User login
What is the risk of adverse outcomes in a woman who develops mild hypertension from OCs?
Women who take oral contraceptives (OCs) have an increased risk of developing new hypertension, which returns to baseline within 1 to 3 months of OC cessation (strength of recommendation [SOR]: A, based on cohort studies).
Among large populations of women with hypertension from all causes, risk of adverse cardiovascular outcomes is increased (SOR: B, based on Framingham data). Women with pre-existing hypertension who take OCs have an increased risk of stroke and myocardial infarction (MI) when compared with hypertensive women who do not (SOR: B, based on case-control studies).
Is an increase of 178 per million woman-years of CV events clinically significant?
Sarina Schrager, MD
University of Wisconsin
In clinical practice, we continually balance the risks and benefits of any treatment. Oral contraceptive pills are the most commonly used reversible form of contraception in the United States. Although this review documents an increased risk of reversible new hypertension for women on OCs and a possibly significant increase in cardiovascular events, the clinical meaning of these data is unclear. Is an increase of 178 per million woman-years of CV events clinically significant? It would be a shame to limit the availability of this effective contraception method to otherwise young and healthy women because of this very rare event.
Evidence summary
No studies specifically examine the risk of adverse outcomes for women who have mild elevations in blood pressure as a result of taking OCs. However, for the general population cardiovascular risk increases 30% for each 10 mm Hg rise in systolic pressure.1
A prospective cohort study found an increased incidence of new hypertension developing among women taking OCs. The study, conducted in the US, included 68,297 female nurses aged 25 to 42 years without a previous diagnosis of hypertension, diabetes, coronary heart disease, stroke, or cancer, who were followed for 4 years. Women were excluded from the study if they had not had a physical exam in the last 2 years or were taking antihypertensive medication at study inception. The nurses self-reported their blood pressure readings via questionnaire; medical records were sampled to validate the accuracy of self-reports. After adjusting for risk factors, current oral contraceptive use increased the risk of developing hypertension (relative risk [RR]=1.8; 95% confidence interval [CI], 1.5–2.3; corresponding to 41.5 new cases of hypertension per 10,000 person-years).2
A systematic review of 8 case control studies (with 4907 cases and 13,443 controls) found an increased risk of stroke and MI among hypertensive women taking combined type OCs vs those not taking OCs. Women with hypertension aged 20 to 24 years had an estimated CV event risk of 312 per million woman-years while taking OCs vs 134 per million woman-years not taking OCs. Among hypertensive women aged 40 to 44 years, the estimated risks were 1213 vs 529 per million woman-years, respectively. Primary endpoints varied across the studies and statistical significance was not given.3
Three studies showed that blood pressure elevations due to taking oral contraceptives returned to baseline with discontinuation of the medication. A prospective cohort study followed 13,358 women who were neither pregnant nor postpartum between the ages of 15 and 60. Women who either initiated or resumed using OCs experienced a statistically significant rise of about 4 mm Hg in the systolic pressure and 1 mm Hg in the diastolic pressure. Women who stopped using OCs experienced significant decreases in both systolic and diastolic components (about 5 mm Hg and 1.5 mm Hg, respectively).1
Similarly, a survey study of 461 women attending family planning clinics found that mean blood pressures were significantly higher for those taking OCs than for those using nonhormonal contraception. Elevated blood pressures correlated with duration of current use of OCs but returned to normal soon after stopping OCs. The mean pressures of those who had stopped OCs more than a month were similar to those of women who had never taken an OC and significantly lower than those of women who were currently taking OCs.4
Finally, a prospective study which followed 32 women who had taken combined OCs for 1 to 3 years and then stopped found that blood pressures returned to pretreatment levels at 3 months. Systolic pressure fell by 9.7 mm Hg and diastolic by 2.9 mm Hg compared with measurements made 1 month before stopping. No cardiovascular complications were reported among women during this study.5
Recommendations from others
The American College of Obstetricians and Gynecologists says that women with well-controlled and monitored hypertension aged 35 years and younger are appropriate candidates for a trial of combination OCs, provided they are otherwise healthy, have no evidence of end-organ vascular disease, and do not smoke cigarettes.6 If blood pressure remains well controlled with careful monitoring several months after initiating OCs, use can be continued.
1. Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA 1977;237:2499-2503.
2. Chasen-Taber L, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation 1996;94:483-489.
3. Curtis K, et al. Contraception for women in selected circumstances. Obstet Gynecol 2002;6:1100-1112.
4. Khaw K-T, Peart WS. Blood pressure and contraceptive use. Br Med J 1982;285:403-407.
5. Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood pressure in women taking oral contraceptives. Br Med J 1974;1:533-535.
6. American College of Obstetricians and Gynecologists (ACOG). The use of hormonal contraception in women with coexisting medical conditions. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2006 June. 20 p. (ACOG practice bulletin; no. 73).
Women who take oral contraceptives (OCs) have an increased risk of developing new hypertension, which returns to baseline within 1 to 3 months of OC cessation (strength of recommendation [SOR]: A, based on cohort studies).
Among large populations of women with hypertension from all causes, risk of adverse cardiovascular outcomes is increased (SOR: B, based on Framingham data). Women with pre-existing hypertension who take OCs have an increased risk of stroke and myocardial infarction (MI) when compared with hypertensive women who do not (SOR: B, based on case-control studies).
Is an increase of 178 per million woman-years of CV events clinically significant?
Sarina Schrager, MD
University of Wisconsin
In clinical practice, we continually balance the risks and benefits of any treatment. Oral contraceptive pills are the most commonly used reversible form of contraception in the United States. Although this review documents an increased risk of reversible new hypertension for women on OCs and a possibly significant increase in cardiovascular events, the clinical meaning of these data is unclear. Is an increase of 178 per million woman-years of CV events clinically significant? It would be a shame to limit the availability of this effective contraception method to otherwise young and healthy women because of this very rare event.
Evidence summary
No studies specifically examine the risk of adverse outcomes for women who have mild elevations in blood pressure as a result of taking OCs. However, for the general population cardiovascular risk increases 30% for each 10 mm Hg rise in systolic pressure.1
A prospective cohort study found an increased incidence of new hypertension developing among women taking OCs. The study, conducted in the US, included 68,297 female nurses aged 25 to 42 years without a previous diagnosis of hypertension, diabetes, coronary heart disease, stroke, or cancer, who were followed for 4 years. Women were excluded from the study if they had not had a physical exam in the last 2 years or were taking antihypertensive medication at study inception. The nurses self-reported their blood pressure readings via questionnaire; medical records were sampled to validate the accuracy of self-reports. After adjusting for risk factors, current oral contraceptive use increased the risk of developing hypertension (relative risk [RR]=1.8; 95% confidence interval [CI], 1.5–2.3; corresponding to 41.5 new cases of hypertension per 10,000 person-years).2
A systematic review of 8 case control studies (with 4907 cases and 13,443 controls) found an increased risk of stroke and MI among hypertensive women taking combined type OCs vs those not taking OCs. Women with hypertension aged 20 to 24 years had an estimated CV event risk of 312 per million woman-years while taking OCs vs 134 per million woman-years not taking OCs. Among hypertensive women aged 40 to 44 years, the estimated risks were 1213 vs 529 per million woman-years, respectively. Primary endpoints varied across the studies and statistical significance was not given.3
Three studies showed that blood pressure elevations due to taking oral contraceptives returned to baseline with discontinuation of the medication. A prospective cohort study followed 13,358 women who were neither pregnant nor postpartum between the ages of 15 and 60. Women who either initiated or resumed using OCs experienced a statistically significant rise of about 4 mm Hg in the systolic pressure and 1 mm Hg in the diastolic pressure. Women who stopped using OCs experienced significant decreases in both systolic and diastolic components (about 5 mm Hg and 1.5 mm Hg, respectively).1
Similarly, a survey study of 461 women attending family planning clinics found that mean blood pressures were significantly higher for those taking OCs than for those using nonhormonal contraception. Elevated blood pressures correlated with duration of current use of OCs but returned to normal soon after stopping OCs. The mean pressures of those who had stopped OCs more than a month were similar to those of women who had never taken an OC and significantly lower than those of women who were currently taking OCs.4
Finally, a prospective study which followed 32 women who had taken combined OCs for 1 to 3 years and then stopped found that blood pressures returned to pretreatment levels at 3 months. Systolic pressure fell by 9.7 mm Hg and diastolic by 2.9 mm Hg compared with measurements made 1 month before stopping. No cardiovascular complications were reported among women during this study.5
Recommendations from others
The American College of Obstetricians and Gynecologists says that women with well-controlled and monitored hypertension aged 35 years and younger are appropriate candidates for a trial of combination OCs, provided they are otherwise healthy, have no evidence of end-organ vascular disease, and do not smoke cigarettes.6 If blood pressure remains well controlled with careful monitoring several months after initiating OCs, use can be continued.
Women who take oral contraceptives (OCs) have an increased risk of developing new hypertension, which returns to baseline within 1 to 3 months of OC cessation (strength of recommendation [SOR]: A, based on cohort studies).
Among large populations of women with hypertension from all causes, risk of adverse cardiovascular outcomes is increased (SOR: B, based on Framingham data). Women with pre-existing hypertension who take OCs have an increased risk of stroke and myocardial infarction (MI) when compared with hypertensive women who do not (SOR: B, based on case-control studies).
Is an increase of 178 per million woman-years of CV events clinically significant?
Sarina Schrager, MD
University of Wisconsin
In clinical practice, we continually balance the risks and benefits of any treatment. Oral contraceptive pills are the most commonly used reversible form of contraception in the United States. Although this review documents an increased risk of reversible new hypertension for women on OCs and a possibly significant increase in cardiovascular events, the clinical meaning of these data is unclear. Is an increase of 178 per million woman-years of CV events clinically significant? It would be a shame to limit the availability of this effective contraception method to otherwise young and healthy women because of this very rare event.
Evidence summary
No studies specifically examine the risk of adverse outcomes for women who have mild elevations in blood pressure as a result of taking OCs. However, for the general population cardiovascular risk increases 30% for each 10 mm Hg rise in systolic pressure.1
A prospective cohort study found an increased incidence of new hypertension developing among women taking OCs. The study, conducted in the US, included 68,297 female nurses aged 25 to 42 years without a previous diagnosis of hypertension, diabetes, coronary heart disease, stroke, or cancer, who were followed for 4 years. Women were excluded from the study if they had not had a physical exam in the last 2 years or were taking antihypertensive medication at study inception. The nurses self-reported their blood pressure readings via questionnaire; medical records were sampled to validate the accuracy of self-reports. After adjusting for risk factors, current oral contraceptive use increased the risk of developing hypertension (relative risk [RR]=1.8; 95% confidence interval [CI], 1.5–2.3; corresponding to 41.5 new cases of hypertension per 10,000 person-years).2
A systematic review of 8 case control studies (with 4907 cases and 13,443 controls) found an increased risk of stroke and MI among hypertensive women taking combined type OCs vs those not taking OCs. Women with hypertension aged 20 to 24 years had an estimated CV event risk of 312 per million woman-years while taking OCs vs 134 per million woman-years not taking OCs. Among hypertensive women aged 40 to 44 years, the estimated risks were 1213 vs 529 per million woman-years, respectively. Primary endpoints varied across the studies and statistical significance was not given.3
Three studies showed that blood pressure elevations due to taking oral contraceptives returned to baseline with discontinuation of the medication. A prospective cohort study followed 13,358 women who were neither pregnant nor postpartum between the ages of 15 and 60. Women who either initiated or resumed using OCs experienced a statistically significant rise of about 4 mm Hg in the systolic pressure and 1 mm Hg in the diastolic pressure. Women who stopped using OCs experienced significant decreases in both systolic and diastolic components (about 5 mm Hg and 1.5 mm Hg, respectively).1
Similarly, a survey study of 461 women attending family planning clinics found that mean blood pressures were significantly higher for those taking OCs than for those using nonhormonal contraception. Elevated blood pressures correlated with duration of current use of OCs but returned to normal soon after stopping OCs. The mean pressures of those who had stopped OCs more than a month were similar to those of women who had never taken an OC and significantly lower than those of women who were currently taking OCs.4
Finally, a prospective study which followed 32 women who had taken combined OCs for 1 to 3 years and then stopped found that blood pressures returned to pretreatment levels at 3 months. Systolic pressure fell by 9.7 mm Hg and diastolic by 2.9 mm Hg compared with measurements made 1 month before stopping. No cardiovascular complications were reported among women during this study.5
Recommendations from others
The American College of Obstetricians and Gynecologists says that women with well-controlled and monitored hypertension aged 35 years and younger are appropriate candidates for a trial of combination OCs, provided they are otherwise healthy, have no evidence of end-organ vascular disease, and do not smoke cigarettes.6 If blood pressure remains well controlled with careful monitoring several months after initiating OCs, use can be continued.
1. Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA 1977;237:2499-2503.
2. Chasen-Taber L, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation 1996;94:483-489.
3. Curtis K, et al. Contraception for women in selected circumstances. Obstet Gynecol 2002;6:1100-1112.
4. Khaw K-T, Peart WS. Blood pressure and contraceptive use. Br Med J 1982;285:403-407.
5. Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood pressure in women taking oral contraceptives. Br Med J 1974;1:533-535.
6. American College of Obstetricians and Gynecologists (ACOG). The use of hormonal contraception in women with coexisting medical conditions. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2006 June. 20 p. (ACOG practice bulletin; no. 73).
1. Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA 1977;237:2499-2503.
2. Chasen-Taber L, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation 1996;94:483-489.
3. Curtis K, et al. Contraception for women in selected circumstances. Obstet Gynecol 2002;6:1100-1112.
4. Khaw K-T, Peart WS. Blood pressure and contraceptive use. Br Med J 1982;285:403-407.
5. Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood pressure in women taking oral contraceptives. Br Med J 1974;1:533-535.
6. American College of Obstetricians and Gynecologists (ACOG). The use of hormonal contraception in women with coexisting medical conditions. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2006 June. 20 p. (ACOG practice bulletin; no. 73).
Evidence-based answers from the Family Physicians Inquiries Network
What is the recommended approach to asymptomatic patients who develop a reactive PPD?
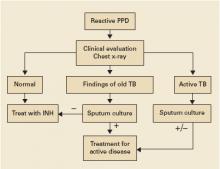
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
Clinical evaluation and chest x-ray are recommended for asymptomatic patients with a positive purified protein derivative (PPD) test result, to exclude the slight possibility of active tuberculosis (TB). Patients with radiographic evidence of old (healed) TB infection should also undergo sputum testing (strength of recommendation [SOR]: C, expert opinion).
Treatment with isoniazid (INH) monotherapy (300 mg/d) reduces progression of latent tuberculosis to active disease (SOR: A, large randomized controlled trials [RCT]), with 9 months as the optimal treatment length (SOR: B, derivation from RCTs). A 3-month course of combined rifampin (600 mg/d) and INH (300 mg/d) is equivalent in efficacy to INH monotherapy and is associated with similar rates of toxicity (SOR: A, meta-analysis of RCTs), but this regimen is not included in Centers for Disease Control and Prevention recommendations.
Address patient concerns about TB and treatment side effects
Richard Guthmann, MD
University of Illinois at Chicago/Advocate Illinois Masonic Family Medicine Residency, Chicago
Patients’ understanding of tuberculosis—the disease, the treatment, and the outcome—poses an important challenge in the care of an asymptomatic PPD-positive patient. These patients may ask, “Will I get sick? Do I have to take the medicine? Are there side effects? And would you take the medicine?” We need to be prepared to answer these questions.
Most patients with a positive PPD will not get active tuberculosis, but when they do it can be serious and it can spread easily. The medication significantly decreases the risk of developing active tuberculosis. The medication side effects are uncommon but can be severe. These side effects are reversible if the medication is stopped promptly. Under the supervision of my physician, I would take the medicine.
Evidence summary
Clinical evaluation with medical history and physical exam, chest radiography, and selected sputum sampling to exclude active tuberculosis are part of the recommended algorithm for all patients who develop a positive PPD (FIGURE).1-3 These recommendations are derived from expert opinion, and their usefulness has not been evaluated in any population-based study of asymptomatic PPD-positive patients.
A comprehensive review of RCTs from the 1950s and 1960s demonstrated that INH treatment of patients with latent tuberculosis infection is effective in decreasing the progression to active tuberculosis.4 A series of double-blinded RCTs performed by the US Public Health Service included 25,923 patients with latent tuberculosis who were randomized to receive either daily INH or placebo for 1 year with 6- to 10-year follow-up. Groups studied included household contacts of patients with active tuberculosis (rate of progression to active disease in placebo group [baseline rate]=27/1000, relative risk with INH [RR]=0.4, number needed to treat [NNT]=63), patients in mental institutions (baseline rate=12/1000, RR=0.3, NNT=121), and patients with x-ray findings of healed tuberculosis (baseline rate=69/1000, RR=0.4, NNT=23).
The optimal length of treatment for PPD-positive patients without active disease was evaluated through 1 double-blinded RCT enrolling 28,000 patients with 5-year follow-up after 12, 24, or 52 weeks of INH or placebo. Active TB developed in 0.35% (24/6919) after 52 weeks of INH compared with 0.49% (34/6965) after 24 weeks (RR=1.4, NNT=708).5 Incidence in the placebo group was 1.4%. Subgroup analysis determined that maximum efficacy with fewest side effects was achieved at 9 months.6 Nine months of INH is also recommended for HIV-positive patients, based on extrapolations from these and other studies.3
INH monotherapy was compared with combination INH and rifampin in a 2005 meta-analysis of 5 RCTs of variable quality involving 1926 patients.7 This meta-analysis found equivalency in risk of active TB and mortality between INH monotherapy for 6 to 12 months and the combination of rifampin and INH for 3 months (pooled risk difference=0%; 95% confidence interval [CI], –1% to 2%). This study also showed similar rates of adverse events in both groups (pooled risk difference=–1%; 95% CI, –7% to 5%). Short-course combination rifampin and pyrazinamide is no longer recommended after an open-label RCT with 589 patients demonstrated severe hepatoxicity in 7.7% (16/207) on a 2-month course of pyrazinamide and rifampin, compared with 1% (2/204) on 6 months of INH (RR=7.9, number needed to harm=15).8 Rifampin monotherapy has only been studied in patients with silicosis in a RCT enrolling 652 participants with latent tuberculosis. A 12-week course of rifampin (600 mg daily) was as effective as 6 months of INH in preventing development of active TB over the next 5 years.9
FIGURE
Suggested workup of asymptomatic, HIV-negative patients with a positive PPD
Source: Am J Respir Crit Care Med 2000;2 Jasmer et al, N Engl J Med 2002.3
Recommendations from others
Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Disease Society of America guidelines recommend targeted screening of high-risk persons followed by further clinical evaluation of all those with a reactive PPD (FIGURE).2,10 The recommended treatment regimen for latent TB is daily INH for 9 months. Less preferable regimens are daily INH for 6 months, or daily rifampin for 4 months in patients who cannot tolerate INH. A 2-month course of rifampin and pyrazinamide is no longer recommended. The recent meta-analysis supporting a 3-month regimen of combination INH and rifampin has not been incorporated into expert guidelines.7
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
1. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
2. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
3. Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med 2002;347:1860-1866.
4. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc 1970;26:28-106.
5. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555-564.
6. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999;3:847-850.
7. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005;40:670-676.
8. Jasmer RM, Saukkonen JJ, Blumberg HM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640-647.
9. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41.
10. Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. MMWR Recomm Rep 2005;54(RR-12):1-81.
Evidence-based answers from the Family Physicians Inquiries Network
What is the recommended evaluation and treatment for elevated serum prolactin?
History and physical examination can distinguish among most physiologic, pharmacologic, or pathologic causes of an elevated serum prolactin level (SPL) (strength of recommendation [SOR]: C, expert opinion). Patients with unexplained elevations of serum prolactin or with a level above 200 ng/mL should undergo imaging of the sella turcica (SOR: C, expert opinion). Mildly elevated SPL due to physiologic causes may be managed expectantly (SOR: B, cohort studies) and pharmacologic elevations may be treated by discontinuing the causative medication (SOR: C, expert opinion). Elevated SPL due to pathologic causes requires both monitoring for complications and treatment of the underlying condition (SOR: C, expert opinion).
Dopamine agonists are effective for patients requiring drug treatment (SOR: B, systematic review of cohort studies), and cabergoline is more effective and better tolerated than bromocriptine (SOR: B, randomized controlled trial [RCT]). Surgery is reserved for symptomatic patients not controlled medically (SOR: C, expert opinion).
Patients with mildly elevated SPLs can be safely watched with testing and symptom monitoring
Allen Daugird, MD
University of North Carolina at Chapel Hill
Most elevated prolactin levels in my practice have been mild and often secondary to medication, though there are a host of causes, as listed in the TABLE. This Clinical Inquiry reassures us that patients with mildly elevated SPLs can be safely watched with serial testing and monitoring symptoms. Obtaining SPLs only on fasting specimens can help improve test accuracy. The feared risk of vision loss due to a macroadenoma seems to be quite small. Patients with significantly elevated SPLs with amenorrhea or infertility deserve referral to clinicians comfortable with using dopamine agonists because of the high rate of success with this treatment.
Evidence summary
An expert guideline recommends a history and physical examination to determine whether an elevated SPL is due to physiologic, pharmacologic, or pathologic causes (TABLE).1 The fasting morning SPL is least variable and correlates best with a disease state.1 Clinical correlation is necessary to reveal false positives (due to biologically inactive forms of prolactin) or false negatives (due to very high SPLs that exceed the ability of the assay). If an elevated SPL is suspected despite a normal laboratory report, retesting with serum diluted 1:100 can identify a false-negative value.2
A detailed drug history is important since drug-induced elevated SPL is common.1 Laboratory evaluation includes thyroid-stimulating hormone, blood urea nitrogen, and creatinine, as well as pregnancy testing when applicable. If no cause of elevated SPL is identified by initial clinical evaluation or if the SPL is greater than 200 ng/mL, experts recommend imaging of the sella turcica with computed tomography or magnetic resonance imaging.1
Physiologic causes. For patients with a mildly elevated SPL due to a physiologic cause, experts recommend expectant management. Patients should be monitored for symptoms of hypogonadism (amenorrhea, infertility, or sexual dysfunction) and have SPL measured at 6- to 12-month intervals.1 In cohort studies, treatment of the underlying cause of elevated SPL reverses secondary physiologic changes of low estrogen or testosterone, and hypogonadism.3-5
Pharmacologic causes. Eliminating a pharmacologic cause may lead to normalization of SPL, although experts recommend psychiatric consultation before discontinuing neuroleptic medications.1
Pathologic causes. Experts advise treating the underlying cause of a pathologic elevation of SPL. Patients with microadenoma should have SPLs monitored to prevent complications of decreased bone mineral density and sexual dysfunction due to persistently elevated SPL. Patients with a macroadenoma (>1 cm) are at risk for tumor growth and require serial imaging studies in addition to treatment of SPL, according to expert opinion.1-3
Medical therapy. Medical therapy with a dopamine agonist is indicated for patients with either symptoms of hypogonadism due to elevated SPL, or neurologic symptoms due to the size of a macroadenoma.1 In a review of 13 cohort studies, bromocriptine improved symptoms and reduced SPLs to normal for 229 of 280 women (82%).6 A cohort study of 27 patients with macroadenomas treated with bromocriptine found 10% to 50% reductions of tumor size.7 A randomized controlled trial treating 459 women having hyperprolactinemic amenorrhea with either cabergoline or bromocriptine achieved a stable normal SPL in 83% and 59%, respectively (P<.001). Adverse effects were common but were less common with cabergoline (68% vs 78%) and resulted in fewer discontinuations (3% vs 12%).8
Surgical therapy. Surgery is indicated for patients unresponsive to or intolerant of medical therapy, or who have visual field loss, cranial nerve palsy, or headache due to macroadenoma.1 A retrospective review of patients who underwent surgical resection found a 40% recurrence rate.9
Recommendations from others
Williams Textbook of Endocrinology includes the recommendations above and advises seeking consultation for patients with mass effects of macroadenomas such as visual field loss, cranial nerve palsy, or headaches; for patients with progressive elevation of SPL despite medical treatment; and for pregnant women.4 Conventional antipsychotic agents are commonly associated with elevated prolactin due to dopamine agonist activity. Some atypical antipsychotics may lead to lower levels of elevated prolactin, transient elevations or marked elevations.10 Experts recommend following serial SPLs, if antipsychotics are truly needed. Psychiatric consultation may assist in making decisions about medication selection. Patients with symptoms (galactorrhea, amenorrhea, headaches, visual disturbances, sexual dysfunction) or levels of 200 or more, should undergo an MRI or CT. Experts recommend monitoring levels every 1 to 3 months.1
TABLE
Physiologic, pharmacologic, and pathologic causes of an elevated serum prolactin level1
| PHYSIOLOGIC |
| Pregnancy |
| Ectopic pregnancy |
| Lactation |
| Nipple stimulation |
| Stress |
| Sleep disorder |
| PHARMACOLGIC |
| Dopamine receptor antagonists: phenothiazines, butyrophenones, thioxanthene, risperidone, metoclopramide, sulpiride, pimozide |
| Dopamine-depleting agents: α-methyldopa, reserpine |
| Hormones: estrogens, antiandrogens |
| Others: danazol, isoniazid, verapamil, cyproheptadine, opiates, H2-blockers (cimetidine), cocaine and marijuana, tricyclic antidepressants |
| PATHOLOGIC |
| Acromegaly |
| Alcoholic cirrhosis |
| Chest wall trauma or tumor |
| Herpes zoster |
| Hypothalamic and pituitary stalk disease |
| Hypothyroidism |
| Pituitary tumors: prolactinomas, adenomas |
| Polycystic ovarian syndrome |
| Renal failure |
| Sarcoidosis |
1. Biller BM, Luciano A, Crosignani PG, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1075-1084.
2. Barkan AL, Chandler WF. Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the “high hook effect”: Case report. Neurosurgery 1998;42:913-915.
3. Sanfilippo JS. Implications of not treating hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1111-1115.
4. Melmed S, Kleinberg D. Physiology and disorders of the pituitary hormone axes. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia, Pa: Saunders; 2003;200-212.
5. Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A. The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab 1989;68:412-418
6. Vance ML, Evans WS, Thorner MO. Drugs five years later. Bromocriptine. Ann Intern Med 1984;100:78-91.
7. Molitch ME, Elton RL, Blackwell RE, Caldwell B, Chang RJ, Jaffe R, et al. Bromocriptine as primary therapy for prolactin-secreting macroadenomas: results of a prospective multicenter study. J Clin Endocrinol Metab 1985;60:698-705.
8. Webster J, Piscitelli G, Polli A, Ferrari C, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med 1994;331:904-909.
9. Abrahamson M, Snyder P. Treatment of hyperprolactin due to lactotroph adenomas and other causes. UpToDate [database]. Waltham, Mass: UpToDate; 2004.
10. Smith S. Effects of antipsychotics on sexual and endocrine function in women: implications in clinical practice. J Clin Psychopharmacol 2003;23(3 Suppl 1):S27-S32.
History and physical examination can distinguish among most physiologic, pharmacologic, or pathologic causes of an elevated serum prolactin level (SPL) (strength of recommendation [SOR]: C, expert opinion). Patients with unexplained elevations of serum prolactin or with a level above 200 ng/mL should undergo imaging of the sella turcica (SOR: C, expert opinion). Mildly elevated SPL due to physiologic causes may be managed expectantly (SOR: B, cohort studies) and pharmacologic elevations may be treated by discontinuing the causative medication (SOR: C, expert opinion). Elevated SPL due to pathologic causes requires both monitoring for complications and treatment of the underlying condition (SOR: C, expert opinion).
Dopamine agonists are effective for patients requiring drug treatment (SOR: B, systematic review of cohort studies), and cabergoline is more effective and better tolerated than bromocriptine (SOR: B, randomized controlled trial [RCT]). Surgery is reserved for symptomatic patients not controlled medically (SOR: C, expert opinion).
Patients with mildly elevated SPLs can be safely watched with testing and symptom monitoring
Allen Daugird, MD
University of North Carolina at Chapel Hill
Most elevated prolactin levels in my practice have been mild and often secondary to medication, though there are a host of causes, as listed in the TABLE. This Clinical Inquiry reassures us that patients with mildly elevated SPLs can be safely watched with serial testing and monitoring symptoms. Obtaining SPLs only on fasting specimens can help improve test accuracy. The feared risk of vision loss due to a macroadenoma seems to be quite small. Patients with significantly elevated SPLs with amenorrhea or infertility deserve referral to clinicians comfortable with using dopamine agonists because of the high rate of success with this treatment.
Evidence summary
An expert guideline recommends a history and physical examination to determine whether an elevated SPL is due to physiologic, pharmacologic, or pathologic causes (TABLE).1 The fasting morning SPL is least variable and correlates best with a disease state.1 Clinical correlation is necessary to reveal false positives (due to biologically inactive forms of prolactin) or false negatives (due to very high SPLs that exceed the ability of the assay). If an elevated SPL is suspected despite a normal laboratory report, retesting with serum diluted 1:100 can identify a false-negative value.2
A detailed drug history is important since drug-induced elevated SPL is common.1 Laboratory evaluation includes thyroid-stimulating hormone, blood urea nitrogen, and creatinine, as well as pregnancy testing when applicable. If no cause of elevated SPL is identified by initial clinical evaluation or if the SPL is greater than 200 ng/mL, experts recommend imaging of the sella turcica with computed tomography or magnetic resonance imaging.1
Physiologic causes. For patients with a mildly elevated SPL due to a physiologic cause, experts recommend expectant management. Patients should be monitored for symptoms of hypogonadism (amenorrhea, infertility, or sexual dysfunction) and have SPL measured at 6- to 12-month intervals.1 In cohort studies, treatment of the underlying cause of elevated SPL reverses secondary physiologic changes of low estrogen or testosterone, and hypogonadism.3-5
Pharmacologic causes. Eliminating a pharmacologic cause may lead to normalization of SPL, although experts recommend psychiatric consultation before discontinuing neuroleptic medications.1
Pathologic causes. Experts advise treating the underlying cause of a pathologic elevation of SPL. Patients with microadenoma should have SPLs monitored to prevent complications of decreased bone mineral density and sexual dysfunction due to persistently elevated SPL. Patients with a macroadenoma (>1 cm) are at risk for tumor growth and require serial imaging studies in addition to treatment of SPL, according to expert opinion.1-3
Medical therapy. Medical therapy with a dopamine agonist is indicated for patients with either symptoms of hypogonadism due to elevated SPL, or neurologic symptoms due to the size of a macroadenoma.1 In a review of 13 cohort studies, bromocriptine improved symptoms and reduced SPLs to normal for 229 of 280 women (82%).6 A cohort study of 27 patients with macroadenomas treated with bromocriptine found 10% to 50% reductions of tumor size.7 A randomized controlled trial treating 459 women having hyperprolactinemic amenorrhea with either cabergoline or bromocriptine achieved a stable normal SPL in 83% and 59%, respectively (P<.001). Adverse effects were common but were less common with cabergoline (68% vs 78%) and resulted in fewer discontinuations (3% vs 12%).8
Surgical therapy. Surgery is indicated for patients unresponsive to or intolerant of medical therapy, or who have visual field loss, cranial nerve palsy, or headache due to macroadenoma.1 A retrospective review of patients who underwent surgical resection found a 40% recurrence rate.9
Recommendations from others
Williams Textbook of Endocrinology includes the recommendations above and advises seeking consultation for patients with mass effects of macroadenomas such as visual field loss, cranial nerve palsy, or headaches; for patients with progressive elevation of SPL despite medical treatment; and for pregnant women.4 Conventional antipsychotic agents are commonly associated with elevated prolactin due to dopamine agonist activity. Some atypical antipsychotics may lead to lower levels of elevated prolactin, transient elevations or marked elevations.10 Experts recommend following serial SPLs, if antipsychotics are truly needed. Psychiatric consultation may assist in making decisions about medication selection. Patients with symptoms (galactorrhea, amenorrhea, headaches, visual disturbances, sexual dysfunction) or levels of 200 or more, should undergo an MRI or CT. Experts recommend monitoring levels every 1 to 3 months.1
TABLE
Physiologic, pharmacologic, and pathologic causes of an elevated serum prolactin level1
| PHYSIOLOGIC |
| Pregnancy |
| Ectopic pregnancy |
| Lactation |
| Nipple stimulation |
| Stress |
| Sleep disorder |
| PHARMACOLGIC |
| Dopamine receptor antagonists: phenothiazines, butyrophenones, thioxanthene, risperidone, metoclopramide, sulpiride, pimozide |
| Dopamine-depleting agents: α-methyldopa, reserpine |
| Hormones: estrogens, antiandrogens |
| Others: danazol, isoniazid, verapamil, cyproheptadine, opiates, H2-blockers (cimetidine), cocaine and marijuana, tricyclic antidepressants |
| PATHOLOGIC |
| Acromegaly |
| Alcoholic cirrhosis |
| Chest wall trauma or tumor |
| Herpes zoster |
| Hypothalamic and pituitary stalk disease |
| Hypothyroidism |
| Pituitary tumors: prolactinomas, adenomas |
| Polycystic ovarian syndrome |
| Renal failure |
| Sarcoidosis |
History and physical examination can distinguish among most physiologic, pharmacologic, or pathologic causes of an elevated serum prolactin level (SPL) (strength of recommendation [SOR]: C, expert opinion). Patients with unexplained elevations of serum prolactin or with a level above 200 ng/mL should undergo imaging of the sella turcica (SOR: C, expert opinion). Mildly elevated SPL due to physiologic causes may be managed expectantly (SOR: B, cohort studies) and pharmacologic elevations may be treated by discontinuing the causative medication (SOR: C, expert opinion). Elevated SPL due to pathologic causes requires both monitoring for complications and treatment of the underlying condition (SOR: C, expert opinion).
Dopamine agonists are effective for patients requiring drug treatment (SOR: B, systematic review of cohort studies), and cabergoline is more effective and better tolerated than bromocriptine (SOR: B, randomized controlled trial [RCT]). Surgery is reserved for symptomatic patients not controlled medically (SOR: C, expert opinion).
Patients with mildly elevated SPLs can be safely watched with testing and symptom monitoring
Allen Daugird, MD
University of North Carolina at Chapel Hill
Most elevated prolactin levels in my practice have been mild and often secondary to medication, though there are a host of causes, as listed in the TABLE. This Clinical Inquiry reassures us that patients with mildly elevated SPLs can be safely watched with serial testing and monitoring symptoms. Obtaining SPLs only on fasting specimens can help improve test accuracy. The feared risk of vision loss due to a macroadenoma seems to be quite small. Patients with significantly elevated SPLs with amenorrhea or infertility deserve referral to clinicians comfortable with using dopamine agonists because of the high rate of success with this treatment.
Evidence summary
An expert guideline recommends a history and physical examination to determine whether an elevated SPL is due to physiologic, pharmacologic, or pathologic causes (TABLE).1 The fasting morning SPL is least variable and correlates best with a disease state.1 Clinical correlation is necessary to reveal false positives (due to biologically inactive forms of prolactin) or false negatives (due to very high SPLs that exceed the ability of the assay). If an elevated SPL is suspected despite a normal laboratory report, retesting with serum diluted 1:100 can identify a false-negative value.2
A detailed drug history is important since drug-induced elevated SPL is common.1 Laboratory evaluation includes thyroid-stimulating hormone, blood urea nitrogen, and creatinine, as well as pregnancy testing when applicable. If no cause of elevated SPL is identified by initial clinical evaluation or if the SPL is greater than 200 ng/mL, experts recommend imaging of the sella turcica with computed tomography or magnetic resonance imaging.1
Physiologic causes. For patients with a mildly elevated SPL due to a physiologic cause, experts recommend expectant management. Patients should be monitored for symptoms of hypogonadism (amenorrhea, infertility, or sexual dysfunction) and have SPL measured at 6- to 12-month intervals.1 In cohort studies, treatment of the underlying cause of elevated SPL reverses secondary physiologic changes of low estrogen or testosterone, and hypogonadism.3-5
Pharmacologic causes. Eliminating a pharmacologic cause may lead to normalization of SPL, although experts recommend psychiatric consultation before discontinuing neuroleptic medications.1
Pathologic causes. Experts advise treating the underlying cause of a pathologic elevation of SPL. Patients with microadenoma should have SPLs monitored to prevent complications of decreased bone mineral density and sexual dysfunction due to persistently elevated SPL. Patients with a macroadenoma (>1 cm) are at risk for tumor growth and require serial imaging studies in addition to treatment of SPL, according to expert opinion.1-3
Medical therapy. Medical therapy with a dopamine agonist is indicated for patients with either symptoms of hypogonadism due to elevated SPL, or neurologic symptoms due to the size of a macroadenoma.1 In a review of 13 cohort studies, bromocriptine improved symptoms and reduced SPLs to normal for 229 of 280 women (82%).6 A cohort study of 27 patients with macroadenomas treated with bromocriptine found 10% to 50% reductions of tumor size.7 A randomized controlled trial treating 459 women having hyperprolactinemic amenorrhea with either cabergoline or bromocriptine achieved a stable normal SPL in 83% and 59%, respectively (P<.001). Adverse effects were common but were less common with cabergoline (68% vs 78%) and resulted in fewer discontinuations (3% vs 12%).8
Surgical therapy. Surgery is indicated for patients unresponsive to or intolerant of medical therapy, or who have visual field loss, cranial nerve palsy, or headache due to macroadenoma.1 A retrospective review of patients who underwent surgical resection found a 40% recurrence rate.9
Recommendations from others
Williams Textbook of Endocrinology includes the recommendations above and advises seeking consultation for patients with mass effects of macroadenomas such as visual field loss, cranial nerve palsy, or headaches; for patients with progressive elevation of SPL despite medical treatment; and for pregnant women.4 Conventional antipsychotic agents are commonly associated with elevated prolactin due to dopamine agonist activity. Some atypical antipsychotics may lead to lower levels of elevated prolactin, transient elevations or marked elevations.10 Experts recommend following serial SPLs, if antipsychotics are truly needed. Psychiatric consultation may assist in making decisions about medication selection. Patients with symptoms (galactorrhea, amenorrhea, headaches, visual disturbances, sexual dysfunction) or levels of 200 or more, should undergo an MRI or CT. Experts recommend monitoring levels every 1 to 3 months.1
TABLE
Physiologic, pharmacologic, and pathologic causes of an elevated serum prolactin level1
| PHYSIOLOGIC |
| Pregnancy |
| Ectopic pregnancy |
| Lactation |
| Nipple stimulation |
| Stress |
| Sleep disorder |
| PHARMACOLGIC |
| Dopamine receptor antagonists: phenothiazines, butyrophenones, thioxanthene, risperidone, metoclopramide, sulpiride, pimozide |
| Dopamine-depleting agents: α-methyldopa, reserpine |
| Hormones: estrogens, antiandrogens |
| Others: danazol, isoniazid, verapamil, cyproheptadine, opiates, H2-blockers (cimetidine), cocaine and marijuana, tricyclic antidepressants |
| PATHOLOGIC |
| Acromegaly |
| Alcoholic cirrhosis |
| Chest wall trauma or tumor |
| Herpes zoster |
| Hypothalamic and pituitary stalk disease |
| Hypothyroidism |
| Pituitary tumors: prolactinomas, adenomas |
| Polycystic ovarian syndrome |
| Renal failure |
| Sarcoidosis |
1. Biller BM, Luciano A, Crosignani PG, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1075-1084.
2. Barkan AL, Chandler WF. Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the “high hook effect”: Case report. Neurosurgery 1998;42:913-915.
3. Sanfilippo JS. Implications of not treating hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1111-1115.
4. Melmed S, Kleinberg D. Physiology and disorders of the pituitary hormone axes. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia, Pa: Saunders; 2003;200-212.
5. Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A. The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab 1989;68:412-418
6. Vance ML, Evans WS, Thorner MO. Drugs five years later. Bromocriptine. Ann Intern Med 1984;100:78-91.
7. Molitch ME, Elton RL, Blackwell RE, Caldwell B, Chang RJ, Jaffe R, et al. Bromocriptine as primary therapy for prolactin-secreting macroadenomas: results of a prospective multicenter study. J Clin Endocrinol Metab 1985;60:698-705.
8. Webster J, Piscitelli G, Polli A, Ferrari C, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med 1994;331:904-909.
9. Abrahamson M, Snyder P. Treatment of hyperprolactin due to lactotroph adenomas and other causes. UpToDate [database]. Waltham, Mass: UpToDate; 2004.
10. Smith S. Effects of antipsychotics on sexual and endocrine function in women: implications in clinical practice. J Clin Psychopharmacol 2003;23(3 Suppl 1):S27-S32.
1. Biller BM, Luciano A, Crosignani PG, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1075-1084.
2. Barkan AL, Chandler WF. Giant pituitary prolactinoma with falsely low serum prolactin: the pitfall of the “high hook effect”: Case report. Neurosurgery 1998;42:913-915.
3. Sanfilippo JS. Implications of not treating hyperprolactinemia. J Reprod Med 1999;44(12 Suppl):1111-1115.
4. Melmed S, Kleinberg D. Physiology and disorders of the pituitary hormone axes. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia, Pa: Saunders; 2003;200-212.
5. Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A. The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab 1989;68:412-418
6. Vance ML, Evans WS, Thorner MO. Drugs five years later. Bromocriptine. Ann Intern Med 1984;100:78-91.
7. Molitch ME, Elton RL, Blackwell RE, Caldwell B, Chang RJ, Jaffe R, et al. Bromocriptine as primary therapy for prolactin-secreting macroadenomas: results of a prospective multicenter study. J Clin Endocrinol Metab 1985;60:698-705.
8. Webster J, Piscitelli G, Polli A, Ferrari C, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med 1994;331:904-909.
9. Abrahamson M, Snyder P. Treatment of hyperprolactin due to lactotroph adenomas and other causes. UpToDate [database]. Waltham, Mass: UpToDate; 2004.
10. Smith S. Effects of antipsychotics on sexual and endocrine function in women: implications in clinical practice. J Clin Psychopharmacol 2003;23(3 Suppl 1):S27-S32.
Evidence-based answers from the Family Physicians Inquiries Network
What treatments prevent miscarriage after recurrent pregnancy loss?
Progesterone produces a small but significant decrease in miscarriage among pregnant women with 3 or more unexplained pregnancy losses (strength of recommendation [SOR]: A, based on a meta-analysis of 3 small randomized controlled trials [RCTs] with wide confidence intervals). Human chorionic gonadotropin (HCG) reduces the rate of recurrent pregnancy loss among women with 2 or more unexplained pregnancy losses (SOR: B, based on a meta-analysis of 4 RCTs with significant methodologic weaknesses).
Four types of immunotherapy are ineffective for preventing miscarriage (SOR: A, based on RCTs and systematic reviews of RCTs). Aspirin therapy is ineffective for preventing recurrent miscarriage for women who do not have an autoimmune explanation for previous pregnancy losses (SOR: A, based on RCTs).
Document your patient’s understanding of the risks and benefits
Beth Damitz, MD
Medical College of Wisconsin
When discussing future childbearing with a woman who has had multiple miscarriages, there are several important issues to address. First, ask what concerns she might have about becoming pregnant again. Second, ascertain how significant another pregnancy loss would be to her. Third, outline the therapeutic options, clearly stating that they alter loss rates but do not guarantee successful delivery. Finally, fully document her understanding of the risks and benefits, including the possibility of treatment failure. Remember, even if the miscarriage rate is reduced from 25% to 20% with treatment, should your patient miscarry, her miscarriage rate is 100%!
Evidence summary
Progesterone. A Cochrane meta-analysis on the use of progesterone to prevent pregnancy loss looked at a subset of 3 small RCTs that evaluated women with 3 or more pregnancy losses. Patients with primary recurrent spontaneous abortion (RSA) (no prior live births), were not differentiated from those with secondary RSA (previous live birth with subsequent miscarriages).
Progesterone administration resulted in a significant reduction in miscarriage compared with placebo (odds ratio [OR]=0.37; 95% confidence interval [CI], 0.17–0.91), independent of administration routes (oral, vaginal, or intramuscular). This benefit was lost in the larger meta-analysis when studies containing women with fewer than 3 pregnancy losses were included.1
Human choriogonadotropin. A meta-analysis reviewed 4 trials (n=180 total) of varying methodological quality, which were constructed to determine if women, with at least 2 consecutive miscarriages of unknown cause, derive any protective effect when they receive HCG during the first trimester. Although the overall outcome favored the use of HCG (OR=0.26 compared with placebo; 95% CI, 0.14–0.52), the trials contained major methodological weaknesses (poor description of methods, no power calculations, selection and unclear randomization techniques).2
Immunotherapy. A systematic review of 22 RCTs evaluating 4 different types of immunotherapy for recurrent miscarriage found no significant improvement in live birth rates. All studies were of high quality with a low level of bias. Only one lacked double-blinding.
Immunotherapy types included: paternal leukocyte immunization (PLI) (11 trials, 596 women) (OR=1.05; 95% CI, 0.75–1.47); intravenous immune globulin (IVIG) (OR=0.98; 95% CI, 0.61–1.58); third-party donor cell immunization (3 trials, 156 women) (OR=1.39; 95% CI, 0.68–2.82); and trophoblast membrane infusion (1 trial, 37 women) (OR=0.40; 95% CI, 0.11–1.45).3
A subsequent RCT comparing PLI with placebo among 79 women with primary RSA of unknown cause again found no significant difference in live birth rates (89% vs 71%, respectively).4 However, an additional RCT evaluating PLI (32 patients) vs placebo (19 patients) among women with unexplained primary RSA did find significantly higher birth rates with PLI (84% vs 25%; P=.001). This small study used different techniques than previous PLI studies.5
A later meta-analysis of 5 RCTs including a total of 246 patients also found that IVIG did not improve the subsequent live birth rate for women with a history of primary or secondary RSA (OR=0.98; 95% CI, 0.45–2.13).6
Aspirin. An RCT involving 54 pregnant women (mean age 32.7 years) with a history of primary RSA of unknown cause (negative standard workup) evaluated 50 mg of aspirin daily (n=27) vs placebo (n=27).7 The method of blinding was not reported.
The live birth rate was identical for the 2 groups (88%). A second (unblinded) trial randomized 805 women from a large referral center (mean age 34 years) with a history of first-trimester RSA (not differentiated between primary and secondary RSA) of unknown cause to either 75 mg of aspirin daily or no treatment.8 There was no significant difference in the live birth rate between those who took aspirin (251/367; 68.4%) and those who did not (278/438; 63.5%; OR=1.24; 95% CI, 0.93–1.67).
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) states that “it has not been shown conclusively that progesterone treatment or corpus luteum support (HCG) influences pregnancy outcome for women with recurrent spontaneous abortion.”9 ACOG does not recommend immunotherapy, citing a lack of demonstrated efficacy (IVIG and PLI), a lack of standards for cell storage and administration, and a risk profile similar to that of blood transfusion (PLI). They recommend “couples with otherwise unexplained recurrent pregnancy loss should be counseled regarding the potential for successful pregnancy without treatment.”
1. Oates-Whitehead RM, Haas DM, Carrier JA. Progestogen for preventing miscarriage. Cochrane Database Syst Rev 2003;(4):CD003511.-
2. Scott JR, Pattison N. Human chorionic gonadotrophin for recurrent miscarriage. Cochrane Database Syst Rev 2000;(2):CD000101.-
3. Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev 2003;(1):CD000112.-
4. Ramhorst R, Agriello E, Zittermann S, et al. Is the paternal mononuclear cells’ immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol 2000;44:129-135.
5. Pandey MK, Agrawal S. Induction of MLR-Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int Immunopharmacol 2004;4:289-298.
6. Practice Committee of the American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril 2004;82 Suppl 1:S199-S200.
7. Tulppala M, Marttunen M. Soderstrom-Anttila V, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997;12:1567-1572.
8. Rai R, Backos M, Baxter N, Chilcott I, Regan L. Recurrent miscarriage-an aspirin a day? Hum Reprod 2000;15:2220-2223.
9. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;78:179-190.
Progesterone produces a small but significant decrease in miscarriage among pregnant women with 3 or more unexplained pregnancy losses (strength of recommendation [SOR]: A, based on a meta-analysis of 3 small randomized controlled trials [RCTs] with wide confidence intervals). Human chorionic gonadotropin (HCG) reduces the rate of recurrent pregnancy loss among women with 2 or more unexplained pregnancy losses (SOR: B, based on a meta-analysis of 4 RCTs with significant methodologic weaknesses).
Four types of immunotherapy are ineffective for preventing miscarriage (SOR: A, based on RCTs and systematic reviews of RCTs). Aspirin therapy is ineffective for preventing recurrent miscarriage for women who do not have an autoimmune explanation for previous pregnancy losses (SOR: A, based on RCTs).
Document your patient’s understanding of the risks and benefits
Beth Damitz, MD
Medical College of Wisconsin
When discussing future childbearing with a woman who has had multiple miscarriages, there are several important issues to address. First, ask what concerns she might have about becoming pregnant again. Second, ascertain how significant another pregnancy loss would be to her. Third, outline the therapeutic options, clearly stating that they alter loss rates but do not guarantee successful delivery. Finally, fully document her understanding of the risks and benefits, including the possibility of treatment failure. Remember, even if the miscarriage rate is reduced from 25% to 20% with treatment, should your patient miscarry, her miscarriage rate is 100%!
Evidence summary
Progesterone. A Cochrane meta-analysis on the use of progesterone to prevent pregnancy loss looked at a subset of 3 small RCTs that evaluated women with 3 or more pregnancy losses. Patients with primary recurrent spontaneous abortion (RSA) (no prior live births), were not differentiated from those with secondary RSA (previous live birth with subsequent miscarriages).
Progesterone administration resulted in a significant reduction in miscarriage compared with placebo (odds ratio [OR]=0.37; 95% confidence interval [CI], 0.17–0.91), independent of administration routes (oral, vaginal, or intramuscular). This benefit was lost in the larger meta-analysis when studies containing women with fewer than 3 pregnancy losses were included.1
Human choriogonadotropin. A meta-analysis reviewed 4 trials (n=180 total) of varying methodological quality, which were constructed to determine if women, with at least 2 consecutive miscarriages of unknown cause, derive any protective effect when they receive HCG during the first trimester. Although the overall outcome favored the use of HCG (OR=0.26 compared with placebo; 95% CI, 0.14–0.52), the trials contained major methodological weaknesses (poor description of methods, no power calculations, selection and unclear randomization techniques).2
Immunotherapy. A systematic review of 22 RCTs evaluating 4 different types of immunotherapy for recurrent miscarriage found no significant improvement in live birth rates. All studies were of high quality with a low level of bias. Only one lacked double-blinding.
Immunotherapy types included: paternal leukocyte immunization (PLI) (11 trials, 596 women) (OR=1.05; 95% CI, 0.75–1.47); intravenous immune globulin (IVIG) (OR=0.98; 95% CI, 0.61–1.58); third-party donor cell immunization (3 trials, 156 women) (OR=1.39; 95% CI, 0.68–2.82); and trophoblast membrane infusion (1 trial, 37 women) (OR=0.40; 95% CI, 0.11–1.45).3
A subsequent RCT comparing PLI with placebo among 79 women with primary RSA of unknown cause again found no significant difference in live birth rates (89% vs 71%, respectively).4 However, an additional RCT evaluating PLI (32 patients) vs placebo (19 patients) among women with unexplained primary RSA did find significantly higher birth rates with PLI (84% vs 25%; P=.001). This small study used different techniques than previous PLI studies.5
A later meta-analysis of 5 RCTs including a total of 246 patients also found that IVIG did not improve the subsequent live birth rate for women with a history of primary or secondary RSA (OR=0.98; 95% CI, 0.45–2.13).6
Aspirin. An RCT involving 54 pregnant women (mean age 32.7 years) with a history of primary RSA of unknown cause (negative standard workup) evaluated 50 mg of aspirin daily (n=27) vs placebo (n=27).7 The method of blinding was not reported.
The live birth rate was identical for the 2 groups (88%). A second (unblinded) trial randomized 805 women from a large referral center (mean age 34 years) with a history of first-trimester RSA (not differentiated between primary and secondary RSA) of unknown cause to either 75 mg of aspirin daily or no treatment.8 There was no significant difference in the live birth rate between those who took aspirin (251/367; 68.4%) and those who did not (278/438; 63.5%; OR=1.24; 95% CI, 0.93–1.67).
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) states that “it has not been shown conclusively that progesterone treatment or corpus luteum support (HCG) influences pregnancy outcome for women with recurrent spontaneous abortion.”9 ACOG does not recommend immunotherapy, citing a lack of demonstrated efficacy (IVIG and PLI), a lack of standards for cell storage and administration, and a risk profile similar to that of blood transfusion (PLI). They recommend “couples with otherwise unexplained recurrent pregnancy loss should be counseled regarding the potential for successful pregnancy without treatment.”
Progesterone produces a small but significant decrease in miscarriage among pregnant women with 3 or more unexplained pregnancy losses (strength of recommendation [SOR]: A, based on a meta-analysis of 3 small randomized controlled trials [RCTs] with wide confidence intervals). Human chorionic gonadotropin (HCG) reduces the rate of recurrent pregnancy loss among women with 2 or more unexplained pregnancy losses (SOR: B, based on a meta-analysis of 4 RCTs with significant methodologic weaknesses).
Four types of immunotherapy are ineffective for preventing miscarriage (SOR: A, based on RCTs and systematic reviews of RCTs). Aspirin therapy is ineffective for preventing recurrent miscarriage for women who do not have an autoimmune explanation for previous pregnancy losses (SOR: A, based on RCTs).
Document your patient’s understanding of the risks and benefits
Beth Damitz, MD
Medical College of Wisconsin
When discussing future childbearing with a woman who has had multiple miscarriages, there are several important issues to address. First, ask what concerns she might have about becoming pregnant again. Second, ascertain how significant another pregnancy loss would be to her. Third, outline the therapeutic options, clearly stating that they alter loss rates but do not guarantee successful delivery. Finally, fully document her understanding of the risks and benefits, including the possibility of treatment failure. Remember, even if the miscarriage rate is reduced from 25% to 20% with treatment, should your patient miscarry, her miscarriage rate is 100%!
Evidence summary
Progesterone. A Cochrane meta-analysis on the use of progesterone to prevent pregnancy loss looked at a subset of 3 small RCTs that evaluated women with 3 or more pregnancy losses. Patients with primary recurrent spontaneous abortion (RSA) (no prior live births), were not differentiated from those with secondary RSA (previous live birth with subsequent miscarriages).
Progesterone administration resulted in a significant reduction in miscarriage compared with placebo (odds ratio [OR]=0.37; 95% confidence interval [CI], 0.17–0.91), independent of administration routes (oral, vaginal, or intramuscular). This benefit was lost in the larger meta-analysis when studies containing women with fewer than 3 pregnancy losses were included.1
Human choriogonadotropin. A meta-analysis reviewed 4 trials (n=180 total) of varying methodological quality, which were constructed to determine if women, with at least 2 consecutive miscarriages of unknown cause, derive any protective effect when they receive HCG during the first trimester. Although the overall outcome favored the use of HCG (OR=0.26 compared with placebo; 95% CI, 0.14–0.52), the trials contained major methodological weaknesses (poor description of methods, no power calculations, selection and unclear randomization techniques).2
Immunotherapy. A systematic review of 22 RCTs evaluating 4 different types of immunotherapy for recurrent miscarriage found no significant improvement in live birth rates. All studies were of high quality with a low level of bias. Only one lacked double-blinding.
Immunotherapy types included: paternal leukocyte immunization (PLI) (11 trials, 596 women) (OR=1.05; 95% CI, 0.75–1.47); intravenous immune globulin (IVIG) (OR=0.98; 95% CI, 0.61–1.58); third-party donor cell immunization (3 trials, 156 women) (OR=1.39; 95% CI, 0.68–2.82); and trophoblast membrane infusion (1 trial, 37 women) (OR=0.40; 95% CI, 0.11–1.45).3
A subsequent RCT comparing PLI with placebo among 79 women with primary RSA of unknown cause again found no significant difference in live birth rates (89% vs 71%, respectively).4 However, an additional RCT evaluating PLI (32 patients) vs placebo (19 patients) among women with unexplained primary RSA did find significantly higher birth rates with PLI (84% vs 25%; P=.001). This small study used different techniques than previous PLI studies.5
A later meta-analysis of 5 RCTs including a total of 246 patients also found that IVIG did not improve the subsequent live birth rate for women with a history of primary or secondary RSA (OR=0.98; 95% CI, 0.45–2.13).6
Aspirin. An RCT involving 54 pregnant women (mean age 32.7 years) with a history of primary RSA of unknown cause (negative standard workup) evaluated 50 mg of aspirin daily (n=27) vs placebo (n=27).7 The method of blinding was not reported.
The live birth rate was identical for the 2 groups (88%). A second (unblinded) trial randomized 805 women from a large referral center (mean age 34 years) with a history of first-trimester RSA (not differentiated between primary and secondary RSA) of unknown cause to either 75 mg of aspirin daily or no treatment.8 There was no significant difference in the live birth rate between those who took aspirin (251/367; 68.4%) and those who did not (278/438; 63.5%; OR=1.24; 95% CI, 0.93–1.67).
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) states that “it has not been shown conclusively that progesterone treatment or corpus luteum support (HCG) influences pregnancy outcome for women with recurrent spontaneous abortion.”9 ACOG does not recommend immunotherapy, citing a lack of demonstrated efficacy (IVIG and PLI), a lack of standards for cell storage and administration, and a risk profile similar to that of blood transfusion (PLI). They recommend “couples with otherwise unexplained recurrent pregnancy loss should be counseled regarding the potential for successful pregnancy without treatment.”
1. Oates-Whitehead RM, Haas DM, Carrier JA. Progestogen for preventing miscarriage. Cochrane Database Syst Rev 2003;(4):CD003511.-
2. Scott JR, Pattison N. Human chorionic gonadotrophin for recurrent miscarriage. Cochrane Database Syst Rev 2000;(2):CD000101.-
3. Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev 2003;(1):CD000112.-
4. Ramhorst R, Agriello E, Zittermann S, et al. Is the paternal mononuclear cells’ immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol 2000;44:129-135.
5. Pandey MK, Agrawal S. Induction of MLR-Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int Immunopharmacol 2004;4:289-298.
6. Practice Committee of the American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril 2004;82 Suppl 1:S199-S200.
7. Tulppala M, Marttunen M. Soderstrom-Anttila V, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997;12:1567-1572.
8. Rai R, Backos M, Baxter N, Chilcott I, Regan L. Recurrent miscarriage-an aspirin a day? Hum Reprod 2000;15:2220-2223.
9. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;78:179-190.
1. Oates-Whitehead RM, Haas DM, Carrier JA. Progestogen for preventing miscarriage. Cochrane Database Syst Rev 2003;(4):CD003511.-
2. Scott JR, Pattison N. Human chorionic gonadotrophin for recurrent miscarriage. Cochrane Database Syst Rev 2000;(2):CD000101.-
3. Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev 2003;(1):CD000112.-
4. Ramhorst R, Agriello E, Zittermann S, et al. Is the paternal mononuclear cells’ immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol 2000;44:129-135.
5. Pandey MK, Agrawal S. Induction of MLR-Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int Immunopharmacol 2004;4:289-298.
6. Practice Committee of the American Society for Reproductive Medicine. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertil Steril 2004;82 Suppl 1:S199-S200.
7. Tulppala M, Marttunen M. Soderstrom-Anttila V, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997;12:1567-1572.
8. Rai R, Backos M, Baxter N, Chilcott I, Regan L. Recurrent miscarriage-an aspirin a day? Hum Reprod 2000;15:2220-2223.
9. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;78:179-190.
Evidence-based answers from the Family Physicians Inquiries Network
Is therapy based on endoscopy results better than empiric therapy for dyspepsia?
In the initial management of dyspepsia for patients without “alarm” symptoms (weight loss, recurrent vomiting, dysphagia, anemia, evidence of bleeding, onset of dyspepsia after age 45 years), therapy based on the results of early endoscopy was not better than empiric acid suppression (antisecretory therapy) or a Helicobacter pylori “test and treat” strategy in reducing symptoms or improving quality of life (strength of recommendation [SOR]: A, based on a systematic review). Results from studies of patient satisfaction comparing early endoscopy with empiric medication therapy are conflicting (SOR: A, based on 2 randomized controlled trials [RCTs]).
Though formal cost analyses are not available, a strategy using “test and treat,” as opposed to early endoscopy, results in significantly fewer endoscopies, which when formally evaluated, may translate into a more cost-effective strategy of care (SOR: A, based on a systematic review). Long-term follow-up suggests that patients receiving “test and treat” therapy may require fewer antisecretory medication prescriptions compared with patients receiving early endoscopy (SOR: B, based on a single RCT).
Test-and-treat for H pylori a reasonable first option
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC
Guidelines for treating dyspepsia have to consider several factors: clinical outcomes, risk vs benefit to the patient, direct and indirect medical costs, and patient preference and satisfaction. This well-constructed review clearly demonstrates there is no significant difference in symptom control between early endoscopy and empiric acid suppression or testing and treating for H pylori. The evidence regarding 2 other outcomes—patient satisfaction and cost (especially if the indirect cost of sick days is considered)—is less clear.
In my experience, testing and treating for H pylori is a reasonable first option, which often avoids long courses of antisecretory therapy or costly endoscopy. I treat patients who are negative for H pylori with 8 weeks of acid suppression therapy, and refer those with persistent symptoms for endoscopy. I follow patients carefully and try to distinguish between symptoms of dyspepsia and reflux, which requires longer courses of acid suppression. For patients with alarm symptoms, I recommend early endoscopy.
Evidence summary
Though individual studies have suggested that therapy based on endoscopy performed before any other study (early endoscopy) may be superior to empiric antisecretory therapy and as efficacious as a “test and treat” strategy in symptom relief, a Cochrane systematic review of 20 RCTs (11 in primary care settings) provides the best evidence on the role of early endoscopy.1
A subgroup analysis of 5 RCTs, which compared early endoscopy with empiric antisecretory therapy (typically for 4 weeks), revealed that early endoscopy demonstrated a trend towards improvement in self-reported symptoms and in dyspepsia symptom relief scores, but the difference was not statistically significant (relative risk [RR]=0.89; 95% confidence interval [CI], 0.77–1.1). Because each study used different symptom scores, the relative risk as calculated may under-represent the true benefit of early endoscopy when compared with empiric antisecretory therapy.
When patient satisfaction was evaluated, results were dependent on the location of care. In a primary care setting, patients undergoing early endoscopy were as satisfied as those receiving empiric antisecretory therapy.2 In a trial of 414 patients randomized after referral to specialty care, patients in the early endoscopy group were more satisfied with their medical care than those receiving empiric antisecretory therapy (RR=0.13; 95% CI, 0.06–0.29).3
Results from studies comparing the benefits of H pylori “test and treat” strategies to early endoscopy are conflicting. A subgroup analysis reported on 3 RCTs from both primary and secondary settings with 931 patients comparing H pylori “test and treat” to initial endoscopy. It found no significant difference in symptom reduction (RR=1.06; 95% CI, 0.98–1.26).1 A recent follow-up study of 1 of the trials included in the Cochrane systematic review reported on outcomes of a “test and treat” vs early endoscopy strategy at 6 years. There was no difference in days without symptoms demonstrated between the 2 groups (mean difference=0.05; 95% CI, –0.03 to 0.14 days).4 Self-reported symptom tracking and a poor response rate (62%) to patient questionnaires reduces the strength of this study’s conclusions.
Formal cost-effective analyses comparing the “test and treat” with early endoscopy strategy have not been done. A subgroup analysis of 4 trials from the Cochrane review (1 from primary care) demonstrated a significant reduction of the number of endoscopies among patients receiving “test and treat” care vs those receiving early endoscopy (RR=0.23; 95% CI, 0.12–0.44). In the long-term follow-up study, fewer antisecretory medication prescriptions were needed by those patients in the “test and treat” group (P=.047).4 These figures are more robust; they were obtained from national registry data rather than personal recall and questionnaire submission.
Recommendations from others
Guidelines from the American Gastroenterological Association for the initial approach to young patients with dyspepsia without alarm symptoms is to first “test and treat” for those testing positive for H pylori, prescribe empiric antisecretory therapy for those testing negative, and proceed with endoscopy for recurrent or persistent dyspepsia at 4 to 8 weeks.5 The American Society for Gastrointestinal Endoscopy does not recommend any of initial endoscopy, empiric antisecretory therapy, or “test and treat” over another for the reduction of symptoms.6 The British Society of Gastroenterology recommends that initial management of dyspepsia consist of empiric acid suppression and H pylori testing. Persons testing positive for H pylori should undergo endoscopy.7 The Institute for Clinical Systems Improvement recommends nonurgent upper endoscopy for those aged 50 years and older with symptoms of uncomplicated dyspepsia. They recommend initial H pylori testing and treating those with positive results, and empiric proton pump inhibitor treatment for 4 weeks for those who are H pylori–negative.8
1. Delaney BC, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003;(2):CD001961.-
2. Delaney BC, Wilson S, Roalfe A, et al. Cost-effectiveness of initial endoscopy for dyspepsia in patients over the age of 50 years: A randomised controlled trial in primary care. Lancet 2000;356:1965-1969.
3. Bytzer P, Hansen JM. Schaffalitzky de Muckadell OB. Empirical H2-blocker therapy or prompt endoscopy in management of dyspepsia. Lancet 1994;343:811-816.
4. Lassen AT, Hallis J. Schaffalitzky de Muckadell OB. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7. year follow-up of a randomised trial. Gut 2004;53:1758-1763.
5. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology 1998;114:579-581.
6. Eisen GM, Dominitz JA, Faigel DO, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc 2001;54:815-817.
7. Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV. Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia Guidelines (1996). Aliment Pharmacol Ther 2000;14:413-420.
8. Institute for Clinical Systems Improvement (ICSI). Dyspepsia and GERD. Bloomington, Minn: ICSI; 2004. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=171. Accessed on July 6, 2005.
In the initial management of dyspepsia for patients without “alarm” symptoms (weight loss, recurrent vomiting, dysphagia, anemia, evidence of bleeding, onset of dyspepsia after age 45 years), therapy based on the results of early endoscopy was not better than empiric acid suppression (antisecretory therapy) or a Helicobacter pylori “test and treat” strategy in reducing symptoms or improving quality of life (strength of recommendation [SOR]: A, based on a systematic review). Results from studies of patient satisfaction comparing early endoscopy with empiric medication therapy are conflicting (SOR: A, based on 2 randomized controlled trials [RCTs]).
Though formal cost analyses are not available, a strategy using “test and treat,” as opposed to early endoscopy, results in significantly fewer endoscopies, which when formally evaluated, may translate into a more cost-effective strategy of care (SOR: A, based on a systematic review). Long-term follow-up suggests that patients receiving “test and treat” therapy may require fewer antisecretory medication prescriptions compared with patients receiving early endoscopy (SOR: B, based on a single RCT).
Test-and-treat for H pylori a reasonable first option
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC
Guidelines for treating dyspepsia have to consider several factors: clinical outcomes, risk vs benefit to the patient, direct and indirect medical costs, and patient preference and satisfaction. This well-constructed review clearly demonstrates there is no significant difference in symptom control between early endoscopy and empiric acid suppression or testing and treating for H pylori. The evidence regarding 2 other outcomes—patient satisfaction and cost (especially if the indirect cost of sick days is considered)—is less clear.
In my experience, testing and treating for H pylori is a reasonable first option, which often avoids long courses of antisecretory therapy or costly endoscopy. I treat patients who are negative for H pylori with 8 weeks of acid suppression therapy, and refer those with persistent symptoms for endoscopy. I follow patients carefully and try to distinguish between symptoms of dyspepsia and reflux, which requires longer courses of acid suppression. For patients with alarm symptoms, I recommend early endoscopy.
Evidence summary
Though individual studies have suggested that therapy based on endoscopy performed before any other study (early endoscopy) may be superior to empiric antisecretory therapy and as efficacious as a “test and treat” strategy in symptom relief, a Cochrane systematic review of 20 RCTs (11 in primary care settings) provides the best evidence on the role of early endoscopy.1
A subgroup analysis of 5 RCTs, which compared early endoscopy with empiric antisecretory therapy (typically for 4 weeks), revealed that early endoscopy demonstrated a trend towards improvement in self-reported symptoms and in dyspepsia symptom relief scores, but the difference was not statistically significant (relative risk [RR]=0.89; 95% confidence interval [CI], 0.77–1.1). Because each study used different symptom scores, the relative risk as calculated may under-represent the true benefit of early endoscopy when compared with empiric antisecretory therapy.
When patient satisfaction was evaluated, results were dependent on the location of care. In a primary care setting, patients undergoing early endoscopy were as satisfied as those receiving empiric antisecretory therapy.2 In a trial of 414 patients randomized after referral to specialty care, patients in the early endoscopy group were more satisfied with their medical care than those receiving empiric antisecretory therapy (RR=0.13; 95% CI, 0.06–0.29).3
Results from studies comparing the benefits of H pylori “test and treat” strategies to early endoscopy are conflicting. A subgroup analysis reported on 3 RCTs from both primary and secondary settings with 931 patients comparing H pylori “test and treat” to initial endoscopy. It found no significant difference in symptom reduction (RR=1.06; 95% CI, 0.98–1.26).1 A recent follow-up study of 1 of the trials included in the Cochrane systematic review reported on outcomes of a “test and treat” vs early endoscopy strategy at 6 years. There was no difference in days without symptoms demonstrated between the 2 groups (mean difference=0.05; 95% CI, –0.03 to 0.14 days).4 Self-reported symptom tracking and a poor response rate (62%) to patient questionnaires reduces the strength of this study’s conclusions.
Formal cost-effective analyses comparing the “test and treat” with early endoscopy strategy have not been done. A subgroup analysis of 4 trials from the Cochrane review (1 from primary care) demonstrated a significant reduction of the number of endoscopies among patients receiving “test and treat” care vs those receiving early endoscopy (RR=0.23; 95% CI, 0.12–0.44). In the long-term follow-up study, fewer antisecretory medication prescriptions were needed by those patients in the “test and treat” group (P=.047).4 These figures are more robust; they were obtained from national registry data rather than personal recall and questionnaire submission.
Recommendations from others
Guidelines from the American Gastroenterological Association for the initial approach to young patients with dyspepsia without alarm symptoms is to first “test and treat” for those testing positive for H pylori, prescribe empiric antisecretory therapy for those testing negative, and proceed with endoscopy for recurrent or persistent dyspepsia at 4 to 8 weeks.5 The American Society for Gastrointestinal Endoscopy does not recommend any of initial endoscopy, empiric antisecretory therapy, or “test and treat” over another for the reduction of symptoms.6 The British Society of Gastroenterology recommends that initial management of dyspepsia consist of empiric acid suppression and H pylori testing. Persons testing positive for H pylori should undergo endoscopy.7 The Institute for Clinical Systems Improvement recommends nonurgent upper endoscopy for those aged 50 years and older with symptoms of uncomplicated dyspepsia. They recommend initial H pylori testing and treating those with positive results, and empiric proton pump inhibitor treatment for 4 weeks for those who are H pylori–negative.8
In the initial management of dyspepsia for patients without “alarm” symptoms (weight loss, recurrent vomiting, dysphagia, anemia, evidence of bleeding, onset of dyspepsia after age 45 years), therapy based on the results of early endoscopy was not better than empiric acid suppression (antisecretory therapy) or a Helicobacter pylori “test and treat” strategy in reducing symptoms or improving quality of life (strength of recommendation [SOR]: A, based on a systematic review). Results from studies of patient satisfaction comparing early endoscopy with empiric medication therapy are conflicting (SOR: A, based on 2 randomized controlled trials [RCTs]).
Though formal cost analyses are not available, a strategy using “test and treat,” as opposed to early endoscopy, results in significantly fewer endoscopies, which when formally evaluated, may translate into a more cost-effective strategy of care (SOR: A, based on a systematic review). Long-term follow-up suggests that patients receiving “test and treat” therapy may require fewer antisecretory medication prescriptions compared with patients receiving early endoscopy (SOR: B, based on a single RCT).
Test-and-treat for H pylori a reasonable first option
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC
Guidelines for treating dyspepsia have to consider several factors: clinical outcomes, risk vs benefit to the patient, direct and indirect medical costs, and patient preference and satisfaction. This well-constructed review clearly demonstrates there is no significant difference in symptom control between early endoscopy and empiric acid suppression or testing and treating for H pylori. The evidence regarding 2 other outcomes—patient satisfaction and cost (especially if the indirect cost of sick days is considered)—is less clear.
In my experience, testing and treating for H pylori is a reasonable first option, which often avoids long courses of antisecretory therapy or costly endoscopy. I treat patients who are negative for H pylori with 8 weeks of acid suppression therapy, and refer those with persistent symptoms for endoscopy. I follow patients carefully and try to distinguish between symptoms of dyspepsia and reflux, which requires longer courses of acid suppression. For patients with alarm symptoms, I recommend early endoscopy.
Evidence summary
Though individual studies have suggested that therapy based on endoscopy performed before any other study (early endoscopy) may be superior to empiric antisecretory therapy and as efficacious as a “test and treat” strategy in symptom relief, a Cochrane systematic review of 20 RCTs (11 in primary care settings) provides the best evidence on the role of early endoscopy.1
A subgroup analysis of 5 RCTs, which compared early endoscopy with empiric antisecretory therapy (typically for 4 weeks), revealed that early endoscopy demonstrated a trend towards improvement in self-reported symptoms and in dyspepsia symptom relief scores, but the difference was not statistically significant (relative risk [RR]=0.89; 95% confidence interval [CI], 0.77–1.1). Because each study used different symptom scores, the relative risk as calculated may under-represent the true benefit of early endoscopy when compared with empiric antisecretory therapy.
When patient satisfaction was evaluated, results were dependent on the location of care. In a primary care setting, patients undergoing early endoscopy were as satisfied as those receiving empiric antisecretory therapy.2 In a trial of 414 patients randomized after referral to specialty care, patients in the early endoscopy group were more satisfied with their medical care than those receiving empiric antisecretory therapy (RR=0.13; 95% CI, 0.06–0.29).3
Results from studies comparing the benefits of H pylori “test and treat” strategies to early endoscopy are conflicting. A subgroup analysis reported on 3 RCTs from both primary and secondary settings with 931 patients comparing H pylori “test and treat” to initial endoscopy. It found no significant difference in symptom reduction (RR=1.06; 95% CI, 0.98–1.26).1 A recent follow-up study of 1 of the trials included in the Cochrane systematic review reported on outcomes of a “test and treat” vs early endoscopy strategy at 6 years. There was no difference in days without symptoms demonstrated between the 2 groups (mean difference=0.05; 95% CI, –0.03 to 0.14 days).4 Self-reported symptom tracking and a poor response rate (62%) to patient questionnaires reduces the strength of this study’s conclusions.
Formal cost-effective analyses comparing the “test and treat” with early endoscopy strategy have not been done. A subgroup analysis of 4 trials from the Cochrane review (1 from primary care) demonstrated a significant reduction of the number of endoscopies among patients receiving “test and treat” care vs those receiving early endoscopy (RR=0.23; 95% CI, 0.12–0.44). In the long-term follow-up study, fewer antisecretory medication prescriptions were needed by those patients in the “test and treat” group (P=.047).4 These figures are more robust; they were obtained from national registry data rather than personal recall and questionnaire submission.
Recommendations from others
Guidelines from the American Gastroenterological Association for the initial approach to young patients with dyspepsia without alarm symptoms is to first “test and treat” for those testing positive for H pylori, prescribe empiric antisecretory therapy for those testing negative, and proceed with endoscopy for recurrent or persistent dyspepsia at 4 to 8 weeks.5 The American Society for Gastrointestinal Endoscopy does not recommend any of initial endoscopy, empiric antisecretory therapy, or “test and treat” over another for the reduction of symptoms.6 The British Society of Gastroenterology recommends that initial management of dyspepsia consist of empiric acid suppression and H pylori testing. Persons testing positive for H pylori should undergo endoscopy.7 The Institute for Clinical Systems Improvement recommends nonurgent upper endoscopy for those aged 50 years and older with symptoms of uncomplicated dyspepsia. They recommend initial H pylori testing and treating those with positive results, and empiric proton pump inhibitor treatment for 4 weeks for those who are H pylori–negative.8
1. Delaney BC, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003;(2):CD001961.-
2. Delaney BC, Wilson S, Roalfe A, et al. Cost-effectiveness of initial endoscopy for dyspepsia in patients over the age of 50 years: A randomised controlled trial in primary care. Lancet 2000;356:1965-1969.
3. Bytzer P, Hansen JM. Schaffalitzky de Muckadell OB. Empirical H2-blocker therapy or prompt endoscopy in management of dyspepsia. Lancet 1994;343:811-816.
4. Lassen AT, Hallis J. Schaffalitzky de Muckadell OB. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7. year follow-up of a randomised trial. Gut 2004;53:1758-1763.
5. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology 1998;114:579-581.
6. Eisen GM, Dominitz JA, Faigel DO, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc 2001;54:815-817.
7. Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV. Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia Guidelines (1996). Aliment Pharmacol Ther 2000;14:413-420.
8. Institute for Clinical Systems Improvement (ICSI). Dyspepsia and GERD. Bloomington, Minn: ICSI; 2004. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=171. Accessed on July 6, 2005.
1. Delaney BC, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003;(2):CD001961.-
2. Delaney BC, Wilson S, Roalfe A, et al. Cost-effectiveness of initial endoscopy for dyspepsia in patients over the age of 50 years: A randomised controlled trial in primary care. Lancet 2000;356:1965-1969.
3. Bytzer P, Hansen JM. Schaffalitzky de Muckadell OB. Empirical H2-blocker therapy or prompt endoscopy in management of dyspepsia. Lancet 1994;343:811-816.
4. Lassen AT, Hallis J. Schaffalitzky de Muckadell OB. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7. year follow-up of a randomised trial. Gut 2004;53:1758-1763.
5. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology 1998;114:579-581.
6. Eisen GM, Dominitz JA, Faigel DO, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc 2001;54:815-817.
7. Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV. Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia Guidelines (1996). Aliment Pharmacol Ther 2000;14:413-420.
8. Institute for Clinical Systems Improvement (ICSI). Dyspepsia and GERD. Bloomington, Minn: ICSI; 2004. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=171. Accessed on July 6, 2005.
Evidence-based answers from the Family Physicians Inquiries Network
Does anticoagulation prevent thrombosis for persons with fractures distal to the hip?
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
Evidence-based answers from the Family Physicians Inquiries Network
Is sputum evaluation useful for patients with community-acquired pneumonia?
No high-quality studies specifically address the utility of sputum Gram stain or culture in the assessment or treatment of community-acquired pneumonia (CAP) or nursing home–acquired pneumonia (NHAP). The available evidence suggests that analysis of the sputum adds little to the care or outcomes of patients with CAP (strength of recommendation [SOR]: B, inconsistent results from non-randomized case control, case series, and a systematic review of disease-oriented evidence).
Evidence summary
Studies investigating the role of sputum Gram stain and culture are both difficult to interpret and compare. The difficulty in obtaining an adequate sputum sample, variation in preparation, levels of skill in interpretation, and the lack of a gold standard for the microbiologic diagnosis of pneumonia all contribute to these difficulties.1
The sole meta-analysis identified 12 studies that met 17 specified study criteria regarding the use of sputum Gram stain for patients with com-munity-acquired pneumococcal pneumonia.1 Sample sizes ranged from 16 to 404; reference standards were most frequently sputum culture but also included culture of transtracheal and bronchial aspirates. Results revealed that patients with community-acquired pneumococcal pneumonia were able to produce a valid sputum sample (≥20 neutrophils, <10 squamous epithe-lial cells per low-power field) 70% of the time; the sensitivity of sputum Gram stain ranged from 15% to 69% (when reviewed by a lab technician); and specificity ranged from 11% to 100%.
Because of the heterogeneity of test characteristics, interpreter skill levels, study populations, and reference standards among the studies in this meta-analysis, no single estimate of Gram stain sensitivity or specificity could be reached. Similarly, information regarding the sensitivity and specificity of sputum culture is lacking. Small studies (n=13–85) using blood culture, transthoracic aspi-rate, or transtracheal aspirate as reference standards in untreated cases of definite pneumococcal pneumonia demonstrate sensitivities ranging from 36% to 100%.2 There are no reliable data regarding the specificity of sputum culture.
Recent nonrandomized studies and case series have called into question the role of sputum analysis in CAP. In a case-control study of 605 patients hospitalized with CAP diagnosed by chest x-ray and either cough, chest pain, auscultatory findings, or leukocytosis, establishing an etiologic diagnosis did not influence the choice of antibiotic therapy, length of hospital stay, or mortality.3 Of the 482 patients who had microbiological diagnostics performed (Mycoplasma pneumoniae serology, respiratory virus serology, blood culture, or sputum culture), only 132 (27%) had a presumptive etiologic diagnosis made. Therapy was narrowed or focused in 49 of the 132 (37%) patients who had a presumptive eti-ologic diagnosis, while 84 of the 350 (24%) without a presumptive diagnosis had their therapy narrowed (P>.05). There was no difference in in-hospi-tal changes of therapy, the proportion of new regi-mens having a narrower antimicrobial spectrum than the initial one, length of hospital stay, death in hospital, or death within 3 months after admission.
A prospective study of 74 patients suggested sputum studies had little use in a highly selected population aged <65 years with nonsevere, uncomplicated CAP and no comorbidities. In the 74 patients who produced a valid sputum sample, Gram stain failed to identify the causative agent in any patient (sensitivity 0%), and sputum cultures identified a pathogen in only 4 patients (sensitivity 5%). All patients responded similarly and, even with the identification of a pathogen in 4 patients, there were no changes in initial empiric antibi-otics.4 In a retrospective case series, 19 of 54 (35%) patients with SCAP did not respond to initial empiric antibiotics and had a change in their antibiotic regimen. There was no difference in mortality between the group that had empiric antibiotic change (11 patients) and the group that had a change based on sputum culture results (3 patients) (relative risk reduction= –0.14; 95% confidence interval, –0.47 to 0.12).5 While these studies suggest the need for re-evaluation of routine sputum analysis, the strength of their conclusions are weakened by lack of randomization, small sample size, inadequate blinding, and lack of control group comparison.
Demographic evidence and nonrandomized trials suggest that patients with CAP who have increased risk of infection from multiple-resistant bacteria, such as patients from long-term care facilities, are a unique population that might need to be evaluated differently. However, the only evidence available regarding the utility of either spu-tum Gram stain or culture for patients with NHAP derives from expert opinion. These authors suggest that determining a causative diagnosis of pneumonia in this population is desirable and postulate that sputum examination would permit recognition of multiply resistant organisms that are being isolated with increasing frequency in long-term care facilities.6,7 However, the same authors acknowledge that the elderly are often too weak or too confused to provide adequate sputum specimens, resulting in a low diagnostic yield, and no data demonstrate that spu-tum evaluation favorably influences the outcome of pneumonia in these patient populations.
Recommendations from others
The Infectious Disease Society of America (IDSA) and the Canadian Infectious Disease Society/Canadian Thoracic Society (CIDS/CTS) recommend routine sputum analysis for all inpa-tients with CAP or NHAP,8,9 while the American Thoracic Society (ATS)10 recommends performing sputum analysis only if a drug-resistant pathogen or an organism not covered by usual empiric therapy is suspected. For those with CAP or NHAP treated as outpatients, the ATS, the IDSA, and the CIDS/CTS recommend microbiological testing only if drug-resistant bacteria or an organism not covered by usual empiric therapy is suspected.
In the outpatient setting, a search for the cause is not likely to be helpful
Jon Neher, MD
Valley Medical Center, Renton, Wash
We are fortunate to have excellent guidelines for the empiric treatment of pneumonia because it is difficult to identify the causative organism. There remain, however, theoretical benefits to uncovering the cause: identification of rare organisms, selection of narrower spectrum antibiotics (lessening the community burden of antibiotic resistance), and better targeting of medications should empiric therapy prove ineffective. In the outpatient setting, a search for the cause is not likely to be helpful. In the inpatient setting—particularly in situations where empiric therapy is failing—desper-ation is a powerful motivator and still prompts use of all options available.
1. Reed WW, Byrd GS, Gates RH, Jr, Howard RS, Weaver MJ. Sputum gram’s stain in community-acquired pneumococ-cal pneumonia. A meta-analysis. West J Med 1996;165:197-204.
2. Skerrett SJ. Diagnostic testing for community-acquired pneumonia. Clin Chest Med 1999;20:531-548.
3. Lidman C, Burman LG, Lagergren A, ÖrtQvist Å. Limited value of routine microbiological diagnostics in patients hospitalized for community-acquired pneumonia. Scand J Infect Dis 2002;34:873-879.
4. Theerthakarai R, El-Halees W, Ismail M, Solis RA, Khan MA. Nonvalue of the initial microbiological studies in the management of nonsevere community-acquired pneumonia. Chest 2001;119:181-184.
5. Sanyal S, Smith PR, Saha AC, Gupta S, Berkowitz L, Homel P. Initial microbiologic studies did not affect outcome in adults hospitalized with community-acquired pneumonia. Am J Respir Crit Care Med 1999;160:346-348.
6. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med 1998;105:319-330.
7. Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004;4:112-124.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community acquired pneumonia in adults. Clin Infect Dis 2000;31:347-382.
9. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian Guidelines for the Initial Management of Community-acquired pneumonia: An Evidence-Based Update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis 2000;31:383-421.
10. Niederman MS, Mandell LA, Anqueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicro-bial therapy and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
No high-quality studies specifically address the utility of sputum Gram stain or culture in the assessment or treatment of community-acquired pneumonia (CAP) or nursing home–acquired pneumonia (NHAP). The available evidence suggests that analysis of the sputum adds little to the care or outcomes of patients with CAP (strength of recommendation [SOR]: B, inconsistent results from non-randomized case control, case series, and a systematic review of disease-oriented evidence).
Evidence summary
Studies investigating the role of sputum Gram stain and culture are both difficult to interpret and compare. The difficulty in obtaining an adequate sputum sample, variation in preparation, levels of skill in interpretation, and the lack of a gold standard for the microbiologic diagnosis of pneumonia all contribute to these difficulties.1
The sole meta-analysis identified 12 studies that met 17 specified study criteria regarding the use of sputum Gram stain for patients with com-munity-acquired pneumococcal pneumonia.1 Sample sizes ranged from 16 to 404; reference standards were most frequently sputum culture but also included culture of transtracheal and bronchial aspirates. Results revealed that patients with community-acquired pneumococcal pneumonia were able to produce a valid sputum sample (≥20 neutrophils, <10 squamous epithe-lial cells per low-power field) 70% of the time; the sensitivity of sputum Gram stain ranged from 15% to 69% (when reviewed by a lab technician); and specificity ranged from 11% to 100%.
Because of the heterogeneity of test characteristics, interpreter skill levels, study populations, and reference standards among the studies in this meta-analysis, no single estimate of Gram stain sensitivity or specificity could be reached. Similarly, information regarding the sensitivity and specificity of sputum culture is lacking. Small studies (n=13–85) using blood culture, transthoracic aspi-rate, or transtracheal aspirate as reference standards in untreated cases of definite pneumococcal pneumonia demonstrate sensitivities ranging from 36% to 100%.2 There are no reliable data regarding the specificity of sputum culture.
Recent nonrandomized studies and case series have called into question the role of sputum analysis in CAP. In a case-control study of 605 patients hospitalized with CAP diagnosed by chest x-ray and either cough, chest pain, auscultatory findings, or leukocytosis, establishing an etiologic diagnosis did not influence the choice of antibiotic therapy, length of hospital stay, or mortality.3 Of the 482 patients who had microbiological diagnostics performed (Mycoplasma pneumoniae serology, respiratory virus serology, blood culture, or sputum culture), only 132 (27%) had a presumptive etiologic diagnosis made. Therapy was narrowed or focused in 49 of the 132 (37%) patients who had a presumptive eti-ologic diagnosis, while 84 of the 350 (24%) without a presumptive diagnosis had their therapy narrowed (P>.05). There was no difference in in-hospi-tal changes of therapy, the proportion of new regi-mens having a narrower antimicrobial spectrum than the initial one, length of hospital stay, death in hospital, or death within 3 months after admission.
A prospective study of 74 patients suggested sputum studies had little use in a highly selected population aged <65 years with nonsevere, uncomplicated CAP and no comorbidities. In the 74 patients who produced a valid sputum sample, Gram stain failed to identify the causative agent in any patient (sensitivity 0%), and sputum cultures identified a pathogen in only 4 patients (sensitivity 5%). All patients responded similarly and, even with the identification of a pathogen in 4 patients, there were no changes in initial empiric antibi-otics.4 In a retrospective case series, 19 of 54 (35%) patients with SCAP did not respond to initial empiric antibiotics and had a change in their antibiotic regimen. There was no difference in mortality between the group that had empiric antibiotic change (11 patients) and the group that had a change based on sputum culture results (3 patients) (relative risk reduction= –0.14; 95% confidence interval, –0.47 to 0.12).5 While these studies suggest the need for re-evaluation of routine sputum analysis, the strength of their conclusions are weakened by lack of randomization, small sample size, inadequate blinding, and lack of control group comparison.
Demographic evidence and nonrandomized trials suggest that patients with CAP who have increased risk of infection from multiple-resistant bacteria, such as patients from long-term care facilities, are a unique population that might need to be evaluated differently. However, the only evidence available regarding the utility of either spu-tum Gram stain or culture for patients with NHAP derives from expert opinion. These authors suggest that determining a causative diagnosis of pneumonia in this population is desirable and postulate that sputum examination would permit recognition of multiply resistant organisms that are being isolated with increasing frequency in long-term care facilities.6,7 However, the same authors acknowledge that the elderly are often too weak or too confused to provide adequate sputum specimens, resulting in a low diagnostic yield, and no data demonstrate that spu-tum evaluation favorably influences the outcome of pneumonia in these patient populations.
Recommendations from others
The Infectious Disease Society of America (IDSA) and the Canadian Infectious Disease Society/Canadian Thoracic Society (CIDS/CTS) recommend routine sputum analysis for all inpa-tients with CAP or NHAP,8,9 while the American Thoracic Society (ATS)10 recommends performing sputum analysis only if a drug-resistant pathogen or an organism not covered by usual empiric therapy is suspected. For those with CAP or NHAP treated as outpatients, the ATS, the IDSA, and the CIDS/CTS recommend microbiological testing only if drug-resistant bacteria or an organism not covered by usual empiric therapy is suspected.
In the outpatient setting, a search for the cause is not likely to be helpful
Jon Neher, MD
Valley Medical Center, Renton, Wash
We are fortunate to have excellent guidelines for the empiric treatment of pneumonia because it is difficult to identify the causative organism. There remain, however, theoretical benefits to uncovering the cause: identification of rare organisms, selection of narrower spectrum antibiotics (lessening the community burden of antibiotic resistance), and better targeting of medications should empiric therapy prove ineffective. In the outpatient setting, a search for the cause is not likely to be helpful. In the inpatient setting—particularly in situations where empiric therapy is failing—desper-ation is a powerful motivator and still prompts use of all options available.
No high-quality studies specifically address the utility of sputum Gram stain or culture in the assessment or treatment of community-acquired pneumonia (CAP) or nursing home–acquired pneumonia (NHAP). The available evidence suggests that analysis of the sputum adds little to the care or outcomes of patients with CAP (strength of recommendation [SOR]: B, inconsistent results from non-randomized case control, case series, and a systematic review of disease-oriented evidence).
Evidence summary
Studies investigating the role of sputum Gram stain and culture are both difficult to interpret and compare. The difficulty in obtaining an adequate sputum sample, variation in preparation, levels of skill in interpretation, and the lack of a gold standard for the microbiologic diagnosis of pneumonia all contribute to these difficulties.1
The sole meta-analysis identified 12 studies that met 17 specified study criteria regarding the use of sputum Gram stain for patients with com-munity-acquired pneumococcal pneumonia.1 Sample sizes ranged from 16 to 404; reference standards were most frequently sputum culture but also included culture of transtracheal and bronchial aspirates. Results revealed that patients with community-acquired pneumococcal pneumonia were able to produce a valid sputum sample (≥20 neutrophils, <10 squamous epithe-lial cells per low-power field) 70% of the time; the sensitivity of sputum Gram stain ranged from 15% to 69% (when reviewed by a lab technician); and specificity ranged from 11% to 100%.
Because of the heterogeneity of test characteristics, interpreter skill levels, study populations, and reference standards among the studies in this meta-analysis, no single estimate of Gram stain sensitivity or specificity could be reached. Similarly, information regarding the sensitivity and specificity of sputum culture is lacking. Small studies (n=13–85) using blood culture, transthoracic aspi-rate, or transtracheal aspirate as reference standards in untreated cases of definite pneumococcal pneumonia demonstrate sensitivities ranging from 36% to 100%.2 There are no reliable data regarding the specificity of sputum culture.
Recent nonrandomized studies and case series have called into question the role of sputum analysis in CAP. In a case-control study of 605 patients hospitalized with CAP diagnosed by chest x-ray and either cough, chest pain, auscultatory findings, or leukocytosis, establishing an etiologic diagnosis did not influence the choice of antibiotic therapy, length of hospital stay, or mortality.3 Of the 482 patients who had microbiological diagnostics performed (Mycoplasma pneumoniae serology, respiratory virus serology, blood culture, or sputum culture), only 132 (27%) had a presumptive etiologic diagnosis made. Therapy was narrowed or focused in 49 of the 132 (37%) patients who had a presumptive eti-ologic diagnosis, while 84 of the 350 (24%) without a presumptive diagnosis had their therapy narrowed (P>.05). There was no difference in in-hospi-tal changes of therapy, the proportion of new regi-mens having a narrower antimicrobial spectrum than the initial one, length of hospital stay, death in hospital, or death within 3 months after admission.
A prospective study of 74 patients suggested sputum studies had little use in a highly selected population aged <65 years with nonsevere, uncomplicated CAP and no comorbidities. In the 74 patients who produced a valid sputum sample, Gram stain failed to identify the causative agent in any patient (sensitivity 0%), and sputum cultures identified a pathogen in only 4 patients (sensitivity 5%). All patients responded similarly and, even with the identification of a pathogen in 4 patients, there were no changes in initial empiric antibi-otics.4 In a retrospective case series, 19 of 54 (35%) patients with SCAP did not respond to initial empiric antibiotics and had a change in their antibiotic regimen. There was no difference in mortality between the group that had empiric antibiotic change (11 patients) and the group that had a change based on sputum culture results (3 patients) (relative risk reduction= –0.14; 95% confidence interval, –0.47 to 0.12).5 While these studies suggest the need for re-evaluation of routine sputum analysis, the strength of their conclusions are weakened by lack of randomization, small sample size, inadequate blinding, and lack of control group comparison.
Demographic evidence and nonrandomized trials suggest that patients with CAP who have increased risk of infection from multiple-resistant bacteria, such as patients from long-term care facilities, are a unique population that might need to be evaluated differently. However, the only evidence available regarding the utility of either spu-tum Gram stain or culture for patients with NHAP derives from expert opinion. These authors suggest that determining a causative diagnosis of pneumonia in this population is desirable and postulate that sputum examination would permit recognition of multiply resistant organisms that are being isolated with increasing frequency in long-term care facilities.6,7 However, the same authors acknowledge that the elderly are often too weak or too confused to provide adequate sputum specimens, resulting in a low diagnostic yield, and no data demonstrate that spu-tum evaluation favorably influences the outcome of pneumonia in these patient populations.
Recommendations from others
The Infectious Disease Society of America (IDSA) and the Canadian Infectious Disease Society/Canadian Thoracic Society (CIDS/CTS) recommend routine sputum analysis for all inpa-tients with CAP or NHAP,8,9 while the American Thoracic Society (ATS)10 recommends performing sputum analysis only if a drug-resistant pathogen or an organism not covered by usual empiric therapy is suspected. For those with CAP or NHAP treated as outpatients, the ATS, the IDSA, and the CIDS/CTS recommend microbiological testing only if drug-resistant bacteria or an organism not covered by usual empiric therapy is suspected.
In the outpatient setting, a search for the cause is not likely to be helpful
Jon Neher, MD
Valley Medical Center, Renton, Wash
We are fortunate to have excellent guidelines for the empiric treatment of pneumonia because it is difficult to identify the causative organism. There remain, however, theoretical benefits to uncovering the cause: identification of rare organisms, selection of narrower spectrum antibiotics (lessening the community burden of antibiotic resistance), and better targeting of medications should empiric therapy prove ineffective. In the outpatient setting, a search for the cause is not likely to be helpful. In the inpatient setting—particularly in situations where empiric therapy is failing—desper-ation is a powerful motivator and still prompts use of all options available.
1. Reed WW, Byrd GS, Gates RH, Jr, Howard RS, Weaver MJ. Sputum gram’s stain in community-acquired pneumococ-cal pneumonia. A meta-analysis. West J Med 1996;165:197-204.
2. Skerrett SJ. Diagnostic testing for community-acquired pneumonia. Clin Chest Med 1999;20:531-548.
3. Lidman C, Burman LG, Lagergren A, ÖrtQvist Å. Limited value of routine microbiological diagnostics in patients hospitalized for community-acquired pneumonia. Scand J Infect Dis 2002;34:873-879.
4. Theerthakarai R, El-Halees W, Ismail M, Solis RA, Khan MA. Nonvalue of the initial microbiological studies in the management of nonsevere community-acquired pneumonia. Chest 2001;119:181-184.
5. Sanyal S, Smith PR, Saha AC, Gupta S, Berkowitz L, Homel P. Initial microbiologic studies did not affect outcome in adults hospitalized with community-acquired pneumonia. Am J Respir Crit Care Med 1999;160:346-348.
6. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med 1998;105:319-330.
7. Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004;4:112-124.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community acquired pneumonia in adults. Clin Infect Dis 2000;31:347-382.
9. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian Guidelines for the Initial Management of Community-acquired pneumonia: An Evidence-Based Update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis 2000;31:383-421.
10. Niederman MS, Mandell LA, Anqueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicro-bial therapy and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
1. Reed WW, Byrd GS, Gates RH, Jr, Howard RS, Weaver MJ. Sputum gram’s stain in community-acquired pneumococ-cal pneumonia. A meta-analysis. West J Med 1996;165:197-204.
2. Skerrett SJ. Diagnostic testing for community-acquired pneumonia. Clin Chest Med 1999;20:531-548.
3. Lidman C, Burman LG, Lagergren A, ÖrtQvist Å. Limited value of routine microbiological diagnostics in patients hospitalized for community-acquired pneumonia. Scand J Infect Dis 2002;34:873-879.
4. Theerthakarai R, El-Halees W, Ismail M, Solis RA, Khan MA. Nonvalue of the initial microbiological studies in the management of nonsevere community-acquired pneumonia. Chest 2001;119:181-184.
5. Sanyal S, Smith PR, Saha AC, Gupta S, Berkowitz L, Homel P. Initial microbiologic studies did not affect outcome in adults hospitalized with community-acquired pneumonia. Am J Respir Crit Care Med 1999;160:346-348.
6. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med 1998;105:319-330.
7. Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004;4:112-124.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community acquired pneumonia in adults. Clin Infect Dis 2000;31:347-382.
9. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian Guidelines for the Initial Management of Community-acquired pneumonia: An Evidence-Based Update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis 2000;31:383-421.
10. Niederman MS, Mandell LA, Anqueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicro-bial therapy and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective treatment for ADHD in children?
Stimulant medication therapy is the most effective treatment for attention deficit/hyperactivity disorder (ADHD) in children, producing significant improvements in symptoms and modest improvements in academic achievement (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Nonpharmacologic therapies, such as behavior therapy, school-based interventions, and family therapy, are not as effective as stimulants but may add modest benefit to the effects of medication (SOR: B, based on 1 RCT).
While atomoxetine (Strattera) improves the symptoms of ADHD (SOR: A, based on multiple RCTs), stimulant medications other than methylphenidate offer no distinct short-term advantages (SOR: A, based on meta-analyses of multiple RCTs). Combination drug therapies offer no significant advantage to stimulants alone unless a comorbid condition is present (SOR: A, based on a meta-analysis of 20 RCTs).
The combination of methylphenidate and clonidine (Catapres) improves symptoms in children with both ADHD and tics (SOR: B, based on 1 RCT). Clonidine is less effective alone and has significant side effects (SOR: B, based on a metaanalysis of nonrandomized trials).
Evidence summary
In numerous systematic reviews, RCTs, and metaanalyses, 70% of children responded to stimulant medications with short-term improvements in ADHD symptoms (inattention and hyperactivity/ impulsivity) and academic achievement. A fortyyear review looked at 135 trials and 413 RCTs of methylphenidate in over 19,000 children with an average age of 8.8 years (range, 8.3–9.4 years) for an average duration of 6 weeks (range, 3.3–8.0 weeks).1-3
Study groups included mostly elementary school–aged male children, with few minorities represented. Comorbid conditions, present in 65% of children with ADHD, were often poorly controlled. Outcome measures varied among studies.3
The effect size from stimulant medication in these studies averaged 0.8 for symptom relief and between 0.4 and 0.5 for academic achievement. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is considered moderate to large.)
A large randomized trial of 579 children with ADHD (20% girls) aged 7 to 9.9 years compared outcomes of 4 treatment strategies: stimulant medication, intensive behavioral treatment, combined stimulant medication and behavioral interventions, and standard community care.4 All children met the DSM-IV. criteria for ADHD Combined Type (the most common type of ADHD in this age group). The stimulant medication strategy included an initial dose titration period followed by monthly 30-minute visits. Intensive behavioral treatment involved child, parent, and school personnel components of therapy. Combination therapy added the regimens for medication and behavioral treatment together. Standard community care consisted of usual (nonsystematic) care, evaluated at 6 different sites.
After 14 months of treatment, children in the medication group and the combined treatment groups showed more improvement in ADHD symptoms than children given intensive behavioral treatment or those who received standard community care. When combined with medication, those treated with behavioral therapy showed slight improvement in social skills, anxiety, aggression, oppositional behavior, and academic achievement over medication alone. At the conclusion of the study, 74% of the 212 children on medication were successfully maintained on methylphenidate alone, 10% required dextroamphetamine, and no children required more than one medication. This study found that higher doses of medication with more frequent office follow-up and regular school contact were important features of successful treatment. Only 40% of families were able to complete the intensive behavioral therapy.
Several short-term reviews and meta-analyses show that side effects from stimulant medications are mild and have short duration.5 More long-term studies are required to evaluate effects on growth. RCTs have limited power to detect rare adverse events that may be better detected by large observational studies.6
Atomoxetine, a specific norepinephrine reuptake inhibitor, is an FDA-approved alternative to stimulants for ADHD treatment in children and adolescents. Based on 3 RCTs7 of 588 children between the ages of 7 and 18 years, atomoxetine showed dose-related improvement in ADHD rating scales. Side effects of atomoxetine are similar to stimulants and include mild but significant increases in blood pressure and pulse.7
A meta-analysis of 11 non-randomized trials using clonidine for ADHD showed a smaller effect size compared with stimulants.8 One RCT of 136 children with ADHD and tics showed improvement of both problems with the use of methylphenidate and clonidine, particularly in combination.9 Second-line medications such as clonidine, pemoline (Cylert), and tricyclic antidepressants have more potential serious side effects and are not well studied.10
Recommendations from others
The American Academy of Pediatrics recommends that clinicians: 1) manage ADHD as a chronic illness, 2) collaborate with parents, the child, and school personnel to define specific desired outcomes, 3) use stimulant or behavioral therapy to improve these outcomes; if one stimulant is not effective at the highest feasible dose, try another, 4) reevaluate the diagnosis, treatment options, adherence, and possible coexisting conditions if treatment is not achieving the desired outcomes, and 5) follow-up regularly with parents, child, and teachers to monitor for progress and adverse effects.11
TABLE
Commonly used medications for ADHD
| Medication | Starting dose | Maximum dose | Monthly cost (generic) |
|---|---|---|---|
| Methylphenidate | 5–10 mg 2–3 times daily | 45 mg/d | $20 |
| Dextroamphetamine | 5 mg 1–2 times daily | 40 mg/d | $18 |
| Amphetamine/Dextroamphetamine | 5 mg 1–2 times daily | 60 mg/d | $50 |
| Atomoxetine | 40 mg once daily | 100 mg/d | $86 |
| Common adverse drug reactions for all ADHD medications: Nervousness, insomnia, dry mouth, anorexia, abdominal pain, nausea, constipation, palpitations, tachycardia. | |||
When patients, parents, and teachers are educated, we achieve better outcomes
Jerry Friemoth, MD
University of Cincinnati
Stimulants and atomoxetine improve symptoms of ADHD quite effectively, making office treatment of ADHD a gratifying experience. Like many other diagnoses, there are numerous medications available to treat ADHD. Becoming familiar with a few and regularly prescribing them makes the treatment of ADHD more comfortable for the physician.
Sometimes patients and parents are hesitant to take medication for ADHD. Education about ADHD, along with trials of behavioral therapy, often improves patient satisfaction and compliance with medication. Likewise, children and adolescents may resist medication because of stigma or feeling unfairly labeled with a disease. Because of this, it is helpful to choose a medication with a long duration, so school dosing can be avoided. Artful negotiation with the patient and parent is beneficial.
In my experience, when patients, parents, and teachers are well-educated about ADHD and use behavioral therapy along with medication, we achieve better outcomes. Useful information for physicians and parents regarding medication use and behavioral therapy are described in the American Academy of Pediatrics ADHD Toolkit available at www.nichq.org/resources/toolkit.
1. Conners CK. Forty years of methylphenidate treatment in Attention-Deficit/Hyperactivity Disorder. J Atten Disord 2002;6 Suppl 1:S17-S30.
2. Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1999;38:1551-1559.
3. Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attentiondeficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Can J Psychiatry 1999;44:1007-1016.
4. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-1086.
5. Smith BH, Waschbusch DA, Willoughby MT, Evans S. The efficacy, safety and practicality of treatments for adolescents with attention-deficit/hyperactivity disorder (ADHD). Clin Child Fam Psychol Rev 2000;3:243-267.
6. Treatment of Attention Deficit/Hyperactivity Disorder. Summary, Evidence Report/Technology Assessment: Number 11, AHCPR Publication No. 99-E017. Rockville, Md: Agency for Health Care Policy and Research; 1999. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1. chapter.14677. Accessed on January 8, 2005.
7. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with ADHD. Pediatrics 2001;108:E83.-
8. Kavale K. The efficacy of stimulant drug treatment for hyperactivity: a meta analysis. J Learn Disabil 1982;15:280-289.
9. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58:527-536.
10. Spencer TJ, Biederman J, Wilens TE, Faraone SV. Novel treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry 2002;63 Suppl 12:16-22.
11. Clinical Practice Guideline: treatment of the school-aged child with attention deficit/hyperactivity disorder. Pediatrics 2001;108:1033-1044.Available at: www.aap.org/policy/s0120.html. Accessed on January 8, 2005.
Stimulant medication therapy is the most effective treatment for attention deficit/hyperactivity disorder (ADHD) in children, producing significant improvements in symptoms and modest improvements in academic achievement (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Nonpharmacologic therapies, such as behavior therapy, school-based interventions, and family therapy, are not as effective as stimulants but may add modest benefit to the effects of medication (SOR: B, based on 1 RCT).
While atomoxetine (Strattera) improves the symptoms of ADHD (SOR: A, based on multiple RCTs), stimulant medications other than methylphenidate offer no distinct short-term advantages (SOR: A, based on meta-analyses of multiple RCTs). Combination drug therapies offer no significant advantage to stimulants alone unless a comorbid condition is present (SOR: A, based on a meta-analysis of 20 RCTs).
The combination of methylphenidate and clonidine (Catapres) improves symptoms in children with both ADHD and tics (SOR: B, based on 1 RCT). Clonidine is less effective alone and has significant side effects (SOR: B, based on a metaanalysis of nonrandomized trials).
Evidence summary
In numerous systematic reviews, RCTs, and metaanalyses, 70% of children responded to stimulant medications with short-term improvements in ADHD symptoms (inattention and hyperactivity/ impulsivity) and academic achievement. A fortyyear review looked at 135 trials and 413 RCTs of methylphenidate in over 19,000 children with an average age of 8.8 years (range, 8.3–9.4 years) for an average duration of 6 weeks (range, 3.3–8.0 weeks).1-3
Study groups included mostly elementary school–aged male children, with few minorities represented. Comorbid conditions, present in 65% of children with ADHD, were often poorly controlled. Outcome measures varied among studies.3
The effect size from stimulant medication in these studies averaged 0.8 for symptom relief and between 0.4 and 0.5 for academic achievement. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is considered moderate to large.)
A large randomized trial of 579 children with ADHD (20% girls) aged 7 to 9.9 years compared outcomes of 4 treatment strategies: stimulant medication, intensive behavioral treatment, combined stimulant medication and behavioral interventions, and standard community care.4 All children met the DSM-IV. criteria for ADHD Combined Type (the most common type of ADHD in this age group). The stimulant medication strategy included an initial dose titration period followed by monthly 30-minute visits. Intensive behavioral treatment involved child, parent, and school personnel components of therapy. Combination therapy added the regimens for medication and behavioral treatment together. Standard community care consisted of usual (nonsystematic) care, evaluated at 6 different sites.
After 14 months of treatment, children in the medication group and the combined treatment groups showed more improvement in ADHD symptoms than children given intensive behavioral treatment or those who received standard community care. When combined with medication, those treated with behavioral therapy showed slight improvement in social skills, anxiety, aggression, oppositional behavior, and academic achievement over medication alone. At the conclusion of the study, 74% of the 212 children on medication were successfully maintained on methylphenidate alone, 10% required dextroamphetamine, and no children required more than one medication. This study found that higher doses of medication with more frequent office follow-up and regular school contact were important features of successful treatment. Only 40% of families were able to complete the intensive behavioral therapy.
Several short-term reviews and meta-analyses show that side effects from stimulant medications are mild and have short duration.5 More long-term studies are required to evaluate effects on growth. RCTs have limited power to detect rare adverse events that may be better detected by large observational studies.6
Atomoxetine, a specific norepinephrine reuptake inhibitor, is an FDA-approved alternative to stimulants for ADHD treatment in children and adolescents. Based on 3 RCTs7 of 588 children between the ages of 7 and 18 years, atomoxetine showed dose-related improvement in ADHD rating scales. Side effects of atomoxetine are similar to stimulants and include mild but significant increases in blood pressure and pulse.7
A meta-analysis of 11 non-randomized trials using clonidine for ADHD showed a smaller effect size compared with stimulants.8 One RCT of 136 children with ADHD and tics showed improvement of both problems with the use of methylphenidate and clonidine, particularly in combination.9 Second-line medications such as clonidine, pemoline (Cylert), and tricyclic antidepressants have more potential serious side effects and are not well studied.10
Recommendations from others
The American Academy of Pediatrics recommends that clinicians: 1) manage ADHD as a chronic illness, 2) collaborate with parents, the child, and school personnel to define specific desired outcomes, 3) use stimulant or behavioral therapy to improve these outcomes; if one stimulant is not effective at the highest feasible dose, try another, 4) reevaluate the diagnosis, treatment options, adherence, and possible coexisting conditions if treatment is not achieving the desired outcomes, and 5) follow-up regularly with parents, child, and teachers to monitor for progress and adverse effects.11
TABLE
Commonly used medications for ADHD
| Medication | Starting dose | Maximum dose | Monthly cost (generic) |
|---|---|---|---|
| Methylphenidate | 5–10 mg 2–3 times daily | 45 mg/d | $20 |
| Dextroamphetamine | 5 mg 1–2 times daily | 40 mg/d | $18 |
| Amphetamine/Dextroamphetamine | 5 mg 1–2 times daily | 60 mg/d | $50 |
| Atomoxetine | 40 mg once daily | 100 mg/d | $86 |
| Common adverse drug reactions for all ADHD medications: Nervousness, insomnia, dry mouth, anorexia, abdominal pain, nausea, constipation, palpitations, tachycardia. | |||
When patients, parents, and teachers are educated, we achieve better outcomes
Jerry Friemoth, MD
University of Cincinnati
Stimulants and atomoxetine improve symptoms of ADHD quite effectively, making office treatment of ADHD a gratifying experience. Like many other diagnoses, there are numerous medications available to treat ADHD. Becoming familiar with a few and regularly prescribing them makes the treatment of ADHD more comfortable for the physician.
Sometimes patients and parents are hesitant to take medication for ADHD. Education about ADHD, along with trials of behavioral therapy, often improves patient satisfaction and compliance with medication. Likewise, children and adolescents may resist medication because of stigma or feeling unfairly labeled with a disease. Because of this, it is helpful to choose a medication with a long duration, so school dosing can be avoided. Artful negotiation with the patient and parent is beneficial.
In my experience, when patients, parents, and teachers are well-educated about ADHD and use behavioral therapy along with medication, we achieve better outcomes. Useful information for physicians and parents regarding medication use and behavioral therapy are described in the American Academy of Pediatrics ADHD Toolkit available at www.nichq.org/resources/toolkit.
Stimulant medication therapy is the most effective treatment for attention deficit/hyperactivity disorder (ADHD) in children, producing significant improvements in symptoms and modest improvements in academic achievement (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Nonpharmacologic therapies, such as behavior therapy, school-based interventions, and family therapy, are not as effective as stimulants but may add modest benefit to the effects of medication (SOR: B, based on 1 RCT).
While atomoxetine (Strattera) improves the symptoms of ADHD (SOR: A, based on multiple RCTs), stimulant medications other than methylphenidate offer no distinct short-term advantages (SOR: A, based on meta-analyses of multiple RCTs). Combination drug therapies offer no significant advantage to stimulants alone unless a comorbid condition is present (SOR: A, based on a meta-analysis of 20 RCTs).
The combination of methylphenidate and clonidine (Catapres) improves symptoms in children with both ADHD and tics (SOR: B, based on 1 RCT). Clonidine is less effective alone and has significant side effects (SOR: B, based on a metaanalysis of nonrandomized trials).
Evidence summary
In numerous systematic reviews, RCTs, and metaanalyses, 70% of children responded to stimulant medications with short-term improvements in ADHD symptoms (inattention and hyperactivity/ impulsivity) and academic achievement. A fortyyear review looked at 135 trials and 413 RCTs of methylphenidate in over 19,000 children with an average age of 8.8 years (range, 8.3–9.4 years) for an average duration of 6 weeks (range, 3.3–8.0 weeks).1-3
Study groups included mostly elementary school–aged male children, with few minorities represented. Comorbid conditions, present in 65% of children with ADHD, were often poorly controlled. Outcome measures varied among studies.3
The effect size from stimulant medication in these studies averaged 0.8 for symptom relief and between 0.4 and 0.5 for academic achievement. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is considered moderate to large.)
A large randomized trial of 579 children with ADHD (20% girls) aged 7 to 9.9 years compared outcomes of 4 treatment strategies: stimulant medication, intensive behavioral treatment, combined stimulant medication and behavioral interventions, and standard community care.4 All children met the DSM-IV. criteria for ADHD Combined Type (the most common type of ADHD in this age group). The stimulant medication strategy included an initial dose titration period followed by monthly 30-minute visits. Intensive behavioral treatment involved child, parent, and school personnel components of therapy. Combination therapy added the regimens for medication and behavioral treatment together. Standard community care consisted of usual (nonsystematic) care, evaluated at 6 different sites.
After 14 months of treatment, children in the medication group and the combined treatment groups showed more improvement in ADHD symptoms than children given intensive behavioral treatment or those who received standard community care. When combined with medication, those treated with behavioral therapy showed slight improvement in social skills, anxiety, aggression, oppositional behavior, and academic achievement over medication alone. At the conclusion of the study, 74% of the 212 children on medication were successfully maintained on methylphenidate alone, 10% required dextroamphetamine, and no children required more than one medication. This study found that higher doses of medication with more frequent office follow-up and regular school contact were important features of successful treatment. Only 40% of families were able to complete the intensive behavioral therapy.
Several short-term reviews and meta-analyses show that side effects from stimulant medications are mild and have short duration.5 More long-term studies are required to evaluate effects on growth. RCTs have limited power to detect rare adverse events that may be better detected by large observational studies.6
Atomoxetine, a specific norepinephrine reuptake inhibitor, is an FDA-approved alternative to stimulants for ADHD treatment in children and adolescents. Based on 3 RCTs7 of 588 children between the ages of 7 and 18 years, atomoxetine showed dose-related improvement in ADHD rating scales. Side effects of atomoxetine are similar to stimulants and include mild but significant increases in blood pressure and pulse.7
A meta-analysis of 11 non-randomized trials using clonidine for ADHD showed a smaller effect size compared with stimulants.8 One RCT of 136 children with ADHD and tics showed improvement of both problems with the use of methylphenidate and clonidine, particularly in combination.9 Second-line medications such as clonidine, pemoline (Cylert), and tricyclic antidepressants have more potential serious side effects and are not well studied.10
Recommendations from others
The American Academy of Pediatrics recommends that clinicians: 1) manage ADHD as a chronic illness, 2) collaborate with parents, the child, and school personnel to define specific desired outcomes, 3) use stimulant or behavioral therapy to improve these outcomes; if one stimulant is not effective at the highest feasible dose, try another, 4) reevaluate the diagnosis, treatment options, adherence, and possible coexisting conditions if treatment is not achieving the desired outcomes, and 5) follow-up regularly with parents, child, and teachers to monitor for progress and adverse effects.11
TABLE
Commonly used medications for ADHD
| Medication | Starting dose | Maximum dose | Monthly cost (generic) |
|---|---|---|---|
| Methylphenidate | 5–10 mg 2–3 times daily | 45 mg/d | $20 |
| Dextroamphetamine | 5 mg 1–2 times daily | 40 mg/d | $18 |
| Amphetamine/Dextroamphetamine | 5 mg 1–2 times daily | 60 mg/d | $50 |
| Atomoxetine | 40 mg once daily | 100 mg/d | $86 |
| Common adverse drug reactions for all ADHD medications: Nervousness, insomnia, dry mouth, anorexia, abdominal pain, nausea, constipation, palpitations, tachycardia. | |||
When patients, parents, and teachers are educated, we achieve better outcomes
Jerry Friemoth, MD
University of Cincinnati
Stimulants and atomoxetine improve symptoms of ADHD quite effectively, making office treatment of ADHD a gratifying experience. Like many other diagnoses, there are numerous medications available to treat ADHD. Becoming familiar with a few and regularly prescribing them makes the treatment of ADHD more comfortable for the physician.
Sometimes patients and parents are hesitant to take medication for ADHD. Education about ADHD, along with trials of behavioral therapy, often improves patient satisfaction and compliance with medication. Likewise, children and adolescents may resist medication because of stigma or feeling unfairly labeled with a disease. Because of this, it is helpful to choose a medication with a long duration, so school dosing can be avoided. Artful negotiation with the patient and parent is beneficial.
In my experience, when patients, parents, and teachers are well-educated about ADHD and use behavioral therapy along with medication, we achieve better outcomes. Useful information for physicians and parents regarding medication use and behavioral therapy are described in the American Academy of Pediatrics ADHD Toolkit available at www.nichq.org/resources/toolkit.
1. Conners CK. Forty years of methylphenidate treatment in Attention-Deficit/Hyperactivity Disorder. J Atten Disord 2002;6 Suppl 1:S17-S30.
2. Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1999;38:1551-1559.
3. Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attentiondeficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Can J Psychiatry 1999;44:1007-1016.
4. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-1086.
5. Smith BH, Waschbusch DA, Willoughby MT, Evans S. The efficacy, safety and practicality of treatments for adolescents with attention-deficit/hyperactivity disorder (ADHD). Clin Child Fam Psychol Rev 2000;3:243-267.
6. Treatment of Attention Deficit/Hyperactivity Disorder. Summary, Evidence Report/Technology Assessment: Number 11, AHCPR Publication No. 99-E017. Rockville, Md: Agency for Health Care Policy and Research; 1999. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1. chapter.14677. Accessed on January 8, 2005.
7. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with ADHD. Pediatrics 2001;108:E83.-
8. Kavale K. The efficacy of stimulant drug treatment for hyperactivity: a meta analysis. J Learn Disabil 1982;15:280-289.
9. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58:527-536.
10. Spencer TJ, Biederman J, Wilens TE, Faraone SV. Novel treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry 2002;63 Suppl 12:16-22.
11. Clinical Practice Guideline: treatment of the school-aged child with attention deficit/hyperactivity disorder. Pediatrics 2001;108:1033-1044.Available at: www.aap.org/policy/s0120.html. Accessed on January 8, 2005.
1. Conners CK. Forty years of methylphenidate treatment in Attention-Deficit/Hyperactivity Disorder. J Atten Disord 2002;6 Suppl 1:S17-S30.
2. Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1999;38:1551-1559.
3. Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attentiondeficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Can J Psychiatry 1999;44:1007-1016.
4. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-1086.
5. Smith BH, Waschbusch DA, Willoughby MT, Evans S. The efficacy, safety and practicality of treatments for adolescents with attention-deficit/hyperactivity disorder (ADHD). Clin Child Fam Psychol Rev 2000;3:243-267.
6. Treatment of Attention Deficit/Hyperactivity Disorder. Summary, Evidence Report/Technology Assessment: Number 11, AHCPR Publication No. 99-E017. Rockville, Md: Agency for Health Care Policy and Research; 1999. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1. chapter.14677. Accessed on January 8, 2005.
7. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with ADHD. Pediatrics 2001;108:E83.-
8. Kavale K. The efficacy of stimulant drug treatment for hyperactivity: a meta analysis. J Learn Disabil 1982;15:280-289.
9. Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58:527-536.
10. Spencer TJ, Biederman J, Wilens TE, Faraone SV. Novel treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry 2002;63 Suppl 12:16-22.
11. Clinical Practice Guideline: treatment of the school-aged child with attention deficit/hyperactivity disorder. Pediatrics 2001;108:1033-1044.Available at: www.aap.org/policy/s0120.html. Accessed on January 8, 2005.
Evidence-based answers from the Family Physicians Inquiries Network
Do antipyretics prolong febrile illness?
Antipyretics appear to have minor and variable effects on the course of febrile illness. Aspirin and acetaminophen do not prolong the course of rhinovirus illness, although they may prolong the period of viral shedding and worsen nasal congestion (strength of recommendation [SOR]: A–, based on small randomized controlled trials).
Acetaminophen did not affect symptoms, overall condition, or time to complete healing in children with varicella, although it increased the time to total scabbing of lesions (SOR: A, based on a small randomized controlled trial). Aspirin and acetaminophen may prolong influenza A illness (SOR: C, based on a poor-quality, retrospective observational study).
Acetaminophen may prolong the course of Shigella sonnei infection (SOR: B–, based on a small retrospective cohort study). It does not affect malaria cure rate, and there are insufficient data to assess clearance of Plasmodium falciparum (SOR: C, based on small randomized controlled trials with heterogeneous results).
Evidence summary
Acetaminophen has a different mechanism of action from other antipyretics. It halts the production of prostaglandin in the brain but not in the periphery, solely lowering fever. Aspirin and other nonsteroidal anti-inflammatory agents inhibit both central and peripheral cyclooxygenase and may cause multiple effects in addition to temperature reduction. Clinical outcome studies of their antipyretic effects are inconclusive.1
A randomized controlled trial involving 60 volunteers given intranasal rhinovirus type 2 monitored the effect of aspirin, acetaminophen, ibuprofen, or placebo on virus shedding, immune response, and clinical status. There was no difference in duration of illness. There was a trend toward longer duration of virus shedding in the aspirin and acetaminophen groups, but serum neutralizing antibody response was suppressed (P<.05 vs placebo). Aspirin and acetaminophen worsened symptoms of turbinate edema and nasal obstruction (P<.05 vs placebo).2
In 2 double-blind trials, 45 adults infected with rhinovirus were given aspirin or placebo for 5 days, beginning on the day after viral exposure (as opposed to the typical use in response to symptoms). Aspirin treatment improved symptoms of conjunctivitis significantly, but did not change the duration of illness. Other symptoms (headache, sneezing, chills, malaise, nasal discharge) were not significantly different. Aspirin increased the amount of viral shedding by 36% in 1 trial and 17% in the other (P<.01), potentially increasing risk of spread.3
In a randomized controlled trial evaluating antipyretic effects on the duration or severity of childhood varicella, 31 children received placebo and 37 received acetaminophen for 4 days. There was no difference in itching, appetite, activity, or overall condition between the 2 groups. Children treated with acetaminophen took 1.1 days longer to total scabbing (P<.05), although the number of days until the appearance of the last new vesicle and the time to total healing were unchanged. The duration of viral shedding was not measured, but it is possible that the delay in healing of lesions would prolong viral shedding as well.4
A retrospective observational study of 54 volunteers demonstrated prolonged illness in subjects infected with influenza A that received antipyretic therapy. Patients who got antipyretics were sick 3.5 days longer than those who did not (8.8 ± 2.3 days vs 5.3 ± 3.0 days; P<.001). Only patients with temperatures >38.9°C on 2 readings 6 hours apart received antipyretics, indicating that the longer course correlated with greater severity of illness as well as with antipyretic use.
In the same study, antipyretics were associated with a trend towards prolonged duration of illness in a group of 21 patients infected with S sonnei (4.6 ± 2.1 days with antipyretics vs 1.9 ± 1.6 days without; P=not significant).5
A Cochrane review examined 3 trials of acetaminophen vs placebo for fever in 128 adults and children with P falciparum malaria. Although fever clearance varied between the trials, the malaria cure rate was similar in all, and the review concluded that data were insufficient to evaluate an effect on parasitemia.6
Recommendations from others
We found no recommendations regarding the use of antipyretics and their effect on the duration of febrile illness.
The risk-benefit ratio of antipyretics may not be as favorable as you think
Jon O. Neher, MD
Valley Medical Center Family Medicine Residency
The doctor’s recommendation, “Take two aspirin and call me in the morning,” is an enduring stereotype, not an evidence-based therapy for a fever. This review reevaluates the simplistic notion that antipyretics are uniformly beneficial and safe in febrile illnesses.
Surprisingly, there appear to be some negative impacts from using antipyretics for common disease states without much clear benefit. It can be argued that the studies are small and purported negative consequences modest. Still, enough evidence exists to warrant more research and to cause clinicians to consider that the risk-to-benefit ratio of these medications may not be as favorable as once thought.
1. Mackowiak PA, Plaisance KI. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci 1998;856:214-223.
2. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis 1990;162:1277-1282.
3. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975;231:1248-1251.
4. Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr 1989;114:1045-1048.
5. Plaisance KI, Kudaravalli S, Wasserman SS, Levine MM, Mackowiak PA. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy 2000;20:1417-1422.
6. Meremikwu M, Logan K, Garner P. Antipyretic measures for treating fever in malaria (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software; 2002.
Antipyretics appear to have minor and variable effects on the course of febrile illness. Aspirin and acetaminophen do not prolong the course of rhinovirus illness, although they may prolong the period of viral shedding and worsen nasal congestion (strength of recommendation [SOR]: A–, based on small randomized controlled trials).
Acetaminophen did not affect symptoms, overall condition, or time to complete healing in children with varicella, although it increased the time to total scabbing of lesions (SOR: A, based on a small randomized controlled trial). Aspirin and acetaminophen may prolong influenza A illness (SOR: C, based on a poor-quality, retrospective observational study).
Acetaminophen may prolong the course of Shigella sonnei infection (SOR: B–, based on a small retrospective cohort study). It does not affect malaria cure rate, and there are insufficient data to assess clearance of Plasmodium falciparum (SOR: C, based on small randomized controlled trials with heterogeneous results).
Evidence summary
Acetaminophen has a different mechanism of action from other antipyretics. It halts the production of prostaglandin in the brain but not in the periphery, solely lowering fever. Aspirin and other nonsteroidal anti-inflammatory agents inhibit both central and peripheral cyclooxygenase and may cause multiple effects in addition to temperature reduction. Clinical outcome studies of their antipyretic effects are inconclusive.1
A randomized controlled trial involving 60 volunteers given intranasal rhinovirus type 2 monitored the effect of aspirin, acetaminophen, ibuprofen, or placebo on virus shedding, immune response, and clinical status. There was no difference in duration of illness. There was a trend toward longer duration of virus shedding in the aspirin and acetaminophen groups, but serum neutralizing antibody response was suppressed (P<.05 vs placebo). Aspirin and acetaminophen worsened symptoms of turbinate edema and nasal obstruction (P<.05 vs placebo).2
In 2 double-blind trials, 45 adults infected with rhinovirus were given aspirin or placebo for 5 days, beginning on the day after viral exposure (as opposed to the typical use in response to symptoms). Aspirin treatment improved symptoms of conjunctivitis significantly, but did not change the duration of illness. Other symptoms (headache, sneezing, chills, malaise, nasal discharge) were not significantly different. Aspirin increased the amount of viral shedding by 36% in 1 trial and 17% in the other (P<.01), potentially increasing risk of spread.3
In a randomized controlled trial evaluating antipyretic effects on the duration or severity of childhood varicella, 31 children received placebo and 37 received acetaminophen for 4 days. There was no difference in itching, appetite, activity, or overall condition between the 2 groups. Children treated with acetaminophen took 1.1 days longer to total scabbing (P<.05), although the number of days until the appearance of the last new vesicle and the time to total healing were unchanged. The duration of viral shedding was not measured, but it is possible that the delay in healing of lesions would prolong viral shedding as well.4
A retrospective observational study of 54 volunteers demonstrated prolonged illness in subjects infected with influenza A that received antipyretic therapy. Patients who got antipyretics were sick 3.5 days longer than those who did not (8.8 ± 2.3 days vs 5.3 ± 3.0 days; P<.001). Only patients with temperatures >38.9°C on 2 readings 6 hours apart received antipyretics, indicating that the longer course correlated with greater severity of illness as well as with antipyretic use.
In the same study, antipyretics were associated with a trend towards prolonged duration of illness in a group of 21 patients infected with S sonnei (4.6 ± 2.1 days with antipyretics vs 1.9 ± 1.6 days without; P=not significant).5
A Cochrane review examined 3 trials of acetaminophen vs placebo for fever in 128 adults and children with P falciparum malaria. Although fever clearance varied between the trials, the malaria cure rate was similar in all, and the review concluded that data were insufficient to evaluate an effect on parasitemia.6
Recommendations from others
We found no recommendations regarding the use of antipyretics and their effect on the duration of febrile illness.
The risk-benefit ratio of antipyretics may not be as favorable as you think
Jon O. Neher, MD
Valley Medical Center Family Medicine Residency
The doctor’s recommendation, “Take two aspirin and call me in the morning,” is an enduring stereotype, not an evidence-based therapy for a fever. This review reevaluates the simplistic notion that antipyretics are uniformly beneficial and safe in febrile illnesses.
Surprisingly, there appear to be some negative impacts from using antipyretics for common disease states without much clear benefit. It can be argued that the studies are small and purported negative consequences modest. Still, enough evidence exists to warrant more research and to cause clinicians to consider that the risk-to-benefit ratio of these medications may not be as favorable as once thought.
Antipyretics appear to have minor and variable effects on the course of febrile illness. Aspirin and acetaminophen do not prolong the course of rhinovirus illness, although they may prolong the period of viral shedding and worsen nasal congestion (strength of recommendation [SOR]: A–, based on small randomized controlled trials).
Acetaminophen did not affect symptoms, overall condition, or time to complete healing in children with varicella, although it increased the time to total scabbing of lesions (SOR: A, based on a small randomized controlled trial). Aspirin and acetaminophen may prolong influenza A illness (SOR: C, based on a poor-quality, retrospective observational study).
Acetaminophen may prolong the course of Shigella sonnei infection (SOR: B–, based on a small retrospective cohort study). It does not affect malaria cure rate, and there are insufficient data to assess clearance of Plasmodium falciparum (SOR: C, based on small randomized controlled trials with heterogeneous results).
Evidence summary
Acetaminophen has a different mechanism of action from other antipyretics. It halts the production of prostaglandin in the brain but not in the periphery, solely lowering fever. Aspirin and other nonsteroidal anti-inflammatory agents inhibit both central and peripheral cyclooxygenase and may cause multiple effects in addition to temperature reduction. Clinical outcome studies of their antipyretic effects are inconclusive.1
A randomized controlled trial involving 60 volunteers given intranasal rhinovirus type 2 monitored the effect of aspirin, acetaminophen, ibuprofen, or placebo on virus shedding, immune response, and clinical status. There was no difference in duration of illness. There was a trend toward longer duration of virus shedding in the aspirin and acetaminophen groups, but serum neutralizing antibody response was suppressed (P<.05 vs placebo). Aspirin and acetaminophen worsened symptoms of turbinate edema and nasal obstruction (P<.05 vs placebo).2
In 2 double-blind trials, 45 adults infected with rhinovirus were given aspirin or placebo for 5 days, beginning on the day after viral exposure (as opposed to the typical use in response to symptoms). Aspirin treatment improved symptoms of conjunctivitis significantly, but did not change the duration of illness. Other symptoms (headache, sneezing, chills, malaise, nasal discharge) were not significantly different. Aspirin increased the amount of viral shedding by 36% in 1 trial and 17% in the other (P<.01), potentially increasing risk of spread.3
In a randomized controlled trial evaluating antipyretic effects on the duration or severity of childhood varicella, 31 children received placebo and 37 received acetaminophen for 4 days. There was no difference in itching, appetite, activity, or overall condition between the 2 groups. Children treated with acetaminophen took 1.1 days longer to total scabbing (P<.05), although the number of days until the appearance of the last new vesicle and the time to total healing were unchanged. The duration of viral shedding was not measured, but it is possible that the delay in healing of lesions would prolong viral shedding as well.4
A retrospective observational study of 54 volunteers demonstrated prolonged illness in subjects infected with influenza A that received antipyretic therapy. Patients who got antipyretics were sick 3.5 days longer than those who did not (8.8 ± 2.3 days vs 5.3 ± 3.0 days; P<.001). Only patients with temperatures >38.9°C on 2 readings 6 hours apart received antipyretics, indicating that the longer course correlated with greater severity of illness as well as with antipyretic use.
In the same study, antipyretics were associated with a trend towards prolonged duration of illness in a group of 21 patients infected with S sonnei (4.6 ± 2.1 days with antipyretics vs 1.9 ± 1.6 days without; P=not significant).5
A Cochrane review examined 3 trials of acetaminophen vs placebo for fever in 128 adults and children with P falciparum malaria. Although fever clearance varied between the trials, the malaria cure rate was similar in all, and the review concluded that data were insufficient to evaluate an effect on parasitemia.6
Recommendations from others
We found no recommendations regarding the use of antipyretics and their effect on the duration of febrile illness.
The risk-benefit ratio of antipyretics may not be as favorable as you think
Jon O. Neher, MD
Valley Medical Center Family Medicine Residency
The doctor’s recommendation, “Take two aspirin and call me in the morning,” is an enduring stereotype, not an evidence-based therapy for a fever. This review reevaluates the simplistic notion that antipyretics are uniformly beneficial and safe in febrile illnesses.
Surprisingly, there appear to be some negative impacts from using antipyretics for common disease states without much clear benefit. It can be argued that the studies are small and purported negative consequences modest. Still, enough evidence exists to warrant more research and to cause clinicians to consider that the risk-to-benefit ratio of these medications may not be as favorable as once thought.
1. Mackowiak PA, Plaisance KI. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci 1998;856:214-223.
2. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis 1990;162:1277-1282.
3. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975;231:1248-1251.
4. Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr 1989;114:1045-1048.
5. Plaisance KI, Kudaravalli S, Wasserman SS, Levine MM, Mackowiak PA. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy 2000;20:1417-1422.
6. Meremikwu M, Logan K, Garner P. Antipyretic measures for treating fever in malaria (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software; 2002.
1. Mackowiak PA, Plaisance KI. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci 1998;856:214-223.
2. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis 1990;162:1277-1282.
3. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975;231:1248-1251.
4. Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr 1989;114:1045-1048.
5. Plaisance KI, Kudaravalli S, Wasserman SS, Levine MM, Mackowiak PA. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy 2000;20:1417-1422.
6. Meremikwu M, Logan K, Garner P. Antipyretic measures for treating fever in malaria (Cochrane Review). The Cochrane Library, Issue 2, 2002. Oxford: Update Software; 2002.
Evidence-based answers from the Family Physicians Inquiries Network
Which infants need lumbar puncture for suspected sepsis?
Evidence from prospective and retrospective clinical trials suggests that for infants <2 months old, only those at high risk for serious bacterial infection by standardized criteria (eg, Rochester classification) require lumbar puncture (strength of recommendation [SOR]: B, based on prospective and retrospective cohort studies). However, expert opinion suggests lumbar puncture on all infants aged 0 to 28 days with suspected sepsis, and all infants aged >2 months who are to receive empiric antibiotics (SOR: C, based on expert opinion).
Evidence summary
Standardized clinical criteria (Table) exist to determine the risk of serious bacterial infection, which includes meningitis; of particular note, these criteria do not require cerebrospinal fluid examination. Infants aged <3 months who fall into the “high-risk” category or appear toxic have 21% probability of a serious bacterial infection, 10% probability of bacteremia, and 2% probability of bacterial meningitis.1 The “low-risk” infants have a correspondingly lower incidence of serious bacterial infection: the negative predictive value of the Rochester classification is 98.9% (95% confidence interval [CI], 97.2–99.6%).2
The negative predictive value for bacterial meningitis (a subset of serious bacterial infection) is even greater. Five studies applied the standardized criteria to febrile infants and monitored them for the development of serious bacterial infection, including meningitis. Two prospective cohort studies of outpatients aged 0 to 2 months used the Rochester criteria to assign infants to risk groups. They studied a total of 1294 infants; 659 (51%) were low-risk. None of the low-risk infants developed bacterial meningitis.2,3
One prospective cohort study of infants aged <1 month hospitalized for fever used a similar method for assessing risk, but added a C-reactive protein value <20 mg/L to criteria for low-risk. Of 250 infants studied, 131 (52%) were low-risk; none of these developed bacterial meningitis.4
A retrospective chart review of 492 infants aged <3 months who were hospitalized due to fever included 108 infants aged <1 month. Thirty percent (114) of the infants aged 1 to 3 months and 67% (72) of the younger infants underwent lumbar puncture at the discretion of the treating physician. All infants were retrospectively assigned to low- or high-risk groups for serious bacterial infection using the Rochester criteria. Of the 296 infants rated “low-risk,” none developed bacterial meningitis. Ten of these infants subsequently developed evidence of another bacterial focus (predominantly urinary tract infection).5
RECOMMENDATIONS FROM OTHERS
The American Academy of Pediatrics has not issued a clinical practice guideline or clinical report addressing this issue. An evidence-based guideline developed at Cincinnati Children’s Hospital Medical Center in 1998 recommends hospitalization and a full sepsis workup (including lumbar puncture) for infants aged <1 month, or infants aged 1 to 2 months who are high-risk.6
A clinical review-based guideline published in 1993 gives the same recommendations.7 The expert panel that devised this guideline emphasized a full sepsis evaluation (including cerebrospinal fluid cultures) for infants <28 days of age “despite the low probability of serious bacterial infections in this age group and the favorable outcome of the children managed to date with careful observation.” For low-risk infants aged 1 to 2 months, lumbar puncture is not necessary unless empiric antibiotics are given; having a cerebrospinal fluid culture prior to empiric antibiotics reduces the concern of partially treated meningitis in the case of clinical deterioration after hospital discharge.6,7
TABLE
How to identify infants at low risk of serious bacterial infection: Rochester Classification
| Febrile infants (temperature ≥38°C, 100.4°F) ≥60 days of age who meet all criteria are at low risk of serious bacterial infection: | |
|---|---|
| General health | Born at ≥37 weeks’ gestation |
| Did not receive perinatal or antenatal antibiotics | |
| Was not treated for unexplained hyperbilirubinemia | |
| Was not hospitalized in the nursery longer than the mother | |
| Has had no hospitalization since discharge | |
| No diagnosed chronic or underlying illnesses | |
| Physical findings | Appears well and nontoxic |
| No evidence of skin, soft tissue, bone, or joint abnormalities, or otitis media | |
| Laboratory findings | Peripheral total white blood cells 5,000–15,000/mm3 |
| Absolute band form leukocytes <1,500/mm3 | |
| Spun urine sediment <10 white blood cells per high power field | |
| Fresh stool smear <5 white blood cells per high power field | |
Evaluating fever in infants: judging the risks
Randy Ward, MD
Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
The evaluation of the febrile infant is often fraught with anxiety. Physicians must balance the potentially devastating consequences of a missed serious bacterial infection with the desire to avoid unnecessary work-ups.
In the past, guidelines have had an extremely conservative viewpoint, essentially grouping all infants by age, and recommended an extensive inpatient work-up regardless of clinical status. The Rochester Criteria have provided guidelines for clinical risk stratification in this age group, allowing a more rational approach to the workup. The above data provide further useful guidance for the appropriate use of lumbar puncture in evaluation of these infants.
1. Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J 1992;11:257-264.
2. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94:390-396.
3. Dagan R, Sofer S, Phillip M, Shachak E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J Pediatr 1988;112:355-360.
4. Chiu CH, Lin TY, Bullard MJ. Identification of febrile neonates unlikely to have bacterial infection. Pediatr Infect Dis J 1997;16:59-63.
5. Brik R, Hamissah R, Shehada N, Berant M. Evaluation of febrile infants under 3 months of age: is routine lumbar puncture warranted?. Isr J Med Sci 1997;33:93-97.
6. Cincinnati Children’s Hospital Medical Center. Evidence based clinical protocol guideline for fever of uncertain source in infants 60 days of age or less. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1998.
7. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med 1993;22:1198-1210.
Evidence from prospective and retrospective clinical trials suggests that for infants <2 months old, only those at high risk for serious bacterial infection by standardized criteria (eg, Rochester classification) require lumbar puncture (strength of recommendation [SOR]: B, based on prospective and retrospective cohort studies). However, expert opinion suggests lumbar puncture on all infants aged 0 to 28 days with suspected sepsis, and all infants aged >2 months who are to receive empiric antibiotics (SOR: C, based on expert opinion).
Evidence summary
Standardized clinical criteria (Table) exist to determine the risk of serious bacterial infection, which includes meningitis; of particular note, these criteria do not require cerebrospinal fluid examination. Infants aged <3 months who fall into the “high-risk” category or appear toxic have 21% probability of a serious bacterial infection, 10% probability of bacteremia, and 2% probability of bacterial meningitis.1 The “low-risk” infants have a correspondingly lower incidence of serious bacterial infection: the negative predictive value of the Rochester classification is 98.9% (95% confidence interval [CI], 97.2–99.6%).2
The negative predictive value for bacterial meningitis (a subset of serious bacterial infection) is even greater. Five studies applied the standardized criteria to febrile infants and monitored them for the development of serious bacterial infection, including meningitis. Two prospective cohort studies of outpatients aged 0 to 2 months used the Rochester criteria to assign infants to risk groups. They studied a total of 1294 infants; 659 (51%) were low-risk. None of the low-risk infants developed bacterial meningitis.2,3
One prospective cohort study of infants aged <1 month hospitalized for fever used a similar method for assessing risk, but added a C-reactive protein value <20 mg/L to criteria for low-risk. Of 250 infants studied, 131 (52%) were low-risk; none of these developed bacterial meningitis.4
A retrospective chart review of 492 infants aged <3 months who were hospitalized due to fever included 108 infants aged <1 month. Thirty percent (114) of the infants aged 1 to 3 months and 67% (72) of the younger infants underwent lumbar puncture at the discretion of the treating physician. All infants were retrospectively assigned to low- or high-risk groups for serious bacterial infection using the Rochester criteria. Of the 296 infants rated “low-risk,” none developed bacterial meningitis. Ten of these infants subsequently developed evidence of another bacterial focus (predominantly urinary tract infection).5
RECOMMENDATIONS FROM OTHERS
The American Academy of Pediatrics has not issued a clinical practice guideline or clinical report addressing this issue. An evidence-based guideline developed at Cincinnati Children’s Hospital Medical Center in 1998 recommends hospitalization and a full sepsis workup (including lumbar puncture) for infants aged <1 month, or infants aged 1 to 2 months who are high-risk.6
A clinical review-based guideline published in 1993 gives the same recommendations.7 The expert panel that devised this guideline emphasized a full sepsis evaluation (including cerebrospinal fluid cultures) for infants <28 days of age “despite the low probability of serious bacterial infections in this age group and the favorable outcome of the children managed to date with careful observation.” For low-risk infants aged 1 to 2 months, lumbar puncture is not necessary unless empiric antibiotics are given; having a cerebrospinal fluid culture prior to empiric antibiotics reduces the concern of partially treated meningitis in the case of clinical deterioration after hospital discharge.6,7
TABLE
How to identify infants at low risk of serious bacterial infection: Rochester Classification
| Febrile infants (temperature ≥38°C, 100.4°F) ≥60 days of age who meet all criteria are at low risk of serious bacterial infection: | |
|---|---|
| General health | Born at ≥37 weeks’ gestation |
| Did not receive perinatal or antenatal antibiotics | |
| Was not treated for unexplained hyperbilirubinemia | |
| Was not hospitalized in the nursery longer than the mother | |
| Has had no hospitalization since discharge | |
| No diagnosed chronic or underlying illnesses | |
| Physical findings | Appears well and nontoxic |
| No evidence of skin, soft tissue, bone, or joint abnormalities, or otitis media | |
| Laboratory findings | Peripheral total white blood cells 5,000–15,000/mm3 |
| Absolute band form leukocytes <1,500/mm3 | |
| Spun urine sediment <10 white blood cells per high power field | |
| Fresh stool smear <5 white blood cells per high power field | |
Evaluating fever in infants: judging the risks
Randy Ward, MD
Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
The evaluation of the febrile infant is often fraught with anxiety. Physicians must balance the potentially devastating consequences of a missed serious bacterial infection with the desire to avoid unnecessary work-ups.
In the past, guidelines have had an extremely conservative viewpoint, essentially grouping all infants by age, and recommended an extensive inpatient work-up regardless of clinical status. The Rochester Criteria have provided guidelines for clinical risk stratification in this age group, allowing a more rational approach to the workup. The above data provide further useful guidance for the appropriate use of lumbar puncture in evaluation of these infants.
Evidence from prospective and retrospective clinical trials suggests that for infants <2 months old, only those at high risk for serious bacterial infection by standardized criteria (eg, Rochester classification) require lumbar puncture (strength of recommendation [SOR]: B, based on prospective and retrospective cohort studies). However, expert opinion suggests lumbar puncture on all infants aged 0 to 28 days with suspected sepsis, and all infants aged >2 months who are to receive empiric antibiotics (SOR: C, based on expert opinion).
Evidence summary
Standardized clinical criteria (Table) exist to determine the risk of serious bacterial infection, which includes meningitis; of particular note, these criteria do not require cerebrospinal fluid examination. Infants aged <3 months who fall into the “high-risk” category or appear toxic have 21% probability of a serious bacterial infection, 10% probability of bacteremia, and 2% probability of bacterial meningitis.1 The “low-risk” infants have a correspondingly lower incidence of serious bacterial infection: the negative predictive value of the Rochester classification is 98.9% (95% confidence interval [CI], 97.2–99.6%).2
The negative predictive value for bacterial meningitis (a subset of serious bacterial infection) is even greater. Five studies applied the standardized criteria to febrile infants and monitored them for the development of serious bacterial infection, including meningitis. Two prospective cohort studies of outpatients aged 0 to 2 months used the Rochester criteria to assign infants to risk groups. They studied a total of 1294 infants; 659 (51%) were low-risk. None of the low-risk infants developed bacterial meningitis.2,3
One prospective cohort study of infants aged <1 month hospitalized for fever used a similar method for assessing risk, but added a C-reactive protein value <20 mg/L to criteria for low-risk. Of 250 infants studied, 131 (52%) were low-risk; none of these developed bacterial meningitis.4
A retrospective chart review of 492 infants aged <3 months who were hospitalized due to fever included 108 infants aged <1 month. Thirty percent (114) of the infants aged 1 to 3 months and 67% (72) of the younger infants underwent lumbar puncture at the discretion of the treating physician. All infants were retrospectively assigned to low- or high-risk groups for serious bacterial infection using the Rochester criteria. Of the 296 infants rated “low-risk,” none developed bacterial meningitis. Ten of these infants subsequently developed evidence of another bacterial focus (predominantly urinary tract infection).5
RECOMMENDATIONS FROM OTHERS
The American Academy of Pediatrics has not issued a clinical practice guideline or clinical report addressing this issue. An evidence-based guideline developed at Cincinnati Children’s Hospital Medical Center in 1998 recommends hospitalization and a full sepsis workup (including lumbar puncture) for infants aged <1 month, or infants aged 1 to 2 months who are high-risk.6
A clinical review-based guideline published in 1993 gives the same recommendations.7 The expert panel that devised this guideline emphasized a full sepsis evaluation (including cerebrospinal fluid cultures) for infants <28 days of age “despite the low probability of serious bacterial infections in this age group and the favorable outcome of the children managed to date with careful observation.” For low-risk infants aged 1 to 2 months, lumbar puncture is not necessary unless empiric antibiotics are given; having a cerebrospinal fluid culture prior to empiric antibiotics reduces the concern of partially treated meningitis in the case of clinical deterioration after hospital discharge.6,7
TABLE
How to identify infants at low risk of serious bacterial infection: Rochester Classification
| Febrile infants (temperature ≥38°C, 100.4°F) ≥60 days of age who meet all criteria are at low risk of serious bacterial infection: | |
|---|---|
| General health | Born at ≥37 weeks’ gestation |
| Did not receive perinatal or antenatal antibiotics | |
| Was not treated for unexplained hyperbilirubinemia | |
| Was not hospitalized in the nursery longer than the mother | |
| Has had no hospitalization since discharge | |
| No diagnosed chronic or underlying illnesses | |
| Physical findings | Appears well and nontoxic |
| No evidence of skin, soft tissue, bone, or joint abnormalities, or otitis media | |
| Laboratory findings | Peripheral total white blood cells 5,000–15,000/mm3 |
| Absolute band form leukocytes <1,500/mm3 | |
| Spun urine sediment <10 white blood cells per high power field | |
| Fresh stool smear <5 white blood cells per high power field | |
Evaluating fever in infants: judging the risks
Randy Ward, MD
Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
The evaluation of the febrile infant is often fraught with anxiety. Physicians must balance the potentially devastating consequences of a missed serious bacterial infection with the desire to avoid unnecessary work-ups.
In the past, guidelines have had an extremely conservative viewpoint, essentially grouping all infants by age, and recommended an extensive inpatient work-up regardless of clinical status. The Rochester Criteria have provided guidelines for clinical risk stratification in this age group, allowing a more rational approach to the workup. The above data provide further useful guidance for the appropriate use of lumbar puncture in evaluation of these infants.
1. Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J 1992;11:257-264.
2. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94:390-396.
3. Dagan R, Sofer S, Phillip M, Shachak E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J Pediatr 1988;112:355-360.
4. Chiu CH, Lin TY, Bullard MJ. Identification of febrile neonates unlikely to have bacterial infection. Pediatr Infect Dis J 1997;16:59-63.
5. Brik R, Hamissah R, Shehada N, Berant M. Evaluation of febrile infants under 3 months of age: is routine lumbar puncture warranted?. Isr J Med Sci 1997;33:93-97.
6. Cincinnati Children’s Hospital Medical Center. Evidence based clinical protocol guideline for fever of uncertain source in infants 60 days of age or less. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1998.
7. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med 1993;22:1198-1210.
1. Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J 1992;11:257-264.
2. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94:390-396.
3. Dagan R, Sofer S, Phillip M, Shachak E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J Pediatr 1988;112:355-360.
4. Chiu CH, Lin TY, Bullard MJ. Identification of febrile neonates unlikely to have bacterial infection. Pediatr Infect Dis J 1997;16:59-63.
5. Brik R, Hamissah R, Shehada N, Berant M. Evaluation of febrile infants under 3 months of age: is routine lumbar puncture warranted?. Isr J Med Sci 1997;33:93-97.
6. Cincinnati Children’s Hospital Medical Center. Evidence based clinical protocol guideline for fever of uncertain source in infants 60 days of age or less. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1998.
7. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med 1993;22:1198-1210.
Evidence-based answers from the Family Physicians Inquiries Network