User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Many unknowns on fertility preservation in transgender patients
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
‘Striking’ increase in childhood obesity during pandemic
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.
The best exercises for BP control? European statement sorts it out
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Long-haul COVID-19 brings welcome attention to POTS
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.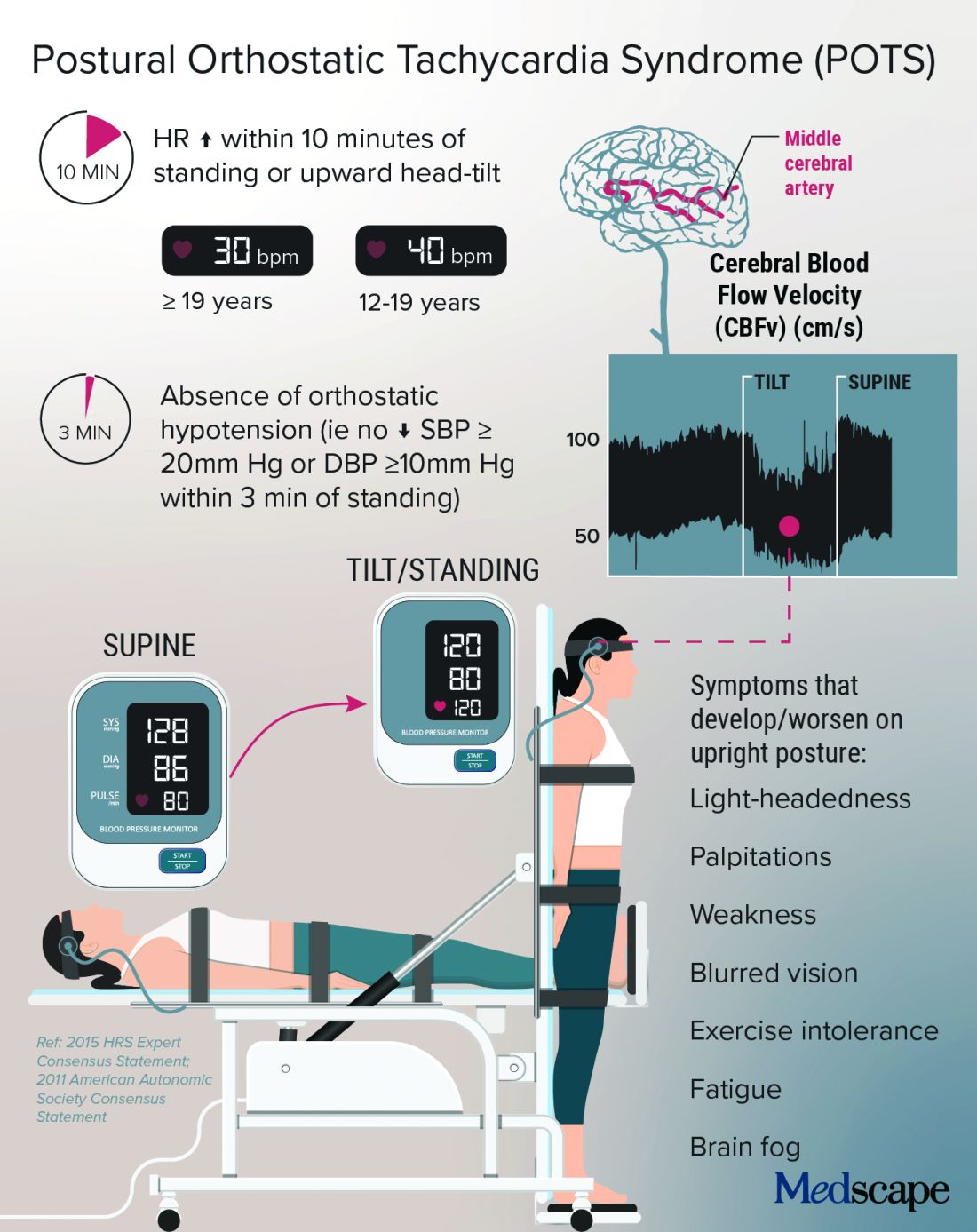
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Step therapy: Inside the fight against insurance companies and fail-first medicine
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
Every day Melissa Fulton, RN, MSN, FNP, APRN-C, shows up to work, she’s ready for another fight. An advanced practice nurse who specializes in multiple sclerosis care, Ms. Fulton said she typically spends more than a third of her time battling it out with insurance companies over drugs she knows her patients need but that insurers don’t want to cover. Instead, they want the patient to first receive less expensive and often less efficacious drugs, even if that goes against recommendations and, in some cases, against the patient’s medical history.
The maddening protocol – familiar to health care providers everywhere – is known as “step therapy.” It forces patients to try alternative medications – medications that often fail – before receiving the one initially prescribed. The process can take weeks or months, which is time that some patients don’t have. Step therapy was sold as a way to lower costs. However, beyond the ethically problematic notion of forcing sick patients to receiver cheaper alternatives that are ineffective, research has also shown it may actually be more costly in the long run.
Ms. Fulton, who works at Saunders Medical Center in Wahoo, Neb., is a veteran in the war against step therapy. She is used to pushing her appeals up the insurance company chain of command, past nonmedical reviewers, until her patient’s case finally lands on the desk of someone with a neurology background. She said that can take three or four appeals – a judge might even get involved – and the patient could still lose. “This happens constantly,” she said, “but we fight like hell.”
Fortunately, life may soon get a little easier for Ms. Fulton. In late March, a bill to restrict step therapy made it through the Nebraska state legislature and is on its way to the governor’s desk. The Step Therapy Reform Act doesn’t outright ban the practice; however, it will put guardrails in place. It requires that insurers respond to appeals within certain time frames, and it creates key exemptions.
When the governor signs off, Nebraska will join more than two dozen other states that already have step therapy restrictions on the books, according to Hannah Lynch, MPS, associate director of federal government relations and health policy at the National Psoriasis Foundation, a leading advocate to reform and protect against the insurance practice. “There’s a lot of frustration out there,” Ms. Lynch said. “It really hinders providers’ ability to make decisions they think will have the best outcomes.”
Driven by coalitions of doctors, nurses, and patients, laws reining in step therapy have been adopted at a relatively quick clip, mostly within the past 5 years. Recent additions include South Dakota and North Carolina, which adopted step therapy laws in 2020, and Arkansas, which passed a law earlier this year.
Ms. Lynch attributed growing support to rising out-of-pocket drug costs and the introduction of biologic drugs, which are often more effective but also more expensive. Like Nebraska’s law, most step therapy reform legislation carves out exemptions and requires timely appeals processes; however, many of the laws still have significant gaps, such as not including certain types of insurance plans.
Ideally, Ms. Lynch said, the protections would apply to all types of health plans that are regulated at the state level, such as Medicaid, state employee health plans, and coverage sold through state insurance exchanges. Closing loopholes in the laws is a top priority for advocates, she added, pointing to work currently underway in Arkansas to extend its new protections to Medicaid expansion patients.
“With so many outside stakeholders, you have to compromise – it’s a give and take,” Ms. Lynch said. Still, when it comes to fighting step therapy, she says, “Any protection on the books is always our first goal when we go into a state.”
Putting patients first
Lisa Arkin, MD, a pediatric dermatologist at the University of Wisconsin–Madison, said she finds herself “swimming upstream every day in the fight with insurance.” Her patients are typically on their second or third stop and have more complex disorders. Dr. Arkin said that the problem with step therapy is that it tries to squeeze all patients into the same box, even if the circumstances don’t fit.
Her state passed restrictions on step therapy in 2019, but the measures only went into effect last year. Under the Wisconsin law, patients can be granted an exemption if an alternative treatment is contraindicated, likely to cause harm, or expected to be ineffective. Patients can also be exempt if their current treatment is working.
Dr. Arkin, an outspoken advocate for curbing step therapy, says the Wisconsin law is “very strong.” However, because it only applies to certain health plans – state employee health plans and those purchased in the state’s health insurance exchange – fewer than half the state’s patients benefit from its protections. She notes that some of the most severe presentations she treats occur in patients who rely on Medicaid coverage and already face barriers to care.
“I’m a doctor who puts up a fuss [with insurers], but that’s not fair – we shouldn’t have to do that,” Dr. Arkin said. “To me, it’s really critical to make this an even playing field so this law affords protection to everyone I see in the clinic.”
Major medical associations caution against step therapy as well. The American Society of Clinical Oncology and the American Medical Association have called out the risks to patient safety and health. In fact, in 2019, after the Centers for Medicare & Medicaid Services gave new authority to Medicare Advantage plans to start using step therapy, dozens of national medical groups called out the agency for allowing a practice that could potentially hurt patients and undercut the physician-patient decision-making process.
Last year, in a new position paper from the American College of Physicians, authors laid out recommendations for combating step therapy’s side effects. These recommendations included making related data transparent to the public and minimizing the policy’s disruptions to care. Jacqueline W. Fincher, MD, MACP, a member of the committee that issued the position paper and who is a primary care physician in Georgia, said such insurance practices need to be designed with “strong input from frontline physicians, not clipboard physicians.
“What we want from insurers is understanding, transparency, and the least burdensome protocol to provide patients the care they need at a cost-effective price they can afford,” said Dr. Fincher, who is also the current president of the ACP. “The focus needs to be on what’s in the patient’s best interest.”
Every year a new fight
“We all dread January,” said Dr. Fincher. That is the worst month, she added, because new health benefits go into effect, which means patients who are responding well to certain treatments may suddenly face new restrictions.
Another aggravating aspect of step therapy? It is often difficult – if not impossible – to access information on specific step therapy protocols in a patient’s health plan in real time in the exam room, where treatment conversations actually take place. In a more patient-centered world, Dr. Fincher said, she would be able to use the electronic health record system to quickly identify whether a patient’s plan covers a particular treatment and, if not, what the alternatives are.
Georgia’s new step therapy law went into effect last year. Like laws in other states, it spells out step therapy exemptions and sets time frames in which insurers must respond to exceptions and appeals. Dr. Fincher, who spoke in favor of the new law, said she’s “happy for any step forward.” Still, the growing burden of prior authorization rules are an utter “time sink” for her and her staff.
“I have to justify my decisions to nondoctors before I even get to a doctor, and that’s really frustrating,” she said. “We’re talking about people here, not widgets.”
Advocates in Nevada are hoping this is the year a step therapy bill will make it into law in their state. As of March, one had yet to be introduced in the state legislature. Tom McCoy, director of state government affairs at the Nevada Chronic Care Collaborative, said existing Nevada law already prohibits nonmedical drug switching during a policy year; however, insurers can still make changes the following year.
A bill to rein in step therapy was proposed previously, Mr. McCoy said, but it never got off the ground. The collaborative, as well as about two dozen organizations representing Nevada providers and patients, are now calling on state lawmakers to make the issue a priority in the current session.
“The health plans have a lot of power – a lot,” Mr. McCoy said. “We’re hoping to get a [legislative] sponsor in 2021 ... but it’s also been a really hard year to connect legislators with patients and doctors, and being able to hear their stories really does make a difference.”
In Nebraska, Marcus Snow, MD, a rheumatologist at Nebraska Medicine, in Omaha, said that the state’s new step therapy law will be a “great first step in helping to provide some guardrails” around the practice. He noted that turnaround requirements for insurer responses are “sorely needed.” However, he said that, because the bill doesn’t apply to all health plans, many Nebraskans still won’t benefit.
Dealing with step therapy is a daily “headache” for Dr. Snow, who says navigating the bureaucracy of prior authorization seems to be getting worse every year. Like his peers around the country, he spends an inordinate amount of time pushing appeals up the insurance company ranks to get access to treatments he believes will be most effective. But Snow says that, more than just being a mountain of tiresome red tape, these practices also intrude on the patient-provider relationship, casting an unsettling sense of uncertainty that the ultimate decision about the best course of action isn’t up to the doctor and patient at all.
“In the end, the insurance company is the judge and jury of my prescription,” Dr. Snow said. “They’d argue I can still prescribe it, but if it costs $70,000 a year – I don’t know who can afford that.”
Ms. Lynch, at the National Psoriasis Foundation, said their step therapy advocacy will continue to take a two-pronged approach. They will push for new and expanded protections at both state and federal levels. Protections are needed at both levels to make sure that all health plans regulated by all entities are covered. In the U.S. Senate and the House, step therapy bills were reintroduced this year. They would apply to health plans subject to the federal Employee Retirement Income Security Act, which governs employer-sponsored health coverage, and could close a big gap in existing protections. Oregon, New Jersey, and Arizona are at the top of the foundation’s advocacy list this year, according to Ms. Lynch.
“Folks are really starting to pay more attention to this issue,” she said. “And hearing those real-world stories and frustrations is definitely one of the most effective tools we have.”
A version of this article first appeared on Medscape.com.
STEP 4: Ongoing semaglutide treatment extends weight loss
Weekly injections with the GLP-1 receptor agonist semaglutide helped people maintain, and even increase, their initial weight loss on the agent when they continued treatment beyond 20 weeks in results from an international, multicenter trial with 803 randomized subjects.
The study “reflects what we always see in practice, that when people lose weight their body then fights to regain it. The results underscore this” by showing what happens when people stop the drug, Domenica M. Rubino, MD, reported at the annual meeting of the Endocrine Society.
The STEP 4 study began with 902 obese or higher-risk people with an average body mass index of about 38 kg/m2 who underwent a 20-week, open-label, run-in phase of weekly subcutaneous injections of semaglutide (Ozempic), during which all subjects gradually up-titrated to the study’s maintenance dosage of 2.4 mg/week and allowing investigators to weed out intolerant, noncompliant, or nonresponsive people. After this phase excluded 99 subjects from continuing, and documented that the remaining 803 patients had already lost an average of 11% of their starting weight, the core of the study kicked in by randomizing them 2:1 to either maintain their weekly semaglutide injections for another 48 weeks or change to placebo injections.
After 48 more weeks, the 535 people who continued active semaglutide treatment lost on average an additional 8% of their weight. Meanwhile, the 268 who switched to placebo gained 7% of the weight they had reached at the 20-week point, for a significant between-group weight-loss difference of about 15% for the study’s primary endpoint. Those maintained on semaglutide for the full 68 weeks had a cumulative average weight loss of about 17%, compared with when they first began treatment, Dr. Rubino said. Concurrently with her report, the results also appeared in an article published online in JAMA.
“It’s reassuring that people who remain on this treatment can sustain weight losses of 15%, and in some cases 20% or more. That’s huge,” Dr. Rubino said in an interview. . After 68 weeks, 40% of the people who maintained their semaglutide treatment had lost at least 20% of their weight, compared with when they first started treatment.
“Preventing weight regain following initial weight loss is a well-known major challenge for people who lose weight,” commented John Clark III, MD, PhD, a weight management specialist at the University of Texas Southwestern Medical Center in Dallas who was not involved with the study. The findings from STEP 4 will be “helpful to have a discussion [with weight-loss patients] about the risks and benefits of continuing to take this medication longer than just a few months and if they want to continue taking the medication after they reach their goal weight,” Dr. Clark noted in an interview. “This new information reinforces that treatment continues to be effective after the short term.”
“This is obesity 101. If a treatment is provided that targets mechanisms of obesity, and then the treatment stops, we should not be surprised that weight regain occurs,” commented Ania M. Jastreboff, MD, PhD, codirector of the Yale Center for Weight Management in New Haven, Conn. “It’s tragic to see patients who, after successful weight loss, suffer regain because the treatment by which they lost weight stopped,” she said in an interview.
The STEP 4 study ran at 73 centers in 10 countries during 2018-2020. It enrolled adults without diabetes and with a BMI of at least 30, or at least 27 if they also had at least one weight-related comorbidity such as hypertension, dyslipidemia, or obstructive sleep apnea. Participants averaged about 47 years of age, almost 80% were women, and about 84% were White, including 8% of Hispanic or Latinx ethnicity.
The adverse-event profile was consistent with findings from trials where semaglutide treated hyperglycemia in patients with type 2 diabetes (semaglutide at a maximum once-weekly dosage of 1 mg has Food and Drug Administration approval for controlling hyperglycemia in patients with type 2 diabetes), as well results from other semaglutide studies and from studies of other agents in the GLP-1 receptor agonist class.
In STEP 4 9% of patients who received semaglutide during the randomized phase and 7% of those randomized to placebo had a serious adverse reaction, and about 2% of those in both treatment arms stopped treatment because of an adverse event. The most common adverse events on semaglutide were gastrointestinal, with diarrhea in 14%, nausea in 14%, constipation in 12%, and vomiting in 10%.
These GI effects are often mitigated by slower dose escalation, eating smaller amounts of food at a time, and not eating beyond the point of feeling full, noted Dr. Jastreboff.
The STEP 4 results follow prior reports from three other large trials – STEP 1, STEP 2, and STEP 3 – that studied the weight-loss effects of weekly semaglutide treatment in adults using varying enrollment criteria and treatment designs. “We’ve seen very consistent results [across all four studies] for efficacy and safety,” said Dr. Rubino, who owns and directs the Washington Center for Weight Management & Research in Arlington, Va.
NovoNordisk, the company that markets semaglutide, submitted data from all four studies to the FDA late last year in an application for a new label for a weight loss indication at the 2.4-mg/week dosage. The company has said it expects an agency decision by June 2021.
Dr. Rubino has been an adviser and consultant to and a speaker on behalf of Novo Nordisk, and she has also been an investigator for studies sponsored by AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr. Clark had no disclosures. Dr. Jastreboff is consultant for and has received research funding from NovoNordisk, and she has also been a consultant to and/or received research from Eli Lilly and Boehringer Ingelheim.
Weekly injections with the GLP-1 receptor agonist semaglutide helped people maintain, and even increase, their initial weight loss on the agent when they continued treatment beyond 20 weeks in results from an international, multicenter trial with 803 randomized subjects.
The study “reflects what we always see in practice, that when people lose weight their body then fights to regain it. The results underscore this” by showing what happens when people stop the drug, Domenica M. Rubino, MD, reported at the annual meeting of the Endocrine Society.
The STEP 4 study began with 902 obese or higher-risk people with an average body mass index of about 38 kg/m2 who underwent a 20-week, open-label, run-in phase of weekly subcutaneous injections of semaglutide (Ozempic), during which all subjects gradually up-titrated to the study’s maintenance dosage of 2.4 mg/week and allowing investigators to weed out intolerant, noncompliant, or nonresponsive people. After this phase excluded 99 subjects from continuing, and documented that the remaining 803 patients had already lost an average of 11% of their starting weight, the core of the study kicked in by randomizing them 2:1 to either maintain their weekly semaglutide injections for another 48 weeks or change to placebo injections.
After 48 more weeks, the 535 people who continued active semaglutide treatment lost on average an additional 8% of their weight. Meanwhile, the 268 who switched to placebo gained 7% of the weight they had reached at the 20-week point, for a significant between-group weight-loss difference of about 15% for the study’s primary endpoint. Those maintained on semaglutide for the full 68 weeks had a cumulative average weight loss of about 17%, compared with when they first began treatment, Dr. Rubino said. Concurrently with her report, the results also appeared in an article published online in JAMA.
“It’s reassuring that people who remain on this treatment can sustain weight losses of 15%, and in some cases 20% or more. That’s huge,” Dr. Rubino said in an interview. . After 68 weeks, 40% of the people who maintained their semaglutide treatment had lost at least 20% of their weight, compared with when they first started treatment.
“Preventing weight regain following initial weight loss is a well-known major challenge for people who lose weight,” commented John Clark III, MD, PhD, a weight management specialist at the University of Texas Southwestern Medical Center in Dallas who was not involved with the study. The findings from STEP 4 will be “helpful to have a discussion [with weight-loss patients] about the risks and benefits of continuing to take this medication longer than just a few months and if they want to continue taking the medication after they reach their goal weight,” Dr. Clark noted in an interview. “This new information reinforces that treatment continues to be effective after the short term.”
“This is obesity 101. If a treatment is provided that targets mechanisms of obesity, and then the treatment stops, we should not be surprised that weight regain occurs,” commented Ania M. Jastreboff, MD, PhD, codirector of the Yale Center for Weight Management in New Haven, Conn. “It’s tragic to see patients who, after successful weight loss, suffer regain because the treatment by which they lost weight stopped,” she said in an interview.
The STEP 4 study ran at 73 centers in 10 countries during 2018-2020. It enrolled adults without diabetes and with a BMI of at least 30, or at least 27 if they also had at least one weight-related comorbidity such as hypertension, dyslipidemia, or obstructive sleep apnea. Participants averaged about 47 years of age, almost 80% were women, and about 84% were White, including 8% of Hispanic or Latinx ethnicity.
The adverse-event profile was consistent with findings from trials where semaglutide treated hyperglycemia in patients with type 2 diabetes (semaglutide at a maximum once-weekly dosage of 1 mg has Food and Drug Administration approval for controlling hyperglycemia in patients with type 2 diabetes), as well results from other semaglutide studies and from studies of other agents in the GLP-1 receptor agonist class.
In STEP 4 9% of patients who received semaglutide during the randomized phase and 7% of those randomized to placebo had a serious adverse reaction, and about 2% of those in both treatment arms stopped treatment because of an adverse event. The most common adverse events on semaglutide were gastrointestinal, with diarrhea in 14%, nausea in 14%, constipation in 12%, and vomiting in 10%.
These GI effects are often mitigated by slower dose escalation, eating smaller amounts of food at a time, and not eating beyond the point of feeling full, noted Dr. Jastreboff.
The STEP 4 results follow prior reports from three other large trials – STEP 1, STEP 2, and STEP 3 – that studied the weight-loss effects of weekly semaglutide treatment in adults using varying enrollment criteria and treatment designs. “We’ve seen very consistent results [across all four studies] for efficacy and safety,” said Dr. Rubino, who owns and directs the Washington Center for Weight Management & Research in Arlington, Va.
NovoNordisk, the company that markets semaglutide, submitted data from all four studies to the FDA late last year in an application for a new label for a weight loss indication at the 2.4-mg/week dosage. The company has said it expects an agency decision by June 2021.
Dr. Rubino has been an adviser and consultant to and a speaker on behalf of Novo Nordisk, and she has also been an investigator for studies sponsored by AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr. Clark had no disclosures. Dr. Jastreboff is consultant for and has received research funding from NovoNordisk, and she has also been a consultant to and/or received research from Eli Lilly and Boehringer Ingelheim.
Weekly injections with the GLP-1 receptor agonist semaglutide helped people maintain, and even increase, their initial weight loss on the agent when they continued treatment beyond 20 weeks in results from an international, multicenter trial with 803 randomized subjects.
The study “reflects what we always see in practice, that when people lose weight their body then fights to regain it. The results underscore this” by showing what happens when people stop the drug, Domenica M. Rubino, MD, reported at the annual meeting of the Endocrine Society.
The STEP 4 study began with 902 obese or higher-risk people with an average body mass index of about 38 kg/m2 who underwent a 20-week, open-label, run-in phase of weekly subcutaneous injections of semaglutide (Ozempic), during which all subjects gradually up-titrated to the study’s maintenance dosage of 2.4 mg/week and allowing investigators to weed out intolerant, noncompliant, or nonresponsive people. After this phase excluded 99 subjects from continuing, and documented that the remaining 803 patients had already lost an average of 11% of their starting weight, the core of the study kicked in by randomizing them 2:1 to either maintain their weekly semaglutide injections for another 48 weeks or change to placebo injections.
After 48 more weeks, the 535 people who continued active semaglutide treatment lost on average an additional 8% of their weight. Meanwhile, the 268 who switched to placebo gained 7% of the weight they had reached at the 20-week point, for a significant between-group weight-loss difference of about 15% for the study’s primary endpoint. Those maintained on semaglutide for the full 68 weeks had a cumulative average weight loss of about 17%, compared with when they first began treatment, Dr. Rubino said. Concurrently with her report, the results also appeared in an article published online in JAMA.
“It’s reassuring that people who remain on this treatment can sustain weight losses of 15%, and in some cases 20% or more. That’s huge,” Dr. Rubino said in an interview. . After 68 weeks, 40% of the people who maintained their semaglutide treatment had lost at least 20% of their weight, compared with when they first started treatment.
“Preventing weight regain following initial weight loss is a well-known major challenge for people who lose weight,” commented John Clark III, MD, PhD, a weight management specialist at the University of Texas Southwestern Medical Center in Dallas who was not involved with the study. The findings from STEP 4 will be “helpful to have a discussion [with weight-loss patients] about the risks and benefits of continuing to take this medication longer than just a few months and if they want to continue taking the medication after they reach their goal weight,” Dr. Clark noted in an interview. “This new information reinforces that treatment continues to be effective after the short term.”
“This is obesity 101. If a treatment is provided that targets mechanisms of obesity, and then the treatment stops, we should not be surprised that weight regain occurs,” commented Ania M. Jastreboff, MD, PhD, codirector of the Yale Center for Weight Management in New Haven, Conn. “It’s tragic to see patients who, after successful weight loss, suffer regain because the treatment by which they lost weight stopped,” she said in an interview.
The STEP 4 study ran at 73 centers in 10 countries during 2018-2020. It enrolled adults without diabetes and with a BMI of at least 30, or at least 27 if they also had at least one weight-related comorbidity such as hypertension, dyslipidemia, or obstructive sleep apnea. Participants averaged about 47 years of age, almost 80% were women, and about 84% were White, including 8% of Hispanic or Latinx ethnicity.
The adverse-event profile was consistent with findings from trials where semaglutide treated hyperglycemia in patients with type 2 diabetes (semaglutide at a maximum once-weekly dosage of 1 mg has Food and Drug Administration approval for controlling hyperglycemia in patients with type 2 diabetes), as well results from other semaglutide studies and from studies of other agents in the GLP-1 receptor agonist class.
In STEP 4 9% of patients who received semaglutide during the randomized phase and 7% of those randomized to placebo had a serious adverse reaction, and about 2% of those in both treatment arms stopped treatment because of an adverse event. The most common adverse events on semaglutide were gastrointestinal, with diarrhea in 14%, nausea in 14%, constipation in 12%, and vomiting in 10%.
These GI effects are often mitigated by slower dose escalation, eating smaller amounts of food at a time, and not eating beyond the point of feeling full, noted Dr. Jastreboff.
The STEP 4 results follow prior reports from three other large trials – STEP 1, STEP 2, and STEP 3 – that studied the weight-loss effects of weekly semaglutide treatment in adults using varying enrollment criteria and treatment designs. “We’ve seen very consistent results [across all four studies] for efficacy and safety,” said Dr. Rubino, who owns and directs the Washington Center for Weight Management & Research in Arlington, Va.
NovoNordisk, the company that markets semaglutide, submitted data from all four studies to the FDA late last year in an application for a new label for a weight loss indication at the 2.4-mg/week dosage. The company has said it expects an agency decision by June 2021.
Dr. Rubino has been an adviser and consultant to and a speaker on behalf of Novo Nordisk, and she has also been an investigator for studies sponsored by AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr. Clark had no disclosures. Dr. Jastreboff is consultant for and has received research funding from NovoNordisk, and she has also been a consultant to and/or received research from Eli Lilly and Boehringer Ingelheim.
FROM ENDO 2021
In U.S., lockdowns added 2 pounds per month
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Vitamin D may protect against COVID-19, especially in Black patients
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Senate confirms Murthy as Surgeon General
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Denosumab now dominant therapy for osteoporosis linked to cancer
Amid a substantial expansion of therapies in several drug classes for the treatment of osteoporosis, there has been a notable increase in the prescription of denosumab for patients with a cancer-related indication.
In an analysis of claims data from January 2009 to March 2020, the bisphosphonate alendronate represented more than 50% of all prescriptions for bone-directed therapies, but growth in the use of the monoclonal antibody denosumab overall and in cancer-related indications particularly was steady throughout the study period.
“In the malignancy cohort, alendronate and zoledronic acid were each used in approximately 30% of individuals at the onset of the study, but use of both then declined,” Sara Cromer, MD, reported at the annual meeting of the Endocrine Society.
For malignancy-based prescriptions, denosumab surpassed either bisphosphonate by 2013 and then continued to rise.
Denosumab use “reached approximately 50% of all bone-directed medication use in the malignancy cohort” by the end of the study period, said Dr. Cromer, a clinical research fellow in endocrinology at Massachusetts General Hospital, Boston.
The claims data for this analysis was drawn from the Clinformatics Data Mart. The analysis was restricted to individuals aged older than 50 years who received a prescription for a bone-directed therapy. The 15.48 million prescriptions evaluated were drawn from 1.46 million unique individuals. The mean age was 69 years, and 89% of those prescribed a drug were women.
Oncologic indications one of two tracked cohorts
In the context of a large expansion of treatment options in several drug classes for osteoporosis, the objective of this claims analysis was to document trends in treatment choice, according to Dr. Cromer. She and her coinvestigators looked at prescriptions overall as well as in two cohorts defined by ICD codes. One included patients prescribed a prescription by an oncologist. The other included everyone else.
When all prescriptions for bone-directed therapy were evaluated over the study period, alendronate was the most commonly prescribed therapy, and its use increased over time. Prescriptions of zoledronic acid also rose, doubling over the study period, but use was very low in the beginning and it never climbed above 5%.
The proportion of prescriptions written for bisphosphonates other than alendronate and zoledronic acid “declined steadily” over the study period, Dr. Cromer reported.
Denosumab, a monoclonal antibody that targets a step in the process important to maturation of osteoclasts, was approved in 2010. It accounted for 10% of all prescriptions for osteoporosis by 2015 and 15% by 2018. It was still rising through the end of the study period.
In contrast, prescriptions of raloxifene, a selective estrogen receptor modulator, began to decline after 2013. In general, the rates of prescriptions for other agents, including some of the more recently approved drugs, such as teriparatide, abaloparatide, and romosozumab, changed very little over the study period. None of these therapies ever represented more than 2% of prescriptions.
When looking at the cohort of patients who received a bone-directed reason for a noncancer indication, the trends “paralleled those in the all-user analysis,” Dr. Cromer reported.
Denosumab use greater in privately insured
In the malignancy cohort, the decline in the use of bisphosphonates and the rise in the use of denosumab were most pronounced in patients who were privately insured. The increased use of denosumab over the study period “outpaced gains in use of other agents despite guidelines,” said Dr. Cromer, referring to the those issued by the Endocrine Society in 2019 .
In those guidelines, written for management of postmenopausal women at high risk of fractures, bisphosphonates are recommended for initial treatment while denosumab is recommended as an alternative. However, those guidelines do not provide specific recommendations for therapies directed at osteoporosis associated with cancer.
Guidelines for this population exist, including one published by the American Society of Clinical Oncology in 2019.
In the ASCO guidelines, oral bisphosphonates, intravenous bisphosphonates, and subcutaneous denosumab were all identified as “efficacious options,” according to Charles L. Shapiro, MD, director of breast cancer translational research, Mount Sinai Health System, New York.
Specifically, “all three of them work to reduce fractures and improve bone density in women with breast cancer in whom you are trying to prevent or treat osteoporosis,” Dr. Shapiro said in an interview.
There might be relative advantages for one therapy over another in specific subgroups defined by type of cancer or stage of cancer, but trials are not definitive for such outcomes as overall survival. Citing one comparative study associating denosumab with an 18% delay to first skeletal event in women with metastatic breast cancer, Dr. Shapiro observed, “I personally don’t consider an 18% delay [for this outcome] to be that clinically meaningful.”
Although major guidelines from ASCO have not so far favored denosumab over any bisphosphonate in routine care, Dr. Shapiro did not rule out the possibility that future studies will show differences.
Dr. Comer and Dr. Shapiro reported no relevant conflicts of interest.
Amid a substantial expansion of therapies in several drug classes for the treatment of osteoporosis, there has been a notable increase in the prescription of denosumab for patients with a cancer-related indication.
In an analysis of claims data from January 2009 to March 2020, the bisphosphonate alendronate represented more than 50% of all prescriptions for bone-directed therapies, but growth in the use of the monoclonal antibody denosumab overall and in cancer-related indications particularly was steady throughout the study period.
“In the malignancy cohort, alendronate and zoledronic acid were each used in approximately 30% of individuals at the onset of the study, but use of both then declined,” Sara Cromer, MD, reported at the annual meeting of the Endocrine Society.
For malignancy-based prescriptions, denosumab surpassed either bisphosphonate by 2013 and then continued to rise.
Denosumab use “reached approximately 50% of all bone-directed medication use in the malignancy cohort” by the end of the study period, said Dr. Cromer, a clinical research fellow in endocrinology at Massachusetts General Hospital, Boston.
The claims data for this analysis was drawn from the Clinformatics Data Mart. The analysis was restricted to individuals aged older than 50 years who received a prescription for a bone-directed therapy. The 15.48 million prescriptions evaluated were drawn from 1.46 million unique individuals. The mean age was 69 years, and 89% of those prescribed a drug were women.
Oncologic indications one of two tracked cohorts
In the context of a large expansion of treatment options in several drug classes for osteoporosis, the objective of this claims analysis was to document trends in treatment choice, according to Dr. Cromer. She and her coinvestigators looked at prescriptions overall as well as in two cohorts defined by ICD codes. One included patients prescribed a prescription by an oncologist. The other included everyone else.
When all prescriptions for bone-directed therapy were evaluated over the study period, alendronate was the most commonly prescribed therapy, and its use increased over time. Prescriptions of zoledronic acid also rose, doubling over the study period, but use was very low in the beginning and it never climbed above 5%.
The proportion of prescriptions written for bisphosphonates other than alendronate and zoledronic acid “declined steadily” over the study period, Dr. Cromer reported.
Denosumab, a monoclonal antibody that targets a step in the process important to maturation of osteoclasts, was approved in 2010. It accounted for 10% of all prescriptions for osteoporosis by 2015 and 15% by 2018. It was still rising through the end of the study period.
In contrast, prescriptions of raloxifene, a selective estrogen receptor modulator, began to decline after 2013. In general, the rates of prescriptions for other agents, including some of the more recently approved drugs, such as teriparatide, abaloparatide, and romosozumab, changed very little over the study period. None of these therapies ever represented more than 2% of prescriptions.
When looking at the cohort of patients who received a bone-directed reason for a noncancer indication, the trends “paralleled those in the all-user analysis,” Dr. Cromer reported.
Denosumab use greater in privately insured
In the malignancy cohort, the decline in the use of bisphosphonates and the rise in the use of denosumab were most pronounced in patients who were privately insured. The increased use of denosumab over the study period “outpaced gains in use of other agents despite guidelines,” said Dr. Cromer, referring to the those issued by the Endocrine Society in 2019 .
In those guidelines, written for management of postmenopausal women at high risk of fractures, bisphosphonates are recommended for initial treatment while denosumab is recommended as an alternative. However, those guidelines do not provide specific recommendations for therapies directed at osteoporosis associated with cancer.
Guidelines for this population exist, including one published by the American Society of Clinical Oncology in 2019.
In the ASCO guidelines, oral bisphosphonates, intravenous bisphosphonates, and subcutaneous denosumab were all identified as “efficacious options,” according to Charles L. Shapiro, MD, director of breast cancer translational research, Mount Sinai Health System, New York.
Specifically, “all three of them work to reduce fractures and improve bone density in women with breast cancer in whom you are trying to prevent or treat osteoporosis,” Dr. Shapiro said in an interview.
There might be relative advantages for one therapy over another in specific subgroups defined by type of cancer or stage of cancer, but trials are not definitive for such outcomes as overall survival. Citing one comparative study associating denosumab with an 18% delay to first skeletal event in women with metastatic breast cancer, Dr. Shapiro observed, “I personally don’t consider an 18% delay [for this outcome] to be that clinically meaningful.”
Although major guidelines from ASCO have not so far favored denosumab over any bisphosphonate in routine care, Dr. Shapiro did not rule out the possibility that future studies will show differences.
Dr. Comer and Dr. Shapiro reported no relevant conflicts of interest.
Amid a substantial expansion of therapies in several drug classes for the treatment of osteoporosis, there has been a notable increase in the prescription of denosumab for patients with a cancer-related indication.
In an analysis of claims data from January 2009 to March 2020, the bisphosphonate alendronate represented more than 50% of all prescriptions for bone-directed therapies, but growth in the use of the monoclonal antibody denosumab overall and in cancer-related indications particularly was steady throughout the study period.
“In the malignancy cohort, alendronate and zoledronic acid were each used in approximately 30% of individuals at the onset of the study, but use of both then declined,” Sara Cromer, MD, reported at the annual meeting of the Endocrine Society.
For malignancy-based prescriptions, denosumab surpassed either bisphosphonate by 2013 and then continued to rise.
Denosumab use “reached approximately 50% of all bone-directed medication use in the malignancy cohort” by the end of the study period, said Dr. Cromer, a clinical research fellow in endocrinology at Massachusetts General Hospital, Boston.
The claims data for this analysis was drawn from the Clinformatics Data Mart. The analysis was restricted to individuals aged older than 50 years who received a prescription for a bone-directed therapy. The 15.48 million prescriptions evaluated were drawn from 1.46 million unique individuals. The mean age was 69 years, and 89% of those prescribed a drug were women.
Oncologic indications one of two tracked cohorts
In the context of a large expansion of treatment options in several drug classes for osteoporosis, the objective of this claims analysis was to document trends in treatment choice, according to Dr. Cromer. She and her coinvestigators looked at prescriptions overall as well as in two cohorts defined by ICD codes. One included patients prescribed a prescription by an oncologist. The other included everyone else.
When all prescriptions for bone-directed therapy were evaluated over the study period, alendronate was the most commonly prescribed therapy, and its use increased over time. Prescriptions of zoledronic acid also rose, doubling over the study period, but use was very low in the beginning and it never climbed above 5%.
The proportion of prescriptions written for bisphosphonates other than alendronate and zoledronic acid “declined steadily” over the study period, Dr. Cromer reported.
Denosumab, a monoclonal antibody that targets a step in the process important to maturation of osteoclasts, was approved in 2010. It accounted for 10% of all prescriptions for osteoporosis by 2015 and 15% by 2018. It was still rising through the end of the study period.
In contrast, prescriptions of raloxifene, a selective estrogen receptor modulator, began to decline after 2013. In general, the rates of prescriptions for other agents, including some of the more recently approved drugs, such as teriparatide, abaloparatide, and romosozumab, changed very little over the study period. None of these therapies ever represented more than 2% of prescriptions.
When looking at the cohort of patients who received a bone-directed reason for a noncancer indication, the trends “paralleled those in the all-user analysis,” Dr. Cromer reported.
Denosumab use greater in privately insured
In the malignancy cohort, the decline in the use of bisphosphonates and the rise in the use of denosumab were most pronounced in patients who were privately insured. The increased use of denosumab over the study period “outpaced gains in use of other agents despite guidelines,” said Dr. Cromer, referring to the those issued by the Endocrine Society in 2019 .
In those guidelines, written for management of postmenopausal women at high risk of fractures, bisphosphonates are recommended for initial treatment while denosumab is recommended as an alternative. However, those guidelines do not provide specific recommendations for therapies directed at osteoporosis associated with cancer.
Guidelines for this population exist, including one published by the American Society of Clinical Oncology in 2019.
In the ASCO guidelines, oral bisphosphonates, intravenous bisphosphonates, and subcutaneous denosumab were all identified as “efficacious options,” according to Charles L. Shapiro, MD, director of breast cancer translational research, Mount Sinai Health System, New York.
Specifically, “all three of them work to reduce fractures and improve bone density in women with breast cancer in whom you are trying to prevent or treat osteoporosis,” Dr. Shapiro said in an interview.
There might be relative advantages for one therapy over another in specific subgroups defined by type of cancer or stage of cancer, but trials are not definitive for such outcomes as overall survival. Citing one comparative study associating denosumab with an 18% delay to first skeletal event in women with metastatic breast cancer, Dr. Shapiro observed, “I personally don’t consider an 18% delay [for this outcome] to be that clinically meaningful.”
Although major guidelines from ASCO have not so far favored denosumab over any bisphosphonate in routine care, Dr. Shapiro did not rule out the possibility that future studies will show differences.
Dr. Comer and Dr. Shapiro reported no relevant conflicts of interest.
FROM ENDO 2021