User login
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
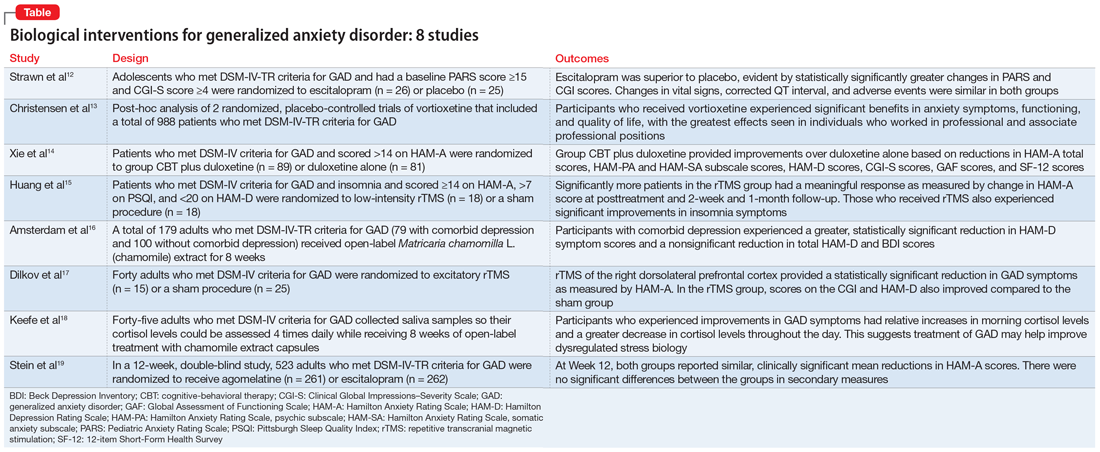
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007