User login
Adult ADHD: 6 studies of nonpharmacologic interventions
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
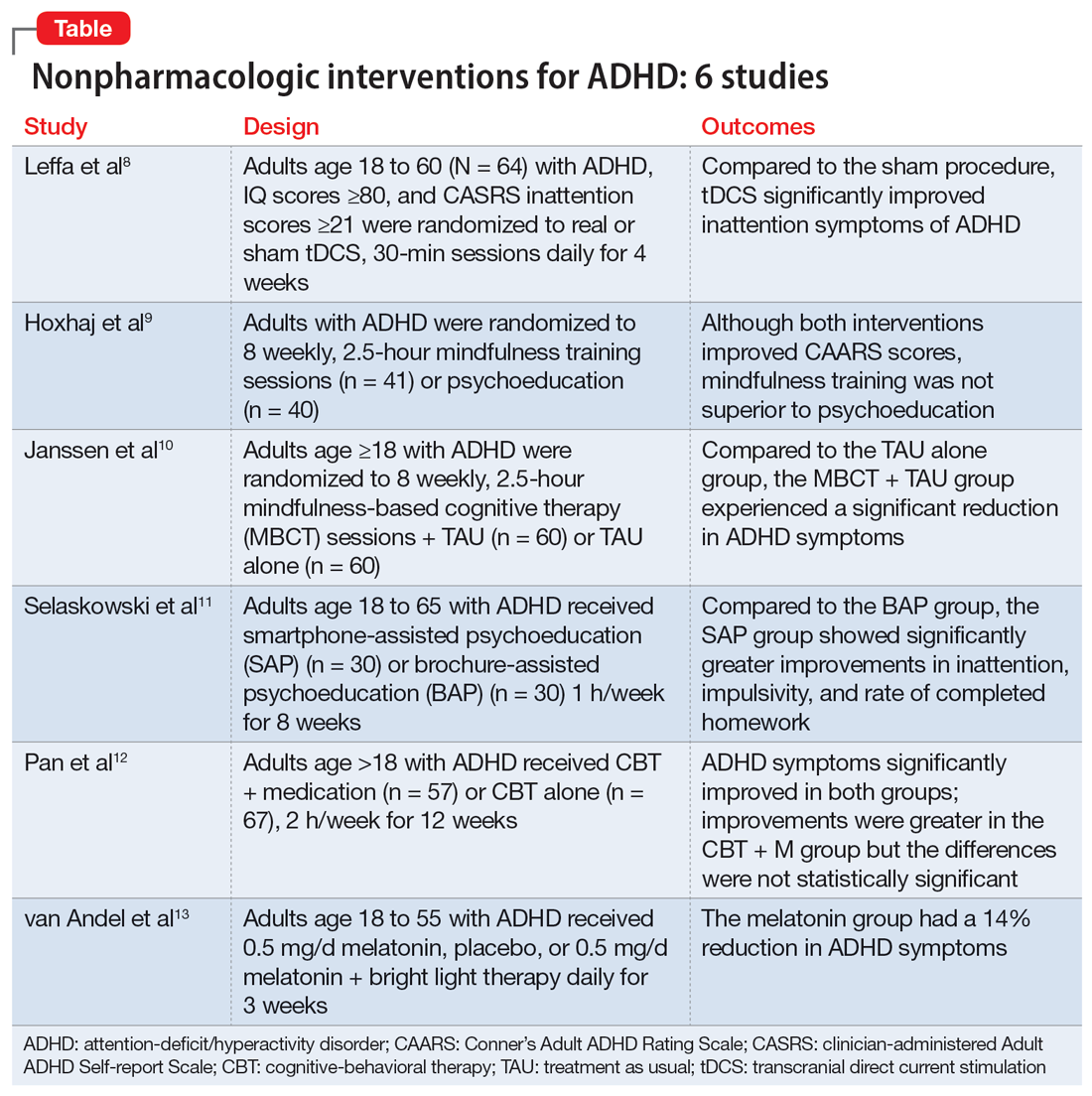
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).
1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
Using apps in clinical practice: 8 studies
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Adult ADHD: 6 studies of pharmacologic interventions
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.
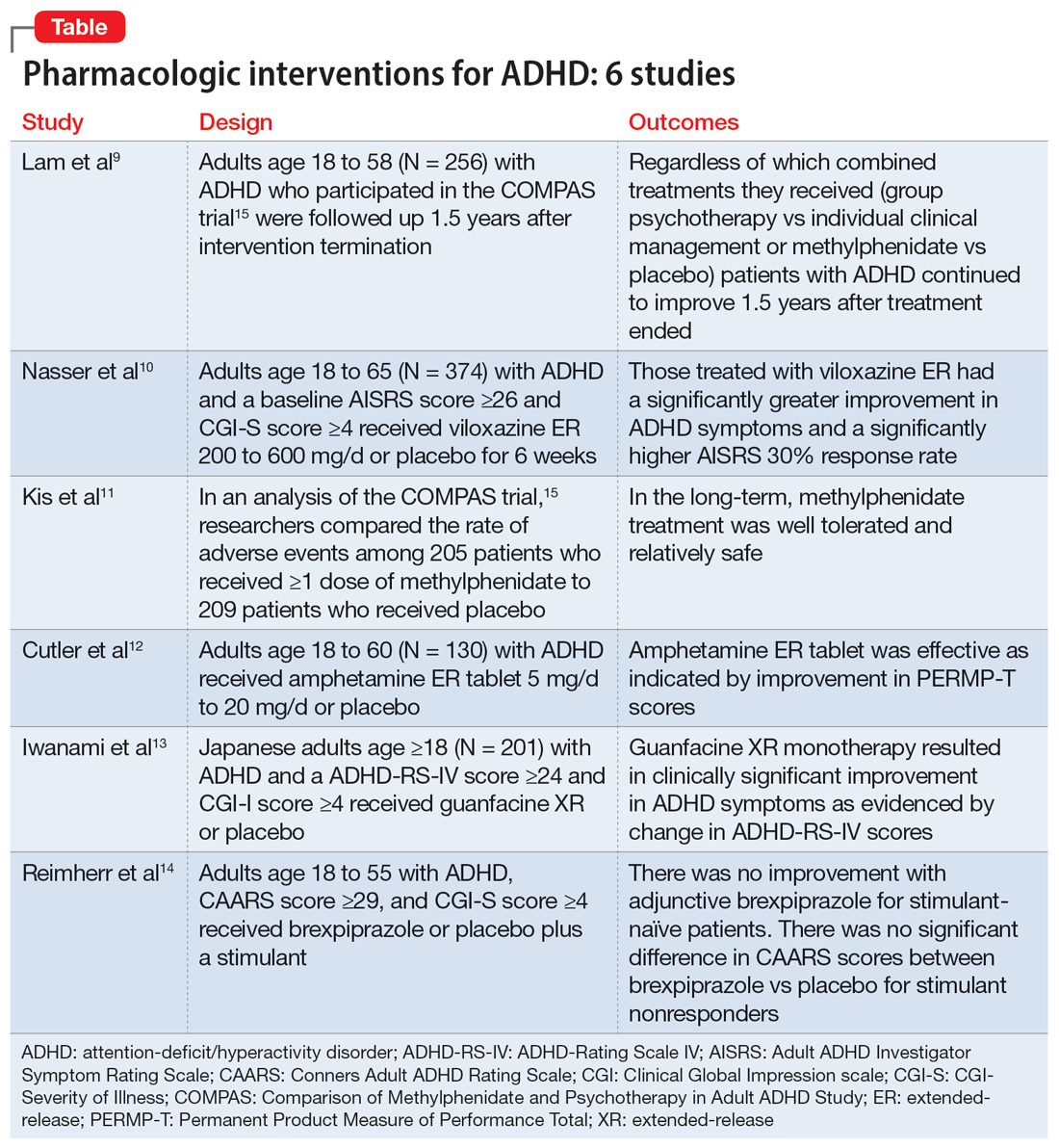
1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Treating PTSD: A review of 8 studies
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Generalized anxiety disorder: 8 studies of psychosocial interventions
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (
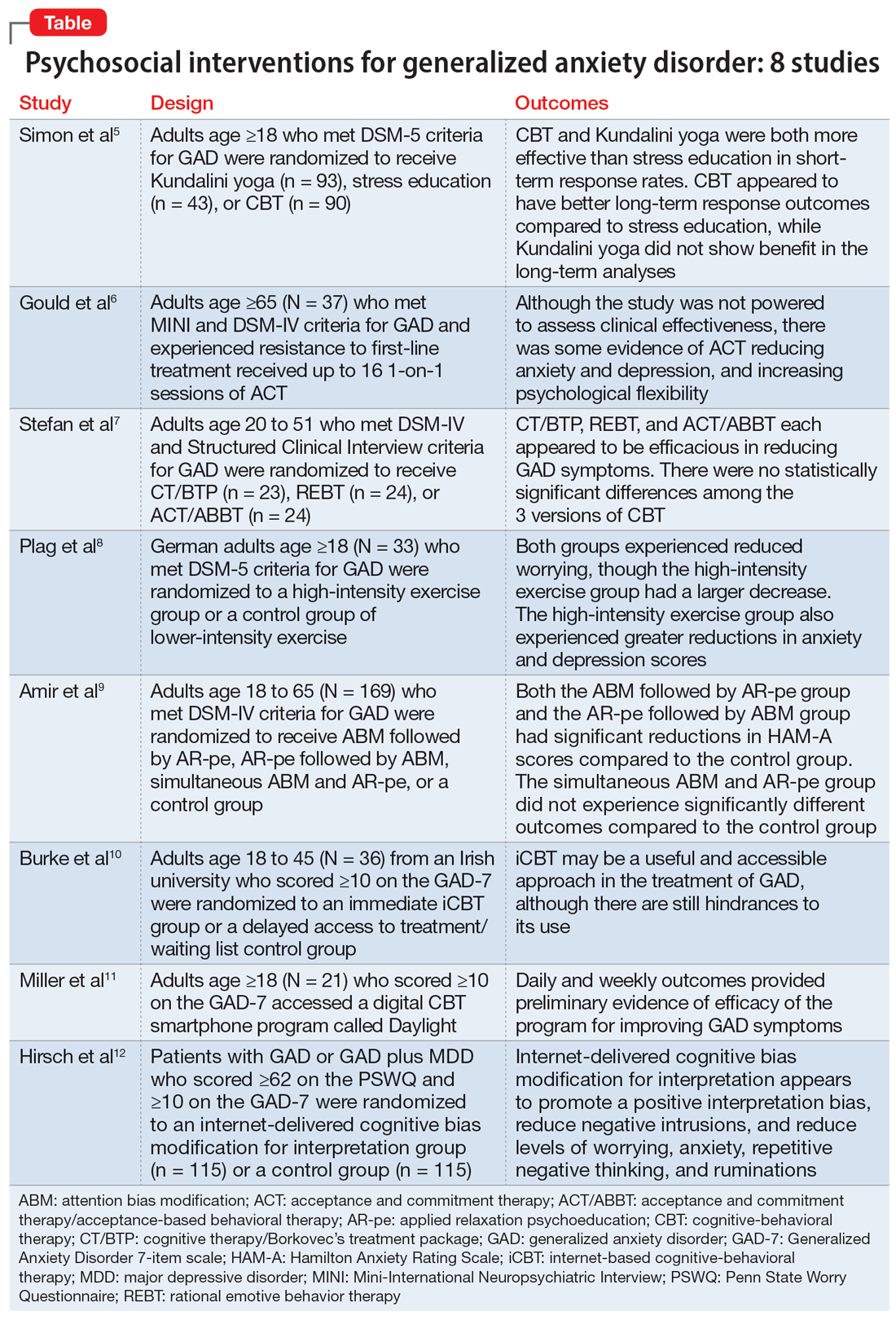
1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
Generalized anxiety disorder: 8 studies of biological interventions
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
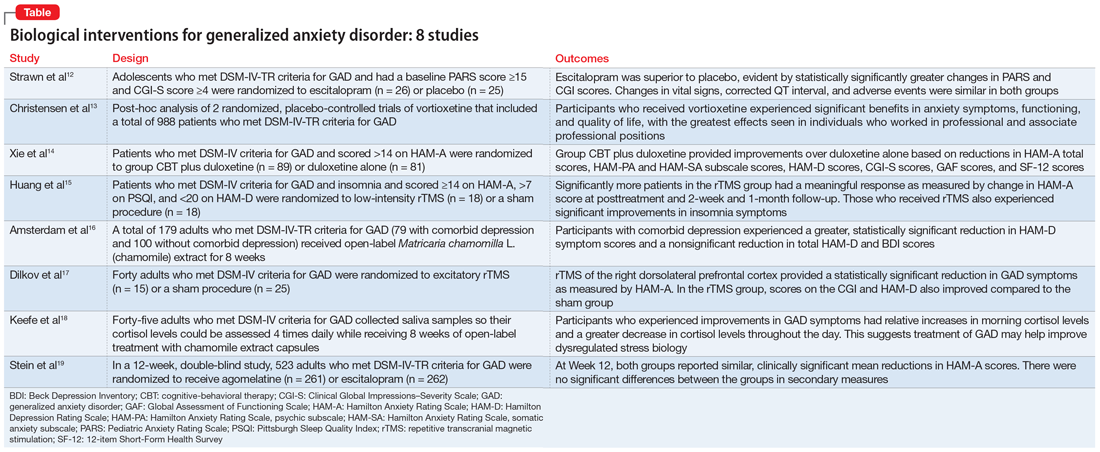
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Treatment augmentation strategies for OCD: A review of 8 studies
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Borderline personality disorder: 6 studies of psychosocial interventions
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD
1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD

1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD

1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
Borderline personality disorder: 6 studies of biological interventions
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.
1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).

1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.