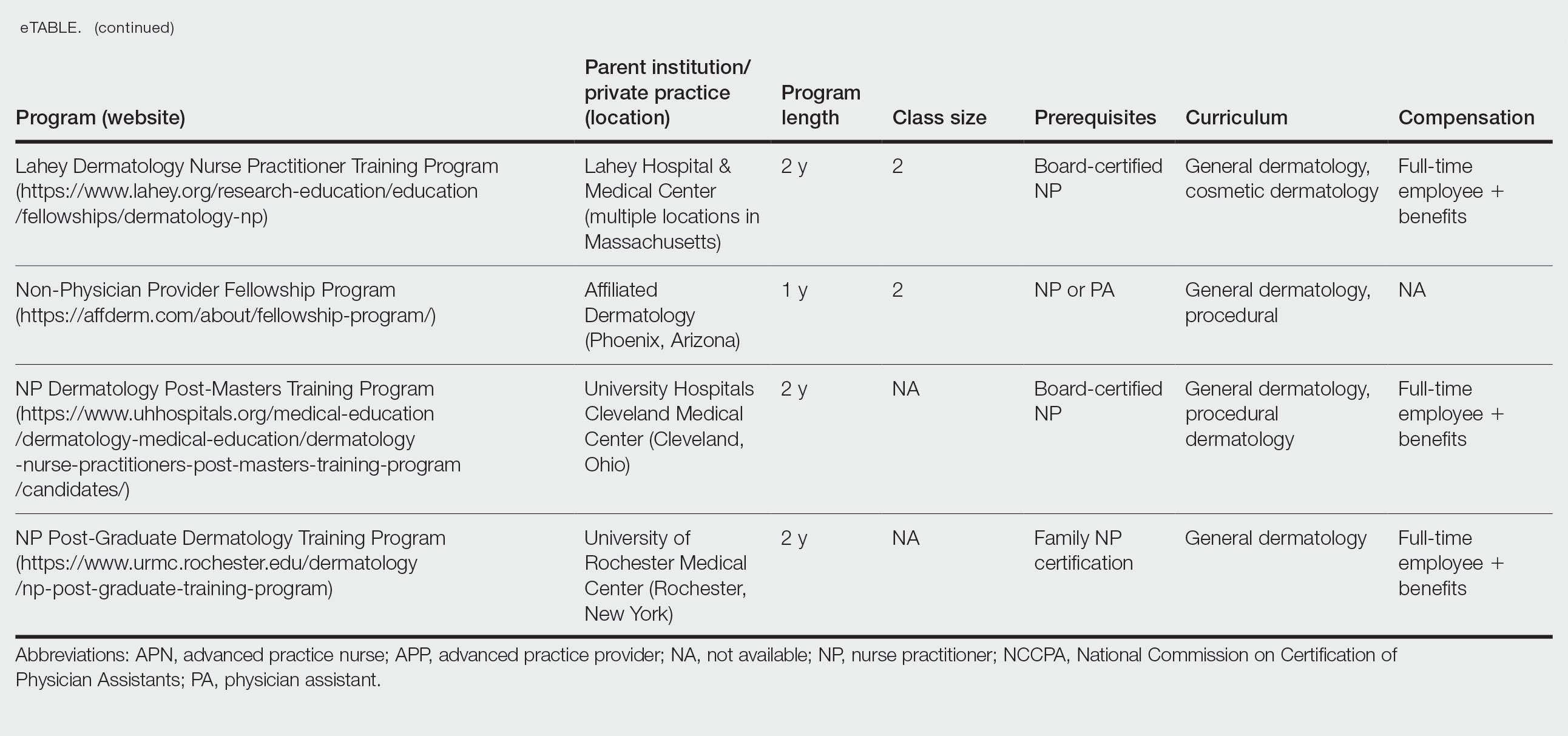
Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.



Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.



Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
Practice Points
- Postgraduate dermatology training programs are available for advanced practice providers (APPs), but they are optional and lack a formal accreditation process.
- Awareness of these programs and the differences between APPs and physician training may help dermatologists provide safe and effective care in collaborative or supervisory roles.
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.
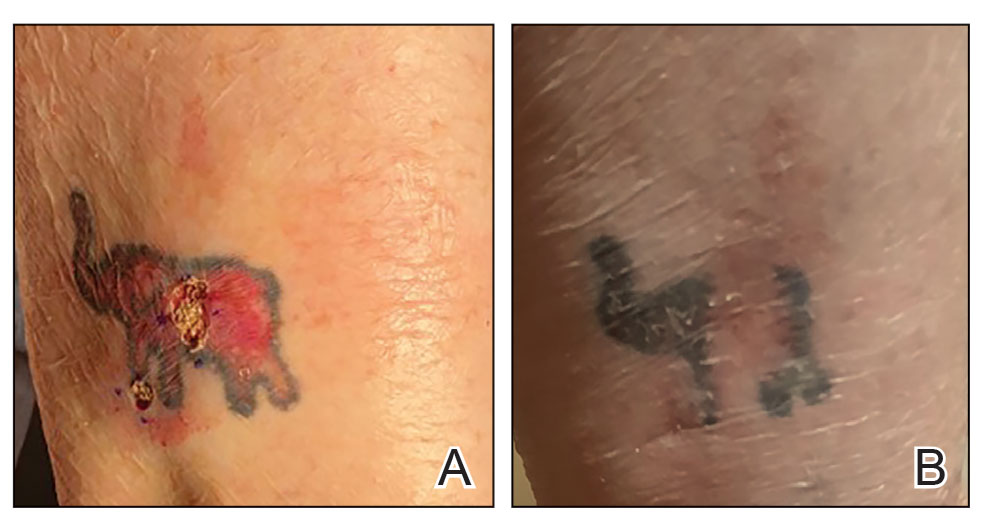
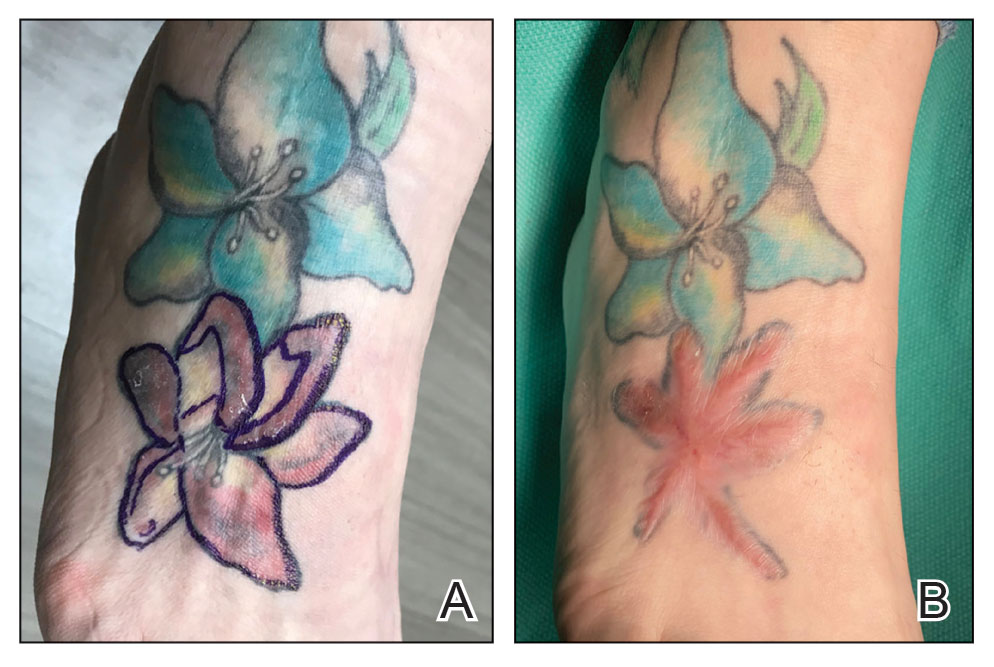
The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.


The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
Practice Gap
Tattoos have become increasingly prevalent in Western culture, with approximately 1 in 4 Americans having at least 1 tattoo. Individuals invest money, time, and even pain in getting tattoos, many of which hold special personal, family, or religious significance.1 Various cutaneous pathologies may arise in areas of the skin with tattoos, including malignancies and inflammatory reactions to tattoo pigment, and in these cases, surgical management may be indicated.2,3
Nonmelanoma skin cancers (NMSCs) such as superficial basal cell carcinomas on broadly sun-damaged areas (eg, trunk, torso), squamous cell carcinomas, reactive keratoacanthomas, and reactive pseudoepitheliomatous squamous hyperplasia diagnosed as squamous cell carcinoma have been reported to occur in or near areas of the skin with tattoos.2 Mohs micrographic surgery (MMS) is the standard of care for removing NMSCs, particularly when they manifest in cosmetically sensitive areas.4 This treatment option allows for careful guided resection of tumors to minimize the risk for recurrence; it also preserves healthy tissue, which typically results in a smaller radial defect after the procedure is complete.
Chronic reactions to tattoo pigment may include granulomatous tattoo reactions and pseudolymphomas.3 Treatment options may include immunosuppressives such as intralesional triamcinolone as well as pigment destruction via lasers5; however, not all tattoos are responsive to these treatments. Surgical excision is an effective and definitive treatment in this context, as tattoo pigment resides in or above the mid dermis to a depth of approximately 400 μm. Intradermal excision effectively removes the antigenic pigment.5
In these clinical scenarios, patients may be hesitant to pursue surgical treatment due to concerns that it may alter tattoo appearance. Many clinicians and surgeons may consider definitive treatment and tattoo preservation to be mutually exclusive, but this is not always the case. We propose a technique that utilizes conservative thickness layers (CTL) to minimize disruption to the appearance of tattoos in MMS for treatment of cutaneous malignancies as well as intradermal excision of tattoo pigment in the setting of chronic inflammatory tattoo reactions.
The Technique
In the appropriate clinical context, CTL can effectively result in defects that heal well by secondary intention and minimize collateral tissue distortion.4 Lesions manifesting in or near tattooed skin often are responsive to treatment with CTL; furthermore, CTL may preserve some deeper tattoo pigment, resulting in only partial loss of the tattooed skin.
Conservative thickness layers are performed intradermally, similar to removing traditional layers in MMS. For treatment of NMSCs, a margin is scored around the lesion, and then the blade is passed carefully under the lesion nearly parallel to the skin through an intradermal plane. It is important to avoid entering the subcuticular fat (Figure 1). The tissue then is processed normally in the Mohs laboratory for complete circumferential margin evaluation. If necessary and possible, subsequent layers also can be performed in the intradermal plane. Once total circumferential margin control is obtained, the wound is allowed to granulate and heal by secondary intention. As these processes occur, we have found that wound contraction is less likely with the dermis intact, resulting in less impact on the overall appearance of the tattoo (Figures 1 and 2). For very thin lesions, resultant defects may retain some residual tattoo pigment. The residual scars also may be responsive to tattoo revision, although a period of monitoring for recurrence should be considered if there is concern that revising the tattoo could obscure early recurrent tumors. From our experience, utilizing CTL for NMSCs that arise within or near tattoos results in favorable preservation of the tattoo appearance and high patient satisfaction.


The procedure is performed similarly for removal of allergenic tattoo pigment, with careful excision to the mid dermis. Since the areas affected by the cutaneous reaction may be relatively large, surgical precision is required to maintain a uniform depth to remove the tattoo pigment and preserve the deep dermis (Figure 2). Once removed, the defect can be left to granulate and heal by secondary intention. If the patient wants to have the tattoo revised in the future, it would be prudent to utilize pigment that the patient has responded favorably to. In our experience, this approach is effective and yields high patient satisfaction and minimizes morbidity.
Practice Implications
Tattoos often hold special meaning for patients; therefore, treatment of pathologies arising in or near tattooed skin should emphasize maintaining the appearance of the tattoo while still being effective. Conservative thickness layers in MMS and intradermal excisions for allergic reactions to tattoo pigment are an effective treatment strategy that clinicians may consider.
One shortcoming of using CTL for MMS is the need for subsequent layers to clear the tumor; however, data suggest that first-stage cure rates are extremely high even with CTL for appropriately selected patients, with clearance of nearly 80% of tumors on the first stage. Tumors that may be most responsive to CTL include exophytic NMSCs and those arising in areas with a thicker dermis, including the back, legs, and scalp, although other locations including the face, hands, shins, ankles, and feet also may be well suited for CTL.4 Another shortcoming of CTL is that skin cancers arising in tattoos may not be considered appropriate for MMS based on the 2012
Conservative thickness layers in MMS and intradermal excisions of tattoo pigment are both effective techniques of minimizing disruption of tattoos while effectively treating patients.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
- Roggenkamp H, Nicholls A, Pierre JM. Tattoos as a window to the psyche: how talking about skin art can inform psychiatric practice. World J Psychiatry. 2017;7:148-158. doi:10.5498/wjp.v7.i3.148
- Rubatto M, Gelato F, Mastorino L, et al. Nonmelanoma skin cancer arising on tattoos. Int J Dermatol. 2023;62:E155-E156. doi:10.1111/ijd.16381
- Atwater AR, Bembry R, Reeder M. Tattoo hypersensitivity reactions: inky business. Cutis. 2020;106:64-67. doi:10.12788/cutis.0028
- Tolkachjov SN, Cappel JA, Bryant EA, et al. Conservative thickness layers in Mohs micrographic surgery. Int J Dermatol. 2018;57:1128-1134. doi:10.1111/ijd.14043
- Sardana K, Ranjan R, Ghunawat S. Optimising laser tattoo removal. J Cutan Aesthet Surg. 2015;8:16-24. doi:10.4103/0974-2077.155068
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j.jaad.2012.06.009.
- Amthor Croley JA. Current controversies in mohs micrographic surgery. Cutis. 2019;104:E29-E31.
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Conservative Thickness Layers to Preserve Tattoo Appearance During Excisional Procedures
Solitary Plaque on the Nose
Solitary Plaque on the Nose
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.
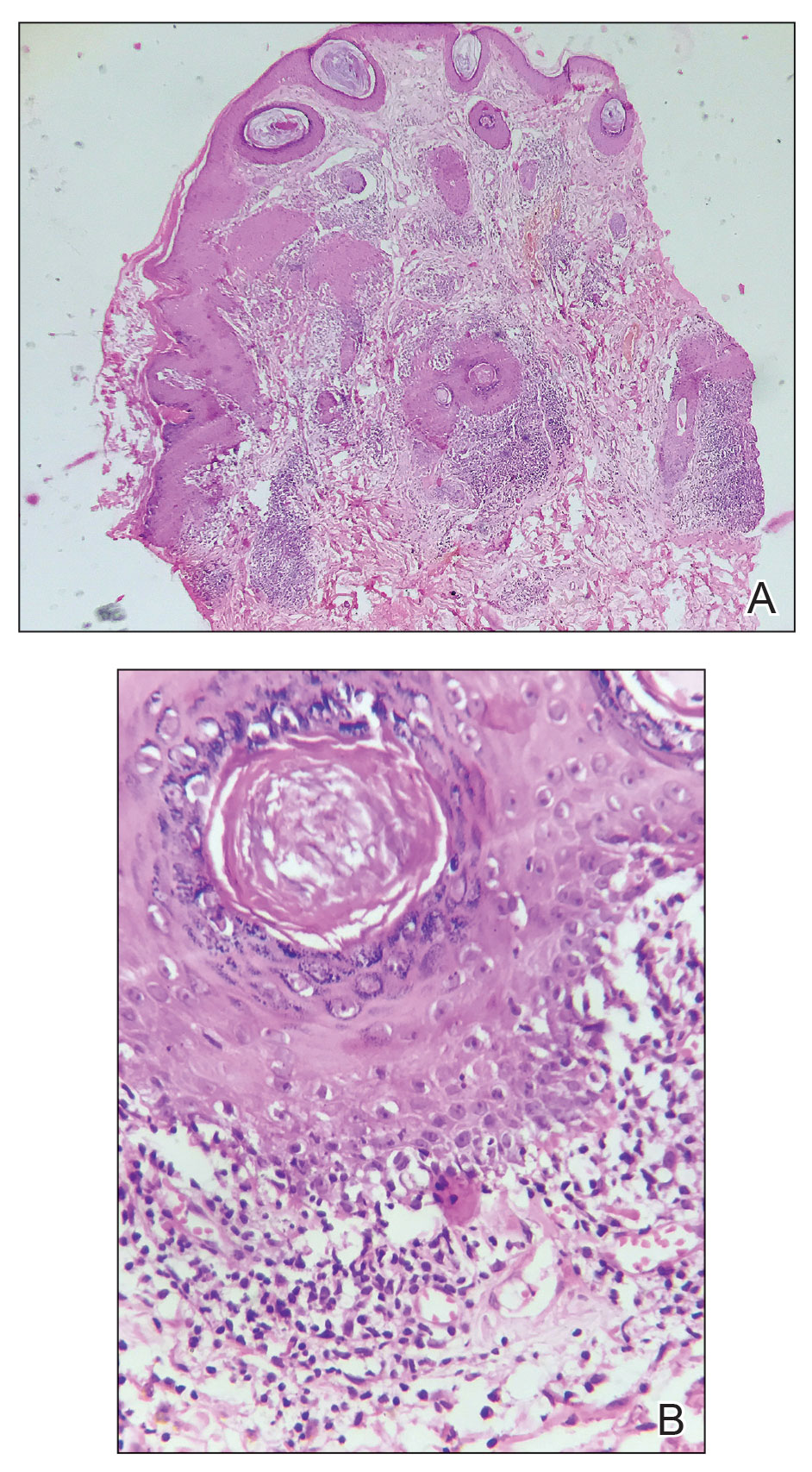
Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.
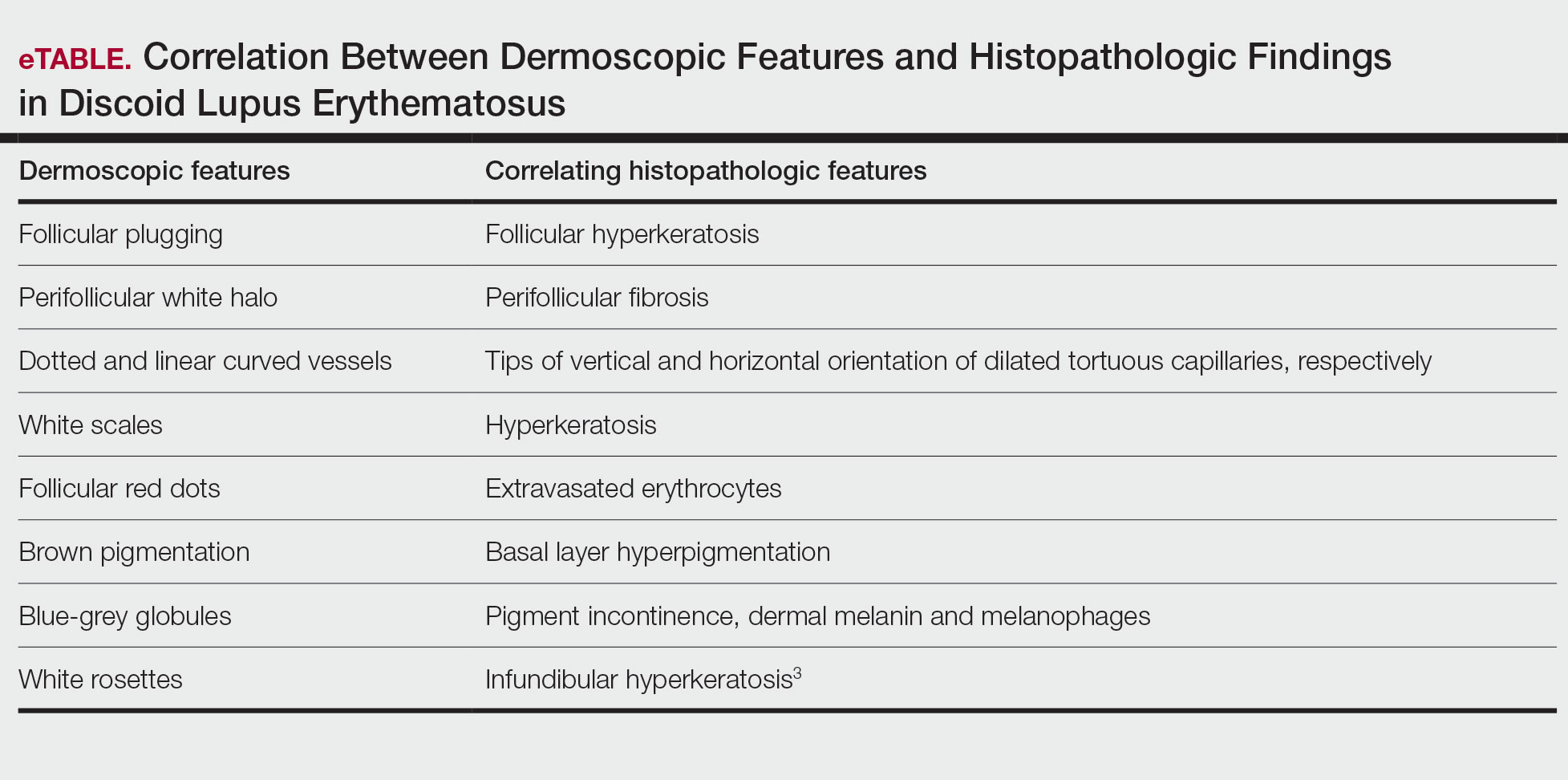
On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.

Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.

On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
The biopsy revealed hyperkeratosis, hypergranulosis, follicular plugging, vacuolar interface dermatitis with apoptotic bodies, dyskeratotic keratinocytes, pigment incontinence, and melanophages. A perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate was noted in the superficial and deep dermis (Figure). Based on the characteristic clinical morphology, dermoscopic features, and histopathology, a diagnosis of discoid lupus erythematosus (DLE) was established. The patient was started on mometasone cream 0.1% and tacrolimus ointment 0.1% once daily, with strict recommendations for photoprotection. However, he subsequently was lost to follow-up, and treatment response could not be assessed.

Lupus erythematosus is a multisystemic autoimmune disease with a predilection for skin involvement that is characterized by the production of autoantibodies against nuclear antigens. Discoid lupus erythematosus is the predominant form of the disease, mostly affecting middle-aged women (female-to-male ratio, 4.1:1).1 Discoid lupus erythematosus usually manifests as well-demarcated, erythematous patches or plaques with partially adherent scales that extend into a patulous follicle. On removal, the scales show horny plugs underneath. This classic finding is known as the carpet tack sign.
As the lesions evolve, they expand with hyperpigmentation at the periphery as well as hypopigmentation, atrophy, scarring, and telangiectasias at the center.2 In our patient, the history of discharge and crusting of the lesion and the presence of slight central atrophy—all of which could be attributed to chronic application of topical medications such as corticosteroids, which can cause epidermal thinning, maceration, and secondary crust formation—raised clinical suspicion of cutaneous infections (eg, cutaneous leishmaniasis, lupus vulgaris) and squamous cell carcinoma. The presence of slightly raised margins upon clinical examination brought basal cell carcinoma (BCC) into the differential.
Dermoscopic features commonly seen in DLE reflect the pathologic findings. Follicular plugging and perifollicular white halos correspond to follicular hyperkeratosis and perifollicular fibrosis, respectively (eTable). Disease duration has been shown to alter the dermoscopic appearance of DLE with early active disease showing radially arranged arborizing blood vessels between perifollicular white halos along with follicular red dots, whereas lesions of longer duration display structureless white areas secondary to dermal fibrosis.3 Additionally, background erythema due to neoangiogenesis and dermal inflammation suggests that the disease is in its active state.

On dermoscopy, pigmentation structures such as brown dots, brown lines, and grey-brown dots and globules were seen more prominently in our patient with skin of color, making the underlying erythema more subtle than in patients with lighter skin types. Dotted and linear vessels also were seen in our patient, but not as prominently as typically is seen in lighter skin types.4
Lupus vulgaris was ruled out in our patient based on the absence of the typical orange to yellowish-orange background with vessels or any histopathologic evidence of epithelioid granulomas.5 Cutaneous leishmaniasis is characterized by polymorphic vascularization, erythema, follicular plugs, yellow-orange structureless areas with scales, and crusts on dermoscopy.6 Squamous cell carcinoma tends to show white structureless areas, looped vessels, and central keratin.7
Superficial BCC also appears as thin plaques or patches bound by a well-circumscribed, slightly raised, irregular margin. However, on dermoscopy, BCC typically exhibits spoke-wheel areas, arborizing vessels, comma vessels, and concentric structures.8
The clinical manifestations of crusting, discharge, and a raised border was atypical, probably owing to the long-term unsupervised application of topical medications, which made the initial diagnosis challenging. Therefore, various differential diagnoses were considered. Dermoscopic evaluation coupled with histology was performed, which ultimately confirmed the diagnosis of DLE.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
- Gopalan G, Gopinath SR, Kothandaramasamy R, et al. A clinical and epidemiological study on discoid lupus erythematosus. Int J Res Dermatol 2018;4:396-402. doi:10.18203/issn.24554529.IntJRes Dermatol20183165
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing 2025. Updated August 28, 2023. Accessed October 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Fathy H, Ghanim BM, Refat S, et al. Dermoscopic criteria of discoid lupus erythematosus: an observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol 2022;88:360-366. doi:10.25259/IJDVL_207_19
- Ankad BS, Gupta A, Nikam BP, et al. Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol 2022;67:5‐11. doi:10.4103/ijd.ijd_591_21
- Jindal R, Chauhan P, Sethi S. Dermoscopy of the diverse spectrum of cutaneous tuberculosis in the skin of color. Dermatol Pract Concept. 2022;12:E2022203. doi:10.5826/dpc.1204a203
- Chauhan P, Adya KA. Dermatoscopy of cutaneous granulomatous disorders. Indian Dermatol Online J. 2021;12:34-44. doi:10.4103 /idoj.IDOJ_543_20.
- Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386-1392. doi:10.1001/archdermatol.2012.2974.
- Vinciullo C, Mada V. Basal cell carcinoma. 10th ed. Wiley: Blackwell Science; 2024.
Solitary Plaque on the Nose
Solitary Plaque on the Nose
A 50-year-old Southeast Asian-Indian man presented to the dermatology clinic with a slightly elevated reddish-purple lesion on the left side of the nose accompanied by intense itching, occasional discharge, and crusting of 5 months’ duration. The patient reported applying multiple unknown topical agents initially prescribed to him by a physician; however, he subsequently continued applying these medications without regular follow-up visits. He had a history of smoking 2 packs per day for 25 years. His family history was unremarkable. Physical examination revealed a well-defined, 1.5×1.5-cm, nontender, scaly, erythematous to violaceous plaque with slightly raised margins, peripheral hyperpigmentation, and slight central atrophy on the left side of the nose. Dermoscopy revealed prominent follicles with a perifollicular halo (red arrow), white scales (black arrow), linear curved and dotted vessels (black circle), blue-grey globules (red circle), brown reticular lines (yellow arrow), and background erythema. General and systemic examination and routine laboratory workup were normal. A biopsy of the lesion was performed.