User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Measurement-Based Treatment to Target Approaches
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Clinical Scenario
Lilly is a 15-year-old girl in her sophomore year of high school. Over the course of a month after a romantic and then a friend-group breakup, her parents have been concerned about her increasing tearfulness every day and retreat from activities to avoid social interactions with others that she once enjoyed so much. She has been missing more and more school, saying that she can’t bear to go, and staying in bed during the days, even on weekends. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment in 2 weeks with you and then again in another 2 weeks. Her parents and she tell you, “I thought she would be better by now.” You feel stuck with how to proceed in the visit. You have correctly identified the problem as depression, started the recommended evidence-based treatments, but the parents and Lilly are looking to you for something more or different. There are not many or other local resources. When and how do you all determine what “better” looks and feels like? Where do you go from here?
Metrics Can Guide Next Steps
This clinical scenario is not uncommon. As a psychiatrist consultant in primary care, I often encounter the following comment and question: “Someone isn’t feeling better. I have them taking an SSRI and doing psychotherapy. What is the next thing to do?” In discussions with supervisees and in training residents, I often say that you will know that your consultations have made a real impact on providers’ practices when these questions shift from “what’s the next medication or treatment” to a more robust baseline and follow-up inventory of symptoms via common and available metrics (PHQ9A, PSC-17 or 30, SCARED) shared with you at the start, the middle, and at other times of treatment. Such metrics can more meaningfully guide your collaborative clinical discussions and decisions.
Tracking baseline metrics and follow-up with treatment interventions is a transformative approach to clinical care. But, in primary care, it’s common that the question around mental health care may not receive the same robust screening and tracking of symptoms which have the power to more thoughtfully guide decision-making, even though this is common in other forms of patient care which have more routine use of more objective data.
Measurement-based treatment to target approaches are well-studied, but not often or always implemented. They involve providing a baseline metric (PHQ9A, Pediatric Symptom Checklist 17 or 30, GAD7, or SCARED), and tracking that metric for response over time using specific scores for decision points.
An Alternative Clinical Scenario
Consider the following alternative scenario for the above patient using a measurement-based treatment to target approach:
Lilly is a 15-year-old girl in her sophomore year of high school with symptoms concerning for depression. A PHQ9A is administered in your appointment, and she scores 20 out of 30, exceeding the threshold score for 11 for depression. You start her on an SSRI and recommend psychotherapy in the form of CBT offered through your office. She returns to the appointment with you in 2 weeks and then again in another 2 weeks. You obtain a PHQ9A at each appointment, and track the change with her and her parents over time.
You share with her and the family that it is common that there will be fluctuations in measurements, and you know that a score change on the PHQ9A greater than 7 is considered a clinically significant, reliable change. So, a PHQ9 score reduction from 20 to 13 would be meaningful progress. While seeking a score within the normal and non-clinical range, the progress can be tracked in a way that allows a more robust monitoring of treatment response. If the scores do not improve, you can see that and act accordingly. This way of using metrics shifts the conversation from “how are you feeling now and today” to tracking symptoms more broadly and tracking those individual symptoms over time, some of which may improve and some which may be trickier to target.
Such a way of tracking common mental health symptoms with a focus on having data at baseline and throughout treatment allows a provider to change or adapt interventions, and to not chase something that can feel ephemeral, such as “feeling happy or looking better.”
For additional information on the measurement-based treatment to target approach, there are resources that share in more depth the research informing this approach, and other and broader real ways to integrate these practices into your own visits:
- Is Treatment Working? Detecting Real Change in the Treatment of Child and Adolescent Depression
- AACAP Clinical Update: Collaborative Mental Health Care for Children and Adolescents in Primary Care
Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
Common Herbicide a Player in Neurodegeneration?
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROINFLAMMATION
Internet Use May Boost Mental Health in Later Life
TOPLINE:
and better self-reported health among adults aged 50 years or older across 23 countries than nonuse, a new cohort study suggests.
METHODOLOGY:
- Data were examined for more than 87,000 adults aged 50 years or older across 23 countries and from six aging cohorts.
- Researchers examined the potential association between internet use and mental health outcomes, including depressive symptoms, life satisfaction, and self-reported health.
- Polygenic scores were used for subset analysis to stratify participants from England and the United States according to their genetic risk for depression.
- Participants were followed up for a median of 6 years.
TAKEAWAY:
- Internet use was linked to consistent benefits across countries, including lower depressive symptoms (pooled average marginal effect [AME], –0.09; 95% CI, –0.12 to –0.07), higher life satisfaction (pooled AME, 0.07; 95% CI, 0.05-0.10), and better self-reported health (pooled AME, 0.15; 95% CI, 0.12-0.17).
- Frequent internet users showed better mental health outcomes than nonusers, and daily internet users showed significant improvements in depressive symptoms and self-reported health in England and the United States.
- Each additional wave of internet use was associated with reduced depressive symptoms (pooled AME, –0.06; 95% CI, –0.09 to –0.04) and improved life satisfaction (pooled AME, 0.05; 95% CI, 0.03-0.07).
- Benefits of internet use were observed across all genetic risk categories for depression in England and the United States, suggesting potential utility regardless of genetic predisposition.
IN PRACTICE:
“Our findings are relevant to public health policies and practices in promoting mental health in later life through the internet, especially in countries with limited internet access and mental health services,” the investigators wrote.
SOURCE:
The study was led by Yan Luo, Department of Data Science, City University of Hong Kong, Hong Kong, China. It was published online November 18 in Nature Human Behaviour.
LIMITATIONS:
The possibility of residual confounding and reverse causation prevented the establishment of direct causality between internet use and mental health. Selection bias may have also existed due to differences in baseline characteristics between the analytic samples and entire populations. Internet use was assessed through self-reported items, which could have led to recall and information bias. Additionally, genetic data were available for participants only from England and the United States.
DISCLOSURES:
The study was funded in part by the National Natural Science Foundation of China. The investigators reported no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
and better self-reported health among adults aged 50 years or older across 23 countries than nonuse, a new cohort study suggests.
METHODOLOGY:
- Data were examined for more than 87,000 adults aged 50 years or older across 23 countries and from six aging cohorts.
- Researchers examined the potential association between internet use and mental health outcomes, including depressive symptoms, life satisfaction, and self-reported health.
- Polygenic scores were used for subset analysis to stratify participants from England and the United States according to their genetic risk for depression.
- Participants were followed up for a median of 6 years.
TAKEAWAY:
- Internet use was linked to consistent benefits across countries, including lower depressive symptoms (pooled average marginal effect [AME], –0.09; 95% CI, –0.12 to –0.07), higher life satisfaction (pooled AME, 0.07; 95% CI, 0.05-0.10), and better self-reported health (pooled AME, 0.15; 95% CI, 0.12-0.17).
- Frequent internet users showed better mental health outcomes than nonusers, and daily internet users showed significant improvements in depressive symptoms and self-reported health in England and the United States.
- Each additional wave of internet use was associated with reduced depressive symptoms (pooled AME, –0.06; 95% CI, –0.09 to –0.04) and improved life satisfaction (pooled AME, 0.05; 95% CI, 0.03-0.07).
- Benefits of internet use were observed across all genetic risk categories for depression in England and the United States, suggesting potential utility regardless of genetic predisposition.
IN PRACTICE:
“Our findings are relevant to public health policies and practices in promoting mental health in later life through the internet, especially in countries with limited internet access and mental health services,” the investigators wrote.
SOURCE:
The study was led by Yan Luo, Department of Data Science, City University of Hong Kong, Hong Kong, China. It was published online November 18 in Nature Human Behaviour.
LIMITATIONS:
The possibility of residual confounding and reverse causation prevented the establishment of direct causality between internet use and mental health. Selection bias may have also existed due to differences in baseline characteristics between the analytic samples and entire populations. Internet use was assessed through self-reported items, which could have led to recall and information bias. Additionally, genetic data were available for participants only from England and the United States.
DISCLOSURES:
The study was funded in part by the National Natural Science Foundation of China. The investigators reported no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
and better self-reported health among adults aged 50 years or older across 23 countries than nonuse, a new cohort study suggests.
METHODOLOGY:
- Data were examined for more than 87,000 adults aged 50 years or older across 23 countries and from six aging cohorts.
- Researchers examined the potential association between internet use and mental health outcomes, including depressive symptoms, life satisfaction, and self-reported health.
- Polygenic scores were used for subset analysis to stratify participants from England and the United States according to their genetic risk for depression.
- Participants were followed up for a median of 6 years.
TAKEAWAY:
- Internet use was linked to consistent benefits across countries, including lower depressive symptoms (pooled average marginal effect [AME], –0.09; 95% CI, –0.12 to –0.07), higher life satisfaction (pooled AME, 0.07; 95% CI, 0.05-0.10), and better self-reported health (pooled AME, 0.15; 95% CI, 0.12-0.17).
- Frequent internet users showed better mental health outcomes than nonusers, and daily internet users showed significant improvements in depressive symptoms and self-reported health in England and the United States.
- Each additional wave of internet use was associated with reduced depressive symptoms (pooled AME, –0.06; 95% CI, –0.09 to –0.04) and improved life satisfaction (pooled AME, 0.05; 95% CI, 0.03-0.07).
- Benefits of internet use were observed across all genetic risk categories for depression in England and the United States, suggesting potential utility regardless of genetic predisposition.
IN PRACTICE:
“Our findings are relevant to public health policies and practices in promoting mental health in later life through the internet, especially in countries with limited internet access and mental health services,” the investigators wrote.
SOURCE:
The study was led by Yan Luo, Department of Data Science, City University of Hong Kong, Hong Kong, China. It was published online November 18 in Nature Human Behaviour.
LIMITATIONS:
The possibility of residual confounding and reverse causation prevented the establishment of direct causality between internet use and mental health. Selection bias may have also existed due to differences in baseline characteristics between the analytic samples and entire populations. Internet use was assessed through self-reported items, which could have led to recall and information bias. Additionally, genetic data were available for participants only from England and the United States.
DISCLOSURES:
The study was funded in part by the National Natural Science Foundation of China. The investigators reported no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
GLP-1s Hold Promise for Addiction but Questions Remain
Glucagon-like peptide 1 receptor agonist (GLP-1) prescriptions for diabetes and obesity treatment are soaring, as is the interest in their potential for treating an array of other conditions. One area in particular is addiction, which, like obesity and diabetes, has been increasing, both in terms of case numbers and deaths from drug overdose, excessive alcohol use, and tobacco/e-cigarettes.
“The evidence is very preliminary and very exciting,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse (NIDA). “The studies have been going on for more than a decade looking at the effects of GLP medications, mostly first generation and predominantly in rodents,” she said.
GLP “drugs like exenatide and liraglutide all reduced consumption of nicotine, of alcohol, of cocaine, and response to opioids,” Volkow said.
Clinical, Real-World Data Promising
Second-generation agents like semaglutide appear to hold greater promise than their first-generation counterparts. Volkow noted that not only is semaglutide a “much more potent drug,” but pointed to recent findings that saw significant declines in heavy drinking days among patients with alcohol use disorder (AUD).
At the Research Society on Alcohol’s annual meeting in June, researchers from the University of North Carolina at Chapel Hill presented findings of a 2-month, phase 2, randomized clinical trial comparing two low doses (0.25 mg/wk, 0.5 mg/wk) of semaglutide with placebo in 48 participants reporting symptoms of AUD. Though preliminary and unpublished, the data showed a reduction in drinking quantity and heavy drinking in the semaglutide vs placebo groups.
Real-world evidence from electronic health records has also underscored the potential benefit of semaglutide in AUD. In a 12-month retrospective cohort analysis of the records of patients with obesity and no prior AUD diagnosis prescribed semaglutide (n = 45,797) or non-GLP-1 anti-obesity medications (naltrexone, topiramate, n = 38,028), semaglutide was associated with a 50% lower risk for a recurrent AUD diagnosis and a 56% significantly lower risk for incidence AUD diagnosis across gender, age group, and race, and in patients with/without type 2 diabetes.
Likewise, findings from another cohort analysis assigned 1306 treatment-naive patients with type 2 diabetes and no prior AUD diagnosis to semaglutide or non-GLP-1 anti-diabetes medications and followed them for 12 months. Compared with people prescribed non-GLP-1 diabetes medications, those who took semaglutide had a 42% lower risk for recurrent alcohol use diagnosis, consistent across gender, age group, and race, whether the person had been diagnosed with obesity.
However, AUD is not the only addiction where semaglutide appears to have potential benefit. Cohort studies conducted by Volkow and her colleagues have suggested as much as a 78% reduced risk or opioid overdose in patients with comorbid obesity and type 2 diabetes) and a 44% reduction in cannabis use disorder in type 2 diabetes patients without a prior cannabis use disorder history.
Unclear Mechanisms, Multiple Theories
It’s not entirely clear how semaglutide provides a path for addicts to reduce their cravings or which patients might benefit most.
Preclinical studies have suggested that GLP-1 receptors are expressed throughout the mesolimbic dopamine system and transmit dopamine directly to reward centers in the forebrain, for example, the nucleus accumbens. The drugs appear to reduce dopamine release and transmission to these reward centers, as well as to areas that are responsible for impulse control.
“What we’re seeing is counteracting mechanisms that allow you to self-regulate are also involved in addiction, but I don’t know to what extent these medications could help strengthen that,” said Volkow.
Henry Kranzler, MD, professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia, has a paper in press looking at genetic correlation between body mass index (BMI) and AUD. “Genetic analysis showed that many of the same genes are working in both disorders but in opposite directions,” he said.
The bottom line is that “they share genetics, but by no means are they the same; this gives us reason to believe that the GLP-1s could be beneficial in obesity but not nearly as beneficial for treating addiction,” said Kranzler.
Behind Closed Doors
Like many people with overweight or obesity who are on semaglutide, Bridget Pilloud, a writer who divides her time between Washington State and Arizona, no longer has any desire to drink.
“I used to really enjoy sitting and slowly sipping an Old Fashioned. I used to really enjoy specific whiskeys. Now, I don’t even like the flavor; the pleasure of drinking is gone,” she said.
Inexplicably, Pilloud said that she’s also given up compulsive shopping; “The hunt and acquisition of it was always really delicious to me,” she said.
Pilloud’s experience is not unique. Angela Fitch, MD, an obesity medicine specialist, co-founder and CMO of knownwell health, and former president of the Obesity Medicine Association, has had patients on semaglutide tell her that they’re not shopping as much.
But self-reports about alcohol consumption are far more common.
A 2023 analysis of social media posts reinforced that the experience is quite common, albeit self-reported.
Researchers used machine learning attribution mapping of 68,250 posts related to GLP-1 or GLP-1/glucose-dependent insulinotropic polypeptide agonists on the Reddit platform. Among the 1580 alcohol-related posts, 71% (1134/1580) of users of either drug said they had reduced cravings and decreased desire to drink. In a remote companion study of 153 people with obesity taking semaglutide (n = 56), tirzepatide (n = 50), or neither (n = 47), there appeared to be a reduced suppression of the desire to consume alcohol, with users reporting fewer drinks and binge episodes than control individuals.
Self-reports also underscored the association between either of the medications and less stimulating/sedative effects of alcohol compared with before starting the medications and to controls.
Behind closed doors, there appears to be as much chatter about the potential of these agents for AUD and other addiction disorders as there are questions about factors like treatment duration, safety of long-term, chronic use, and dosage.
“We don’t have data around people with normal weight and how much risk that is to them if they start taking these medications for addiction and reduce their BMI as low as 18,” said Fitch.
There’s also the question of when and how to wean patients off the medications, a consideration that is quite important for patients with addiction problems, said Volkow.
“What happens when you become addicted to drugs is that you start to degrade social support systems needed for well-being,” she explained. “The big difference with drugs versus foods is that you can live happily with no drugs at all, whereas you die if you don’t eat. So, there are greater challenges in the ability to change the environment (eg, help stabilize everyday life so people have alternative reinforcers) when you remove the reward.”
Additional considerations range from overuse and the development of treatment-resistant obesity to the need to ensure that patients on these drugs receive ongoing management and, of course, access, noted Fitch.
Still, the NIDA coffers are open. “We’re waiting for proposals,” said Volkow.
Fitch is cofounder and CMO of knownwell health. Volkow reported no relevant financial relationships. Kranzler is a member of advisory boards for Altimmune, Clearmind Medicine, Dicerna Pharmaceuticals, Enthion Pharmaceuticals, Eli Lilly and Company, and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharma and Altimmune; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the past 3 years by Alkermes, Dicerna Pharmaceuticals, Ethypharm, Imbrium, Indivior, Kinnov, Eli Lilly, Otsuka, and Pear; and a holder of US patent 10,900,082 titled: “Genotype-guided dosing of opioid agonists,” issued on January 26, 2021.
A version of this article appeared on Medscape.com.
Glucagon-like peptide 1 receptor agonist (GLP-1) prescriptions for diabetes and obesity treatment are soaring, as is the interest in their potential for treating an array of other conditions. One area in particular is addiction, which, like obesity and diabetes, has been increasing, both in terms of case numbers and deaths from drug overdose, excessive alcohol use, and tobacco/e-cigarettes.
“The evidence is very preliminary and very exciting,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse (NIDA). “The studies have been going on for more than a decade looking at the effects of GLP medications, mostly first generation and predominantly in rodents,” she said.
GLP “drugs like exenatide and liraglutide all reduced consumption of nicotine, of alcohol, of cocaine, and response to opioids,” Volkow said.
Clinical, Real-World Data Promising
Second-generation agents like semaglutide appear to hold greater promise than their first-generation counterparts. Volkow noted that not only is semaglutide a “much more potent drug,” but pointed to recent findings that saw significant declines in heavy drinking days among patients with alcohol use disorder (AUD).
At the Research Society on Alcohol’s annual meeting in June, researchers from the University of North Carolina at Chapel Hill presented findings of a 2-month, phase 2, randomized clinical trial comparing two low doses (0.25 mg/wk, 0.5 mg/wk) of semaglutide with placebo in 48 participants reporting symptoms of AUD. Though preliminary and unpublished, the data showed a reduction in drinking quantity and heavy drinking in the semaglutide vs placebo groups.
Real-world evidence from electronic health records has also underscored the potential benefit of semaglutide in AUD. In a 12-month retrospective cohort analysis of the records of patients with obesity and no prior AUD diagnosis prescribed semaglutide (n = 45,797) or non-GLP-1 anti-obesity medications (naltrexone, topiramate, n = 38,028), semaglutide was associated with a 50% lower risk for a recurrent AUD diagnosis and a 56% significantly lower risk for incidence AUD diagnosis across gender, age group, and race, and in patients with/without type 2 diabetes.
Likewise, findings from another cohort analysis assigned 1306 treatment-naive patients with type 2 diabetes and no prior AUD diagnosis to semaglutide or non-GLP-1 anti-diabetes medications and followed them for 12 months. Compared with people prescribed non-GLP-1 diabetes medications, those who took semaglutide had a 42% lower risk for recurrent alcohol use diagnosis, consistent across gender, age group, and race, whether the person had been diagnosed with obesity.
However, AUD is not the only addiction where semaglutide appears to have potential benefit. Cohort studies conducted by Volkow and her colleagues have suggested as much as a 78% reduced risk or opioid overdose in patients with comorbid obesity and type 2 diabetes) and a 44% reduction in cannabis use disorder in type 2 diabetes patients without a prior cannabis use disorder history.
Unclear Mechanisms, Multiple Theories
It’s not entirely clear how semaglutide provides a path for addicts to reduce their cravings or which patients might benefit most.
Preclinical studies have suggested that GLP-1 receptors are expressed throughout the mesolimbic dopamine system and transmit dopamine directly to reward centers in the forebrain, for example, the nucleus accumbens. The drugs appear to reduce dopamine release and transmission to these reward centers, as well as to areas that are responsible for impulse control.
“What we’re seeing is counteracting mechanisms that allow you to self-regulate are also involved in addiction, but I don’t know to what extent these medications could help strengthen that,” said Volkow.
Henry Kranzler, MD, professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia, has a paper in press looking at genetic correlation between body mass index (BMI) and AUD. “Genetic analysis showed that many of the same genes are working in both disorders but in opposite directions,” he said.
The bottom line is that “they share genetics, but by no means are they the same; this gives us reason to believe that the GLP-1s could be beneficial in obesity but not nearly as beneficial for treating addiction,” said Kranzler.
Behind Closed Doors
Like many people with overweight or obesity who are on semaglutide, Bridget Pilloud, a writer who divides her time between Washington State and Arizona, no longer has any desire to drink.
“I used to really enjoy sitting and slowly sipping an Old Fashioned. I used to really enjoy specific whiskeys. Now, I don’t even like the flavor; the pleasure of drinking is gone,” she said.
Inexplicably, Pilloud said that she’s also given up compulsive shopping; “The hunt and acquisition of it was always really delicious to me,” she said.
Pilloud’s experience is not unique. Angela Fitch, MD, an obesity medicine specialist, co-founder and CMO of knownwell health, and former president of the Obesity Medicine Association, has had patients on semaglutide tell her that they’re not shopping as much.
But self-reports about alcohol consumption are far more common.
A 2023 analysis of social media posts reinforced that the experience is quite common, albeit self-reported.
Researchers used machine learning attribution mapping of 68,250 posts related to GLP-1 or GLP-1/glucose-dependent insulinotropic polypeptide agonists on the Reddit platform. Among the 1580 alcohol-related posts, 71% (1134/1580) of users of either drug said they had reduced cravings and decreased desire to drink. In a remote companion study of 153 people with obesity taking semaglutide (n = 56), tirzepatide (n = 50), or neither (n = 47), there appeared to be a reduced suppression of the desire to consume alcohol, with users reporting fewer drinks and binge episodes than control individuals.
Self-reports also underscored the association between either of the medications and less stimulating/sedative effects of alcohol compared with before starting the medications and to controls.
Behind closed doors, there appears to be as much chatter about the potential of these agents for AUD and other addiction disorders as there are questions about factors like treatment duration, safety of long-term, chronic use, and dosage.
“We don’t have data around people with normal weight and how much risk that is to them if they start taking these medications for addiction and reduce their BMI as low as 18,” said Fitch.
There’s also the question of when and how to wean patients off the medications, a consideration that is quite important for patients with addiction problems, said Volkow.
“What happens when you become addicted to drugs is that you start to degrade social support systems needed for well-being,” she explained. “The big difference with drugs versus foods is that you can live happily with no drugs at all, whereas you die if you don’t eat. So, there are greater challenges in the ability to change the environment (eg, help stabilize everyday life so people have alternative reinforcers) when you remove the reward.”
Additional considerations range from overuse and the development of treatment-resistant obesity to the need to ensure that patients on these drugs receive ongoing management and, of course, access, noted Fitch.
Still, the NIDA coffers are open. “We’re waiting for proposals,” said Volkow.
Fitch is cofounder and CMO of knownwell health. Volkow reported no relevant financial relationships. Kranzler is a member of advisory boards for Altimmune, Clearmind Medicine, Dicerna Pharmaceuticals, Enthion Pharmaceuticals, Eli Lilly and Company, and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharma and Altimmune; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the past 3 years by Alkermes, Dicerna Pharmaceuticals, Ethypharm, Imbrium, Indivior, Kinnov, Eli Lilly, Otsuka, and Pear; and a holder of US patent 10,900,082 titled: “Genotype-guided dosing of opioid agonists,” issued on January 26, 2021.
A version of this article appeared on Medscape.com.
Glucagon-like peptide 1 receptor agonist (GLP-1) prescriptions for diabetes and obesity treatment are soaring, as is the interest in their potential for treating an array of other conditions. One area in particular is addiction, which, like obesity and diabetes, has been increasing, both in terms of case numbers and deaths from drug overdose, excessive alcohol use, and tobacco/e-cigarettes.
“The evidence is very preliminary and very exciting,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse (NIDA). “The studies have been going on for more than a decade looking at the effects of GLP medications, mostly first generation and predominantly in rodents,” she said.
GLP “drugs like exenatide and liraglutide all reduced consumption of nicotine, of alcohol, of cocaine, and response to opioids,” Volkow said.
Clinical, Real-World Data Promising
Second-generation agents like semaglutide appear to hold greater promise than their first-generation counterparts. Volkow noted that not only is semaglutide a “much more potent drug,” but pointed to recent findings that saw significant declines in heavy drinking days among patients with alcohol use disorder (AUD).
At the Research Society on Alcohol’s annual meeting in June, researchers from the University of North Carolina at Chapel Hill presented findings of a 2-month, phase 2, randomized clinical trial comparing two low doses (0.25 mg/wk, 0.5 mg/wk) of semaglutide with placebo in 48 participants reporting symptoms of AUD. Though preliminary and unpublished, the data showed a reduction in drinking quantity and heavy drinking in the semaglutide vs placebo groups.
Real-world evidence from electronic health records has also underscored the potential benefit of semaglutide in AUD. In a 12-month retrospective cohort analysis of the records of patients with obesity and no prior AUD diagnosis prescribed semaglutide (n = 45,797) or non-GLP-1 anti-obesity medications (naltrexone, topiramate, n = 38,028), semaglutide was associated with a 50% lower risk for a recurrent AUD diagnosis and a 56% significantly lower risk for incidence AUD diagnosis across gender, age group, and race, and in patients with/without type 2 diabetes.
Likewise, findings from another cohort analysis assigned 1306 treatment-naive patients with type 2 diabetes and no prior AUD diagnosis to semaglutide or non-GLP-1 anti-diabetes medications and followed them for 12 months. Compared with people prescribed non-GLP-1 diabetes medications, those who took semaglutide had a 42% lower risk for recurrent alcohol use diagnosis, consistent across gender, age group, and race, whether the person had been diagnosed with obesity.
However, AUD is not the only addiction where semaglutide appears to have potential benefit. Cohort studies conducted by Volkow and her colleagues have suggested as much as a 78% reduced risk or opioid overdose in patients with comorbid obesity and type 2 diabetes) and a 44% reduction in cannabis use disorder in type 2 diabetes patients without a prior cannabis use disorder history.
Unclear Mechanisms, Multiple Theories
It’s not entirely clear how semaglutide provides a path for addicts to reduce their cravings or which patients might benefit most.
Preclinical studies have suggested that GLP-1 receptors are expressed throughout the mesolimbic dopamine system and transmit dopamine directly to reward centers in the forebrain, for example, the nucleus accumbens. The drugs appear to reduce dopamine release and transmission to these reward centers, as well as to areas that are responsible for impulse control.
“What we’re seeing is counteracting mechanisms that allow you to self-regulate are also involved in addiction, but I don’t know to what extent these medications could help strengthen that,” said Volkow.
Henry Kranzler, MD, professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia, has a paper in press looking at genetic correlation between body mass index (BMI) and AUD. “Genetic analysis showed that many of the same genes are working in both disorders but in opposite directions,” he said.
The bottom line is that “they share genetics, but by no means are they the same; this gives us reason to believe that the GLP-1s could be beneficial in obesity but not nearly as beneficial for treating addiction,” said Kranzler.
Behind Closed Doors
Like many people with overweight or obesity who are on semaglutide, Bridget Pilloud, a writer who divides her time between Washington State and Arizona, no longer has any desire to drink.
“I used to really enjoy sitting and slowly sipping an Old Fashioned. I used to really enjoy specific whiskeys. Now, I don’t even like the flavor; the pleasure of drinking is gone,” she said.
Inexplicably, Pilloud said that she’s also given up compulsive shopping; “The hunt and acquisition of it was always really delicious to me,” she said.
Pilloud’s experience is not unique. Angela Fitch, MD, an obesity medicine specialist, co-founder and CMO of knownwell health, and former president of the Obesity Medicine Association, has had patients on semaglutide tell her that they’re not shopping as much.
But self-reports about alcohol consumption are far more common.
A 2023 analysis of social media posts reinforced that the experience is quite common, albeit self-reported.
Researchers used machine learning attribution mapping of 68,250 posts related to GLP-1 or GLP-1/glucose-dependent insulinotropic polypeptide agonists on the Reddit platform. Among the 1580 alcohol-related posts, 71% (1134/1580) of users of either drug said they had reduced cravings and decreased desire to drink. In a remote companion study of 153 people with obesity taking semaglutide (n = 56), tirzepatide (n = 50), or neither (n = 47), there appeared to be a reduced suppression of the desire to consume alcohol, with users reporting fewer drinks and binge episodes than control individuals.
Self-reports also underscored the association between either of the medications and less stimulating/sedative effects of alcohol compared with before starting the medications and to controls.
Behind closed doors, there appears to be as much chatter about the potential of these agents for AUD and other addiction disorders as there are questions about factors like treatment duration, safety of long-term, chronic use, and dosage.
“We don’t have data around people with normal weight and how much risk that is to them if they start taking these medications for addiction and reduce their BMI as low as 18,” said Fitch.
There’s also the question of when and how to wean patients off the medications, a consideration that is quite important for patients with addiction problems, said Volkow.
“What happens when you become addicted to drugs is that you start to degrade social support systems needed for well-being,” she explained. “The big difference with drugs versus foods is that you can live happily with no drugs at all, whereas you die if you don’t eat. So, there are greater challenges in the ability to change the environment (eg, help stabilize everyday life so people have alternative reinforcers) when you remove the reward.”
Additional considerations range from overuse and the development of treatment-resistant obesity to the need to ensure that patients on these drugs receive ongoing management and, of course, access, noted Fitch.
Still, the NIDA coffers are open. “We’re waiting for proposals,” said Volkow.
Fitch is cofounder and CMO of knownwell health. Volkow reported no relevant financial relationships. Kranzler is a member of advisory boards for Altimmune, Clearmind Medicine, Dicerna Pharmaceuticals, Enthion Pharmaceuticals, Eli Lilly and Company, and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharma and Altimmune; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the past 3 years by Alkermes, Dicerna Pharmaceuticals, Ethypharm, Imbrium, Indivior, Kinnov, Eli Lilly, Otsuka, and Pear; and a holder of US patent 10,900,082 titled: “Genotype-guided dosing of opioid agonists,” issued on January 26, 2021.
A version of this article appeared on Medscape.com.
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
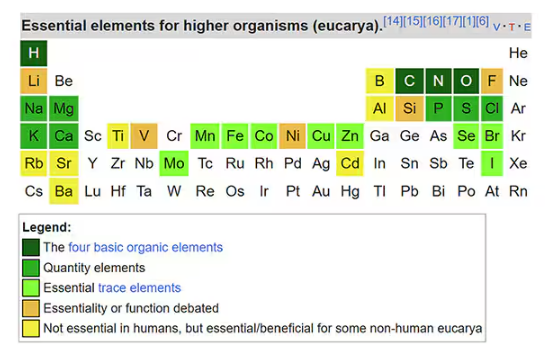
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
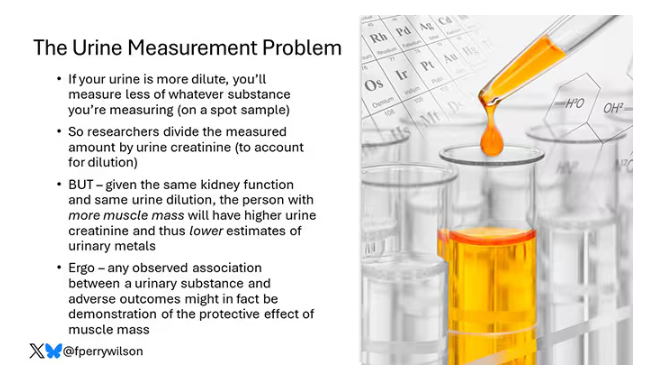
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
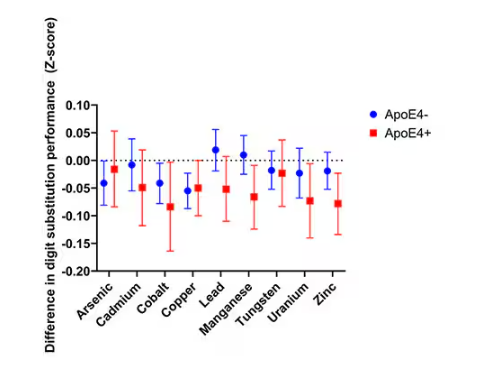
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
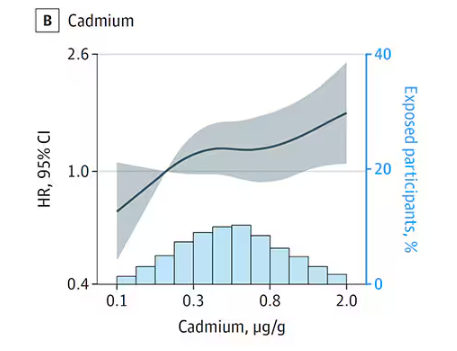
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
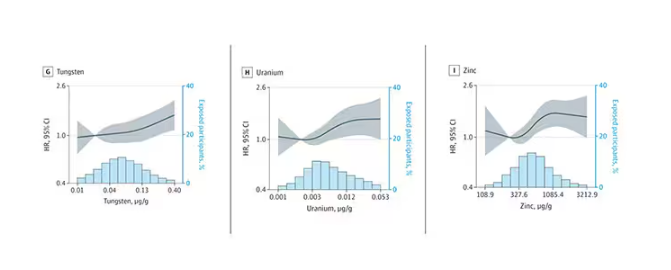
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
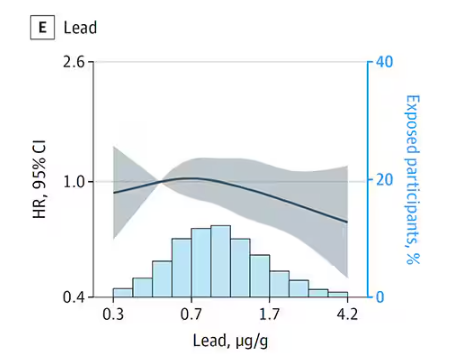
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
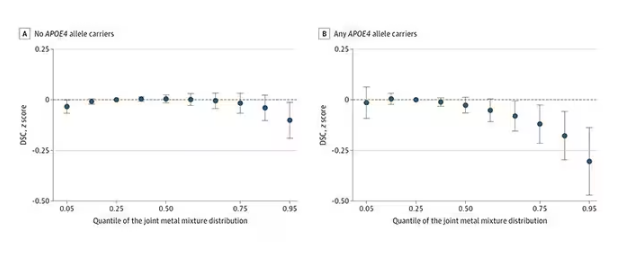
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Watch That Attitude: Is There Ageism in Healthcare?
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
Microplastics Have Been Found in the Human Brain. Now What?
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New Data: The Most Promising Treatments for Long COVID
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Long COVID is a symptom-driven disease, meaning that with no cure, physicians primarily treat the symptoms their patients are experiencing. But as 2024 winds down, researchers have begun to pinpoint a number of treatments that are bringing relief to the 17 million Americans diagnosed with long COVID.
Here’s a current look at what research has identified as some of the most promising treatments.
Low-Dose Naltrexone
Some research suggests that low-dose naltrexone may be helpful for patients suffering from brain fog, pain, sleep issues, and fatigue, said Ziyad Al-Aly, MD, a global expert on long COVID and chief of research and development at the Veterans Affairs St Louis Health Care System in Missouri.
Low-dose naltrexone is an anti-inflammatory agent currently approved by the Food and Drug Administration for the treatment of alcohol and opioid dependence.
“We don’t know the mechanism for how the medication works, and for that matter, we don’t really understand what causes brain fog. But perhaps its anti-inflammatory properties seem to help, and for some patients, low-dose naltrexone has been helpful,” said Al-Aly.
A March 2024 study found that both fatigue and pain were improved in patients taking low-dose naltrexone. In another study, published in the June 2024 issue of Frontiers in Medicine, researchers found that low-dose naltrexone was associated with improvement of several clinical symptoms related to long COVID such as fatigue, poor sleep quality, brain fog, post-exertional malaise, and headache.
Selective Serotonin Reuptake Inhibitors (SSRIs) and Antidepressants
In 2023, University of Pennsylvania researchers uncovered a link between long COVID and lower levels of serotonin in the body. This helped point to the potential treatment of using SSRIs to treat the condition.
For patients who have overlapping psychiatric issues that go along with brain fog, SSRIs prescribed to treat depression and other mental health conditions, as well as the antidepressant Wellbutrin, have been shown effective at dealing with concentration issues, brain fog, and depression, said Nisha Viswanathan, MD, director of the University of California, Los Angeles (UCLA) Long COVID Program at UCLA Health.
A study published in the November 2023 issue of the journal Scientific Reports found that SSRIs led to a “considerable reduction of symptoms,” especially brain fog, fatigue, sensory overload, and overall improved functioning. Low-dose Abilify, which contains aripiprazole, an antipsychotic medication, has also been found to be effective for cognitive issues caused by long COVID.
“Abilify is traditionally used for the treatment of schizophrenia or other psychotic disorders, but in a low-dose format, there is some data to suggest that it can also be anti-inflammatory and helpful for cognitive issues like brain fog,” said Viswanathan.
Modafinil
Modafinil, a medication previously used for managing narcolepsy, has also been shown effective for the treatment of fatigue and neurocognitive deficits caused by long COVID, said Viswanathan, adding that it’s another medication that she’s found useful for a number of her patients.
It’s thought that these cognitive symptoms are caused by an inflammatory cytokine release that leads to excessive stimulation of neurotransmitters in the body. According to a June 2024 article in the American Journal of Psychiatry, “Modafinil can therapeutically act on these pathways, which possibly contributed to the symptomatic improvement.” But the medication has not been studied widely in patients with long COVID and has been shown to have interactions with other medications.
Metformin
Some research has shown that metformin, a well-known diabetes medication, reduces instances of long COVID when taken during the illness’s acute phase. It seems to boost metabolic function in patients.
“It makes sense that it would work because it seems to have anti-inflammatory effects on the body,” said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland, Ohio. McComsey added that it may reduce the viral persistence that causes some forms of long COVID.
A study published in the October 2023 issue of the journal The Lancet Infectious Diseases found that metformin seemed to reduce instances of long COVID in patients who took it after being diagnosed with acute COVID. It seems less effective in patients who already have long COVID.
Antihistamines
Other data suggest that some patients with long COVID showed improvement after taking antihistamines. Research has shown that long COVID symptoms improved in 29% of patients with long COVID.
While researchers aren’t sure why antihistamines work to quell long COVID, the thought is that, when mast cells, a white blood cell that’s part of the immune system, shed granules and cause an inflammatory reaction, they release a lot of histamines. Antihistamine medications like famotidine block histamine receptors in the body, improving symptoms like brain fog, difficulty breathing, and elevated heart rate in patients.
“For some patients, these can be a lifesaver,” said David Putrino, the Nash Family Director of the Cohen Center for Recovery from Complex Chronic Illness and a national leader in the treatment of long COVID.
Putrino cautions patients toward taking these and other medications haphazardly without fully understanding that all treatments have risks, especially if they’re taking a number of them.
“Often patients are told that there’s no risk to trying something, but physicians should be counseling their patients and reminding them that there is a risk that includes medication sensitivities and medication interactions,” said Putrino.
The good news is that doctors have begun to identify some treatments that seem to be working in their patients, but we still don’t have the large-scale clinical trials to identify which treatments will work for certain patients and why.
There’s still so much we don’t know, and for physicians on the front lines of treating long COVID, it’s still largely a guessing game. “This is a constellation of symptoms; it’s not just one thing,” said Al-Aly. And while a treatment might be wildly effective for one patient, it might be ineffective or worse, problematic, for another.
A version of this article first appeared on Medscape.com.
Winter Depression: How to Make the ‘SAD’ Diagnosis
’Tis the season for recognizing seasonal affective disorder (SAD). Just don’t expect to find SAD in diagnostic handbooks.
As a memorable term, SAD “stuck in the general public, and to some extent among health professionals,” said Scott Patten, MD, PhD, professor of psychiatry and epidemiology at the University of Calgary in Alberta, Canada. “But it’s important to emphasize that that’s not an officially recognized diagnosis by the major classifications.”
Researchers coined the term SAD 40 years ago to describe a pattern of depression that sets in during the fall or winter and remits in the spring or summer.
Clinicians are diagnosing the disorder, albeit without that exact moniker.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the condition is considered a subtype of major depression.
So, for patients who meet criteria for recurrent major depressive disorder, the specifier “with seasonal pattern” might be applied.
The subtype covers cases where depressive episodes have followed a seasonal pattern for at least 2 years. Typically, onset occurs in the fall or winter followed by remission in the spring or summer. The opposite pattern is possible but less common.
When stressors such as seasonal unemployment better explain the pattern, the seasonal specifier should not be used, according to the manual. Bipolar disorder can follow a seasonal pattern as well.
Researchers estimate SAD affects about 5% of adults in the United States. The diagnosis is more common in women than in men, and more prevalent farther from the equator.
One Hallmark Symptom?
DSM-5 highlights characteristic features of winter depression, including:
- Loss of energy
- Hypersomnia
- A craving for carbohydrates
- Overeating
- Weight gain
Kelly Rohan, PhD, a researcher at the University of Vermont, Burlington, who has studied SAD since the 1990s, sees one symptom as a possible hallmark for the disorder: fatigue.
“I’ve personally never met someone who met the full diagnostic criteria for the seasonal pattern that did not have fatigue as one of their symptoms,” Rohan said. “In theory, they could exist, but I have spoken to hundreds of people with seasonal depression, and I have never met them if, in fact, they do exist.”
That differs from nonseasonal depression, for which insomnia is a more common problem with sleep, Patten said.
Clinicians look for at least five symptoms of depression that cause substantial impairment and distress for at least 2 weeks, such as pervasive sadness, difficulty concentrating, low self-esteem, or loss of interest in hobbies.
An average episode of winter depression can last 5 months, however, Rohan said. “That’s a long time to be in a major depressive episode.”
Seeing Subsyndromal Cases
In people who do not meet criteria for major depression with a seasonal pattern, the change of seasons still can affect energy levels and mood. Some patients have “subsyndromal SAD” and may benefit from treatments that have been developed for SAD such as bright light therapy, said Paul Desan, MD, PhD, director of the Winter Depression Research Clinic at Yale School of Medicine in New Haven, Connecticut.
“Many people come to our clinic because they have seasonal changes that don’t meet the full criteria for depression, but nevertheless, they want help,” Desan said.
The 1984 paper that introduced the term SAD explored artificial bright light as a promising treatment for the condition. The researchers had heard from dozens of patients with “recurrent depressions that occur annually at the same time each year,” and bright light appeared to help alleviate their symptoms.
Subsequent trials have found the approach effective. Even in nonseasonal depression, bright light therapy may increase the likelihood of remission, a recent meta-analysis found. Light therapy also may bolster the effectiveness of antidepressant medication in nonseasonal major depressive disorder, a randomized trial has shown.
Other treatments for SAD include cognitive behavioral therapy (CBT) and bupropion XL, which is approved as a preventive medication. Other drugs for major depressive disorder may be used.
Quest for Biomarkers
To better understand SAD and how available treatments work, Rohan is conducting a study that examines potential biomarkers in patients treated with light therapy or CBT. She and her colleagues are examining circadian phase angle difference (how well internal clocks match daily routines) and post-illumination pupil response (how the pupil constricts after a light turns off). They also are measuring participants’ pupil responses and brain activity upon seeing words that are associated with winter or summer (like “blizzard,” “icy,” “sunshine,” and “picnics.”)
Studies have shown treating patients to remission with CBT reduces the risk for recurrence in subsequent years, relative to other treatment approaches, Rohan said. That may be because CBT gives people tools to avoid slipping into another depressive episode.
Avoid Self-Diagnosis
Rohan cautions patients against self-diagnosis and treatment.
“Having a conversation with your doctor is a good starting point,” she said. “Just because you can walk into Costco and walk out with a light box doesn’t mean that you should.”
Light therapy can have side effects, including headaches, eye strain, and making patients feel wired, and it can be a challenge to determine the right dose, Rohan said.
Desan’s clinic website provides information about available devices for light therapy for patients who are looking to try this approach, but Desan agrees clinicians — especially primary care clinicians — can play a crucial role in helping patients. In more serious cases, a mental health expert may be necessary.
To start light therapy, Desan’s clinic typically recommends patients try 30 minutes of 10,000 lux bright light — roughly the brightness of being outside on a sunny day — before 8 AM for a 4-week trial.
Still, other specific issues might explain why a patient is struggling during winter months, Patten said. For example, people might experience financial stress around the holidays or consume excessive amounts of alcohol during that time.
“It’s important for clinicians to think broadly about it,” Patten said. “It might not always be light therapy or a medication. It might be focusing on some other aspect of what is going on for them in the winter.”
Rohan’s research is funded by the National Institute of Mental Health, and she receives royalties for a manual on treating SAD with CBT. Patten and Desan had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
’Tis the season for recognizing seasonal affective disorder (SAD). Just don’t expect to find SAD in diagnostic handbooks.
As a memorable term, SAD “stuck in the general public, and to some extent among health professionals,” said Scott Patten, MD, PhD, professor of psychiatry and epidemiology at the University of Calgary in Alberta, Canada. “But it’s important to emphasize that that’s not an officially recognized diagnosis by the major classifications.”
Researchers coined the term SAD 40 years ago to describe a pattern of depression that sets in during the fall or winter and remits in the spring or summer.
Clinicians are diagnosing the disorder, albeit without that exact moniker.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the condition is considered a subtype of major depression.
So, for patients who meet criteria for recurrent major depressive disorder, the specifier “with seasonal pattern” might be applied.
The subtype covers cases where depressive episodes have followed a seasonal pattern for at least 2 years. Typically, onset occurs in the fall or winter followed by remission in the spring or summer. The opposite pattern is possible but less common.
When stressors such as seasonal unemployment better explain the pattern, the seasonal specifier should not be used, according to the manual. Bipolar disorder can follow a seasonal pattern as well.
Researchers estimate SAD affects about 5% of adults in the United States. The diagnosis is more common in women than in men, and more prevalent farther from the equator.
One Hallmark Symptom?
DSM-5 highlights characteristic features of winter depression, including:
- Loss of energy
- Hypersomnia
- A craving for carbohydrates
- Overeating
- Weight gain
Kelly Rohan, PhD, a researcher at the University of Vermont, Burlington, who has studied SAD since the 1990s, sees one symptom as a possible hallmark for the disorder: fatigue.
“I’ve personally never met someone who met the full diagnostic criteria for the seasonal pattern that did not have fatigue as one of their symptoms,” Rohan said. “In theory, they could exist, but I have spoken to hundreds of people with seasonal depression, and I have never met them if, in fact, they do exist.”
That differs from nonseasonal depression, for which insomnia is a more common problem with sleep, Patten said.
Clinicians look for at least five symptoms of depression that cause substantial impairment and distress for at least 2 weeks, such as pervasive sadness, difficulty concentrating, low self-esteem, or loss of interest in hobbies.
An average episode of winter depression can last 5 months, however, Rohan said. “That’s a long time to be in a major depressive episode.”
Seeing Subsyndromal Cases
In people who do not meet criteria for major depression with a seasonal pattern, the change of seasons still can affect energy levels and mood. Some patients have “subsyndromal SAD” and may benefit from treatments that have been developed for SAD such as bright light therapy, said Paul Desan, MD, PhD, director of the Winter Depression Research Clinic at Yale School of Medicine in New Haven, Connecticut.
“Many people come to our clinic because they have seasonal changes that don’t meet the full criteria for depression, but nevertheless, they want help,” Desan said.
The 1984 paper that introduced the term SAD explored artificial bright light as a promising treatment for the condition. The researchers had heard from dozens of patients with “recurrent depressions that occur annually at the same time each year,” and bright light appeared to help alleviate their symptoms.
Subsequent trials have found the approach effective. Even in nonseasonal depression, bright light therapy may increase the likelihood of remission, a recent meta-analysis found. Light therapy also may bolster the effectiveness of antidepressant medication in nonseasonal major depressive disorder, a randomized trial has shown.
Other treatments for SAD include cognitive behavioral therapy (CBT) and bupropion XL, which is approved as a preventive medication. Other drugs for major depressive disorder may be used.
Quest for Biomarkers
To better understand SAD and how available treatments work, Rohan is conducting a study that examines potential biomarkers in patients treated with light therapy or CBT. She and her colleagues are examining circadian phase angle difference (how well internal clocks match daily routines) and post-illumination pupil response (how the pupil constricts after a light turns off). They also are measuring participants’ pupil responses and brain activity upon seeing words that are associated with winter or summer (like “blizzard,” “icy,” “sunshine,” and “picnics.”)
Studies have shown treating patients to remission with CBT reduces the risk for recurrence in subsequent years, relative to other treatment approaches, Rohan said. That may be because CBT gives people tools to avoid slipping into another depressive episode.
Avoid Self-Diagnosis
Rohan cautions patients against self-diagnosis and treatment.
“Having a conversation with your doctor is a good starting point,” she said. “Just because you can walk into Costco and walk out with a light box doesn’t mean that you should.”
Light therapy can have side effects, including headaches, eye strain, and making patients feel wired, and it can be a challenge to determine the right dose, Rohan said.
Desan’s clinic website provides information about available devices for light therapy for patients who are looking to try this approach, but Desan agrees clinicians — especially primary care clinicians — can play a crucial role in helping patients. In more serious cases, a mental health expert may be necessary.
To start light therapy, Desan’s clinic typically recommends patients try 30 minutes of 10,000 lux bright light — roughly the brightness of being outside on a sunny day — before 8 AM for a 4-week trial.
Still, other specific issues might explain why a patient is struggling during winter months, Patten said. For example, people might experience financial stress around the holidays or consume excessive amounts of alcohol during that time.
“It’s important for clinicians to think broadly about it,” Patten said. “It might not always be light therapy or a medication. It might be focusing on some other aspect of what is going on for them in the winter.”
Rohan’s research is funded by the National Institute of Mental Health, and she receives royalties for a manual on treating SAD with CBT. Patten and Desan had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
’Tis the season for recognizing seasonal affective disorder (SAD). Just don’t expect to find SAD in diagnostic handbooks.
As a memorable term, SAD “stuck in the general public, and to some extent among health professionals,” said Scott Patten, MD, PhD, professor of psychiatry and epidemiology at the University of Calgary in Alberta, Canada. “But it’s important to emphasize that that’s not an officially recognized diagnosis by the major classifications.”
Researchers coined the term SAD 40 years ago to describe a pattern of depression that sets in during the fall or winter and remits in the spring or summer.
Clinicians are diagnosing the disorder, albeit without that exact moniker.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the condition is considered a subtype of major depression.
So, for patients who meet criteria for recurrent major depressive disorder, the specifier “with seasonal pattern” might be applied.
The subtype covers cases where depressive episodes have followed a seasonal pattern for at least 2 years. Typically, onset occurs in the fall or winter followed by remission in the spring or summer. The opposite pattern is possible but less common.
When stressors such as seasonal unemployment better explain the pattern, the seasonal specifier should not be used, according to the manual. Bipolar disorder can follow a seasonal pattern as well.
Researchers estimate SAD affects about 5% of adults in the United States. The diagnosis is more common in women than in men, and more prevalent farther from the equator.
One Hallmark Symptom?
DSM-5 highlights characteristic features of winter depression, including:
- Loss of energy
- Hypersomnia
- A craving for carbohydrates
- Overeating
- Weight gain
Kelly Rohan, PhD, a researcher at the University of Vermont, Burlington, who has studied SAD since the 1990s, sees one symptom as a possible hallmark for the disorder: fatigue.
“I’ve personally never met someone who met the full diagnostic criteria for the seasonal pattern that did not have fatigue as one of their symptoms,” Rohan said. “In theory, they could exist, but I have spoken to hundreds of people with seasonal depression, and I have never met them if, in fact, they do exist.”
That differs from nonseasonal depression, for which insomnia is a more common problem with sleep, Patten said.
Clinicians look for at least five symptoms of depression that cause substantial impairment and distress for at least 2 weeks, such as pervasive sadness, difficulty concentrating, low self-esteem, or loss of interest in hobbies.
An average episode of winter depression can last 5 months, however, Rohan said. “That’s a long time to be in a major depressive episode.”
Seeing Subsyndromal Cases
In people who do not meet criteria for major depression with a seasonal pattern, the change of seasons still can affect energy levels and mood. Some patients have “subsyndromal SAD” and may benefit from treatments that have been developed for SAD such as bright light therapy, said Paul Desan, MD, PhD, director of the Winter Depression Research Clinic at Yale School of Medicine in New Haven, Connecticut.
“Many people come to our clinic because they have seasonal changes that don’t meet the full criteria for depression, but nevertheless, they want help,” Desan said.
The 1984 paper that introduced the term SAD explored artificial bright light as a promising treatment for the condition. The researchers had heard from dozens of patients with “recurrent depressions that occur annually at the same time each year,” and bright light appeared to help alleviate their symptoms.
Subsequent trials have found the approach effective. Even in nonseasonal depression, bright light therapy may increase the likelihood of remission, a recent meta-analysis found. Light therapy also may bolster the effectiveness of antidepressant medication in nonseasonal major depressive disorder, a randomized trial has shown.
Other treatments for SAD include cognitive behavioral therapy (CBT) and bupropion XL, which is approved as a preventive medication. Other drugs for major depressive disorder may be used.
Quest for Biomarkers
To better understand SAD and how available treatments work, Rohan is conducting a study that examines potential biomarkers in patients treated with light therapy or CBT. She and her colleagues are examining circadian phase angle difference (how well internal clocks match daily routines) and post-illumination pupil response (how the pupil constricts after a light turns off). They also are measuring participants’ pupil responses and brain activity upon seeing words that are associated with winter or summer (like “blizzard,” “icy,” “sunshine,” and “picnics.”)
Studies have shown treating patients to remission with CBT reduces the risk for recurrence in subsequent years, relative to other treatment approaches, Rohan said. That may be because CBT gives people tools to avoid slipping into another depressive episode.
Avoid Self-Diagnosis
Rohan cautions patients against self-diagnosis and treatment.
“Having a conversation with your doctor is a good starting point,” she said. “Just because you can walk into Costco and walk out with a light box doesn’t mean that you should.”
Light therapy can have side effects, including headaches, eye strain, and making patients feel wired, and it can be a challenge to determine the right dose, Rohan said.
Desan’s clinic website provides information about available devices for light therapy for patients who are looking to try this approach, but Desan agrees clinicians — especially primary care clinicians — can play a crucial role in helping patients. In more serious cases, a mental health expert may be necessary.
To start light therapy, Desan’s clinic typically recommends patients try 30 minutes of 10,000 lux bright light — roughly the brightness of being outside on a sunny day — before 8 AM for a 4-week trial.
Still, other specific issues might explain why a patient is struggling during winter months, Patten said. For example, people might experience financial stress around the holidays or consume excessive amounts of alcohol during that time.
“It’s important for clinicians to think broadly about it,” Patten said. “It might not always be light therapy or a medication. It might be focusing on some other aspect of what is going on for them in the winter.”
Rohan’s research is funded by the National Institute of Mental Health, and she receives royalties for a manual on treating SAD with CBT. Patten and Desan had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Diabetes Drugs Promising for Alcohol Use Disorder
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.

