User login
Chronic inflammatory disease patients at greater risk of major CV events
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Patients with RA, PsA, and psoriasis have similarly elevated risk for major adverse cardiovascular events when compared with the general population.
Major finding: The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49), compared with matched controls.
Data source: A population-based, longitudinal cohort study of 41,752 RA patients, 8,706 PsA patients, 138,424 psoriasis patients, and 81,573 matched controls.
Disclosures: The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Abatacept + methotrexate makes early drug-free RA remission possible
Patients with early rheumatoid arthritis who achieved remission after 1 year of treatment with abatacept and methotrexate had a small but significantly greater rate of sustained remission for another 6 months following withdrawal of all drugs when compared with patients who took methotrexate alone in a randomized phase IIIb study.
The AVERT (Assessing Very Early Rheumatoid Arthritis Treatment) study randomized 351 early rheumatoid arthritis (RA) patients who were methotrexate naive, tested positive for anticitrullinated peptide–2 antibody, and had a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of more than 3.2 to weekly doses of 125 mg abatacept plus methotrexate, abatacept monotherapy, or methotrexate alone. Methotrexate was initiated at 7.5 mg/wk and was then titrated to 15-20 mg/wk in 6-8 weeks, but the protocol allowed for 10 mg or less per week in patients who could not tolerate higher doses.
In one of two primary endpoints, significantly more patients in the combination arm achieved remission (DAS28-CRP <2.6) at 12 months (60.9%), compared with patients who took methotrexate alone (45.2%), for an odds ratio of 2.01 (P = .010). The rate was 42.5% in the abatacept monotherapy arm, Dr. Paul Emery of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) and his colleagues reported (Ann. Rheum. Dis. 2014 Nov. 3 [doi:10.1136/annrheumdis-2014-206106]).
At the end of the first 12 months, patients who had achieved a DAS28-CRP score of less than 3.2 entered a 12-month treatment withdrawal period. Remission at both 12 and 18 months – the second primary endpoint – occurred in 14.8% of patients on combination therapy and 7.8% of those on methotrexate alone (OR, 2.51; P = .045). A total of 12.4% of abatacept monotherapy patients achieved remission at both 12 and 18 months.
The results suggest that in early RA drug-free remission may be possible following treatment with abatacept, the researchers said.
In the 12-month treatment period, the serious adverse events were reported in 12.1% taking abatacept monotherapy, 6.7% taking combination therapy, and 7.8% taking methotrexate alone.
The study was sponsored by Bristol-Myers Squibb, which manufactures abatacept (Orencia). Dr. Emery reported receiving consulting fees and grant support from Bristol-Myers Squibb as well as other companies that market drugs for RA. Many of his coauthors reported similar disclosures. Two authors are employees of Bristol-Myers Squibb.
Patients with early rheumatoid arthritis who achieved remission after 1 year of treatment with abatacept and methotrexate had a small but significantly greater rate of sustained remission for another 6 months following withdrawal of all drugs when compared with patients who took methotrexate alone in a randomized phase IIIb study.
The AVERT (Assessing Very Early Rheumatoid Arthritis Treatment) study randomized 351 early rheumatoid arthritis (RA) patients who were methotrexate naive, tested positive for anticitrullinated peptide–2 antibody, and had a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of more than 3.2 to weekly doses of 125 mg abatacept plus methotrexate, abatacept monotherapy, or methotrexate alone. Methotrexate was initiated at 7.5 mg/wk and was then titrated to 15-20 mg/wk in 6-8 weeks, but the protocol allowed for 10 mg or less per week in patients who could not tolerate higher doses.
In one of two primary endpoints, significantly more patients in the combination arm achieved remission (DAS28-CRP <2.6) at 12 months (60.9%), compared with patients who took methotrexate alone (45.2%), for an odds ratio of 2.01 (P = .010). The rate was 42.5% in the abatacept monotherapy arm, Dr. Paul Emery of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) and his colleagues reported (Ann. Rheum. Dis. 2014 Nov. 3 [doi:10.1136/annrheumdis-2014-206106]).
At the end of the first 12 months, patients who had achieved a DAS28-CRP score of less than 3.2 entered a 12-month treatment withdrawal period. Remission at both 12 and 18 months – the second primary endpoint – occurred in 14.8% of patients on combination therapy and 7.8% of those on methotrexate alone (OR, 2.51; P = .045). A total of 12.4% of abatacept monotherapy patients achieved remission at both 12 and 18 months.
The results suggest that in early RA drug-free remission may be possible following treatment with abatacept, the researchers said.
In the 12-month treatment period, the serious adverse events were reported in 12.1% taking abatacept monotherapy, 6.7% taking combination therapy, and 7.8% taking methotrexate alone.
The study was sponsored by Bristol-Myers Squibb, which manufactures abatacept (Orencia). Dr. Emery reported receiving consulting fees and grant support from Bristol-Myers Squibb as well as other companies that market drugs for RA. Many of his coauthors reported similar disclosures. Two authors are employees of Bristol-Myers Squibb.
Patients with early rheumatoid arthritis who achieved remission after 1 year of treatment with abatacept and methotrexate had a small but significantly greater rate of sustained remission for another 6 months following withdrawal of all drugs when compared with patients who took methotrexate alone in a randomized phase IIIb study.
The AVERT (Assessing Very Early Rheumatoid Arthritis Treatment) study randomized 351 early rheumatoid arthritis (RA) patients who were methotrexate naive, tested positive for anticitrullinated peptide–2 antibody, and had a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of more than 3.2 to weekly doses of 125 mg abatacept plus methotrexate, abatacept monotherapy, or methotrexate alone. Methotrexate was initiated at 7.5 mg/wk and was then titrated to 15-20 mg/wk in 6-8 weeks, but the protocol allowed for 10 mg or less per week in patients who could not tolerate higher doses.
In one of two primary endpoints, significantly more patients in the combination arm achieved remission (DAS28-CRP <2.6) at 12 months (60.9%), compared with patients who took methotrexate alone (45.2%), for an odds ratio of 2.01 (P = .010). The rate was 42.5% in the abatacept monotherapy arm, Dr. Paul Emery of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) and his colleagues reported (Ann. Rheum. Dis. 2014 Nov. 3 [doi:10.1136/annrheumdis-2014-206106]).
At the end of the first 12 months, patients who had achieved a DAS28-CRP score of less than 3.2 entered a 12-month treatment withdrawal period. Remission at both 12 and 18 months – the second primary endpoint – occurred in 14.8% of patients on combination therapy and 7.8% of those on methotrexate alone (OR, 2.51; P = .045). A total of 12.4% of abatacept monotherapy patients achieved remission at both 12 and 18 months.
The results suggest that in early RA drug-free remission may be possible following treatment with abatacept, the researchers said.
In the 12-month treatment period, the serious adverse events were reported in 12.1% taking abatacept monotherapy, 6.7% taking combination therapy, and 7.8% taking methotrexate alone.
The study was sponsored by Bristol-Myers Squibb, which manufactures abatacept (Orencia). Dr. Emery reported receiving consulting fees and grant support from Bristol-Myers Squibb as well as other companies that market drugs for RA. Many of his coauthors reported similar disclosures. Two authors are employees of Bristol-Myers Squibb.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Early RA drug-free remission may be possible following treatment with abatacept.
Major finding: About 15% of patients who were in remission after taking abatacept and methotrexate for 1 year continued to be in remission for another 6 months after stopping all drugs, compared with about 8% of those taking methotrexate alone.
Data source: A phase IIIb randomized study of 351 early RA patients who were methotrexate naive, were anti-CCP2 positive, and had DAS28 scores of more than 3.2.
Disclosures: The study was sponsored by Bristol-Myers Squibb, which manufactures abatacept (Orencia). Dr. Emery reported receiving consulting fees and grant support from Bristol-Myers Squibb as well as other companies that market drugs for RA. Many of his coauthors reported similar disclosures. Two authors are employees of Bristol-Myers Squibb.
Tocilizumab and tofacitinib increase lipids in RA patients
Rheumatoid arthritis patients treated with the biologic tocilizumab and the Janus kinase inhibitor tofacitinib had significant changes to their lipid levels in a meta-analysis of randomized, controlled trials.
No changes in lipid profiles were observed for patients taking anti–tumor necrosis factor–alpha inhibitors in the analysis of 25 randomized, controlled trials of chronic inflammatory arthritis. The study, according to lead author Alejandro Souto of the rheumatology unit at Complejo Hospitalario Universitario de Santiago (Spain) de Compostela and his colleagues, is the first study to summarize all the data about lipid changes in patients treated with biologics and tofacitinib.
The authors found that RA patients who took tocilizumab were significantly more likely to have hypercholesterolemia (>240 mg/dL), high HDL cholesterol levels (>60 mg/dL), and high LDL cholesterol levels (>130 mg/dL) at the end of the trial than did placebo patients. In RA trials, tofacitinib treatment led to significantly greater increases in levels of HDL cholesterol (13.00 mg/dL for 5 mg twice daily and 15.21 mg/dL for 10 mg twice daily) and LDL cholesterol (11.20 mg/dL for 5 mg twice daily and 15.42 mg/dL for 10 mg twice daily) than in those who took placebo, all of which were calculated with a weight mean difference (or mean percentage increase) for the continuous variable.
The exact mechanism by which the drugs affect lipid levels is unclear. “Whether the encountered changes relate to the control of inflammation or an independent mechanism of action remains to be determined,” the study authors wrote (Arthritis Rheumatol. 2014 Oct. 9 [doi:10.1002/art.38894]).
Changes in lipid levels could not be entirely explained by reduced inflammation because several drugs for RA were effective at reducing inflammation, but only tocilizumab and tofacitinib produced significant changes in lipid profiles, they noted. Results from animal models of arthritis showed decreased levels of interleukin (IL)-6 after the administration of tofacitinib, suggesting the drug may change lipid patterns by inhibiting the inflammatory cytokine.
“Interestingly, the modulation of inflammation seems to lead to lower cardiovascular events and a reduction in mortality despite increasing lipid levels,” they wrote. “It is possible that hypercholesterolemia produced by JAK [Janus kinase] inhibitors may be caused by the inhibition of IL-6 signaling.”
The long-term consequence for cardiovascular outcomes in this population should be assessed, the authors said.
The review had several limitations, which affected the generalization of their findings, the authors said. These included a lack of data on the effects of rituximab and abatacept on lipids, or in spondyloarthritis treated with biologics. It was also limited by the fact that only 25 of 307 studies contained data on lipid pattern changes and the heterogeneous way of presenting the data.
The study was supported by an unrestricted grant from Pfizer. Two authors reported having ties to Pfizer as well as other companies that market drugs for inflammatory arthritis.
Rheumatoid arthritis patients treated with the biologic tocilizumab and the Janus kinase inhibitor tofacitinib had significant changes to their lipid levels in a meta-analysis of randomized, controlled trials.
No changes in lipid profiles were observed for patients taking anti–tumor necrosis factor–alpha inhibitors in the analysis of 25 randomized, controlled trials of chronic inflammatory arthritis. The study, according to lead author Alejandro Souto of the rheumatology unit at Complejo Hospitalario Universitario de Santiago (Spain) de Compostela and his colleagues, is the first study to summarize all the data about lipid changes in patients treated with biologics and tofacitinib.
The authors found that RA patients who took tocilizumab were significantly more likely to have hypercholesterolemia (>240 mg/dL), high HDL cholesterol levels (>60 mg/dL), and high LDL cholesterol levels (>130 mg/dL) at the end of the trial than did placebo patients. In RA trials, tofacitinib treatment led to significantly greater increases in levels of HDL cholesterol (13.00 mg/dL for 5 mg twice daily and 15.21 mg/dL for 10 mg twice daily) and LDL cholesterol (11.20 mg/dL for 5 mg twice daily and 15.42 mg/dL for 10 mg twice daily) than in those who took placebo, all of which were calculated with a weight mean difference (or mean percentage increase) for the continuous variable.
The exact mechanism by which the drugs affect lipid levels is unclear. “Whether the encountered changes relate to the control of inflammation or an independent mechanism of action remains to be determined,” the study authors wrote (Arthritis Rheumatol. 2014 Oct. 9 [doi:10.1002/art.38894]).
Changes in lipid levels could not be entirely explained by reduced inflammation because several drugs for RA were effective at reducing inflammation, but only tocilizumab and tofacitinib produced significant changes in lipid profiles, they noted. Results from animal models of arthritis showed decreased levels of interleukin (IL)-6 after the administration of tofacitinib, suggesting the drug may change lipid patterns by inhibiting the inflammatory cytokine.
“Interestingly, the modulation of inflammation seems to lead to lower cardiovascular events and a reduction in mortality despite increasing lipid levels,” they wrote. “It is possible that hypercholesterolemia produced by JAK [Janus kinase] inhibitors may be caused by the inhibition of IL-6 signaling.”
The long-term consequence for cardiovascular outcomes in this population should be assessed, the authors said.
The review had several limitations, which affected the generalization of their findings, the authors said. These included a lack of data on the effects of rituximab and abatacept on lipids, or in spondyloarthritis treated with biologics. It was also limited by the fact that only 25 of 307 studies contained data on lipid pattern changes and the heterogeneous way of presenting the data.
The study was supported by an unrestricted grant from Pfizer. Two authors reported having ties to Pfizer as well as other companies that market drugs for inflammatory arthritis.
Rheumatoid arthritis patients treated with the biologic tocilizumab and the Janus kinase inhibitor tofacitinib had significant changes to their lipid levels in a meta-analysis of randomized, controlled trials.
No changes in lipid profiles were observed for patients taking anti–tumor necrosis factor–alpha inhibitors in the analysis of 25 randomized, controlled trials of chronic inflammatory arthritis. The study, according to lead author Alejandro Souto of the rheumatology unit at Complejo Hospitalario Universitario de Santiago (Spain) de Compostela and his colleagues, is the first study to summarize all the data about lipid changes in patients treated with biologics and tofacitinib.
The authors found that RA patients who took tocilizumab were significantly more likely to have hypercholesterolemia (>240 mg/dL), high HDL cholesterol levels (>60 mg/dL), and high LDL cholesterol levels (>130 mg/dL) at the end of the trial than did placebo patients. In RA trials, tofacitinib treatment led to significantly greater increases in levels of HDL cholesterol (13.00 mg/dL for 5 mg twice daily and 15.21 mg/dL for 10 mg twice daily) and LDL cholesterol (11.20 mg/dL for 5 mg twice daily and 15.42 mg/dL for 10 mg twice daily) than in those who took placebo, all of which were calculated with a weight mean difference (or mean percentage increase) for the continuous variable.
The exact mechanism by which the drugs affect lipid levels is unclear. “Whether the encountered changes relate to the control of inflammation or an independent mechanism of action remains to be determined,” the study authors wrote (Arthritis Rheumatol. 2014 Oct. 9 [doi:10.1002/art.38894]).
Changes in lipid levels could not be entirely explained by reduced inflammation because several drugs for RA were effective at reducing inflammation, but only tocilizumab and tofacitinib produced significant changes in lipid profiles, they noted. Results from animal models of arthritis showed decreased levels of interleukin (IL)-6 after the administration of tofacitinib, suggesting the drug may change lipid patterns by inhibiting the inflammatory cytokine.
“Interestingly, the modulation of inflammation seems to lead to lower cardiovascular events and a reduction in mortality despite increasing lipid levels,” they wrote. “It is possible that hypercholesterolemia produced by JAK [Janus kinase] inhibitors may be caused by the inhibition of IL-6 signaling.”
The long-term consequence for cardiovascular outcomes in this population should be assessed, the authors said.
The review had several limitations, which affected the generalization of their findings, the authors said. These included a lack of data on the effects of rituximab and abatacept on lipids, or in spondyloarthritis treated with biologics. It was also limited by the fact that only 25 of 307 studies contained data on lipid pattern changes and the heterogeneous way of presenting the data.
The study was supported by an unrestricted grant from Pfizer. Two authors reported having ties to Pfizer as well as other companies that market drugs for inflammatory arthritis.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The biologic tocilizumab and the JAK inhibitor increase lipid levels in RA patients.
Major finding: Tocilizumab and tofacitinib increased levels of HDL, LDL, and total cholesterol.
Data source: A systematic meta-analysis of 25 randomized, controlled trials.
Disclosures: The study was supported by an unrestricted grant from Pfizer. Two authors reported having ties to Pfizer as well as other companies that market drugs for inflammatory arthritis.
Hand exercise program worthwhile, cost effective in RA patients
A tailored hand exercise program for rheumatoid arthritis patients proved to be a cost-effective intervention for restoring and maintaining hand function in a randomized trial.
Sarah E. Lamb, D.Phil., of the Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences at the University of Oxford (England), and her colleagues from the SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand) trial randomly assigned 490 adults with rheumatoid arthritis who reported hand pain or dysfunction to usual care or a tailored program of strengthening and stretching hand exercises at a hospital and in the home.
The exercise group’s improvement was more than double that of the usual care group. Hand function at 12 months improved by 3.6 points in the usual care group (95% confidence interval, 1.5-5.7) and 7.9 points in the exercise group (95% CI, 6.0-9.9) as measured by the Michigan Hand Outcomes Questionnaire (Lancet 2014 Oct. 10.[doi:10.1016/S0140-6736 (14)60998-3]).
The cost of the exercise program was $250 USD per patient and cost per quality-adjusted life-year was $15,316 USD.
“A tailored hand exercise program is a worthwhile, low-cost intervention as an adjunct to a range of drug regimens,” the researchers concluded.
In an accompanying editorial, Christina H. Opava, Ph.D., of the Karolinska Institute in Stockholm, and Mathilda Björk, Ph.D., of Linköping University and Jönköping University, both in Sweden, wrote that successful interventions that were also cost effective were important, particularly because RA drugs are so expensive (Lancet 2014 Oct. 10 [doi:10.1016/S0140-6736(14)61285-X]).
“The use of hand exercise also has the potential to be integrated into e-health, via a web-based solution or interactive monitoring, which could further increase cost effectiveness and adherence,” they wrote.
However the positive results of the study might be jeopardized by bias introduced by selecting patients who were motivated to travel to hospital and follow a daily exercise program at home, they said.
Before the exercise program was implemented into clinical practice, it was also important to train therapists to ensure appropriate delivery of the intervention. This was especially important as the program included the use of extensive behavioral change support techniques that are not yet self-evident parts of physical therapy and occupational therapy practice, Dr. Opava and Dr. Björk said.
The trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. None of the study or editorial authors had anything to disclose.
A tailored hand exercise program for rheumatoid arthritis patients proved to be a cost-effective intervention for restoring and maintaining hand function in a randomized trial.
Sarah E. Lamb, D.Phil., of the Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences at the University of Oxford (England), and her colleagues from the SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand) trial randomly assigned 490 adults with rheumatoid arthritis who reported hand pain or dysfunction to usual care or a tailored program of strengthening and stretching hand exercises at a hospital and in the home.
The exercise group’s improvement was more than double that of the usual care group. Hand function at 12 months improved by 3.6 points in the usual care group (95% confidence interval, 1.5-5.7) and 7.9 points in the exercise group (95% CI, 6.0-9.9) as measured by the Michigan Hand Outcomes Questionnaire (Lancet 2014 Oct. 10.[doi:10.1016/S0140-6736 (14)60998-3]).
The cost of the exercise program was $250 USD per patient and cost per quality-adjusted life-year was $15,316 USD.
“A tailored hand exercise program is a worthwhile, low-cost intervention as an adjunct to a range of drug regimens,” the researchers concluded.
In an accompanying editorial, Christina H. Opava, Ph.D., of the Karolinska Institute in Stockholm, and Mathilda Björk, Ph.D., of Linköping University and Jönköping University, both in Sweden, wrote that successful interventions that were also cost effective were important, particularly because RA drugs are so expensive (Lancet 2014 Oct. 10 [doi:10.1016/S0140-6736(14)61285-X]).
“The use of hand exercise also has the potential to be integrated into e-health, via a web-based solution or interactive monitoring, which could further increase cost effectiveness and adherence,” they wrote.
However the positive results of the study might be jeopardized by bias introduced by selecting patients who were motivated to travel to hospital and follow a daily exercise program at home, they said.
Before the exercise program was implemented into clinical practice, it was also important to train therapists to ensure appropriate delivery of the intervention. This was especially important as the program included the use of extensive behavioral change support techniques that are not yet self-evident parts of physical therapy and occupational therapy practice, Dr. Opava and Dr. Björk said.
The trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. None of the study or editorial authors had anything to disclose.
A tailored hand exercise program for rheumatoid arthritis patients proved to be a cost-effective intervention for restoring and maintaining hand function in a randomized trial.
Sarah E. Lamb, D.Phil., of the Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences at the University of Oxford (England), and her colleagues from the SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand) trial randomly assigned 490 adults with rheumatoid arthritis who reported hand pain or dysfunction to usual care or a tailored program of strengthening and stretching hand exercises at a hospital and in the home.
The exercise group’s improvement was more than double that of the usual care group. Hand function at 12 months improved by 3.6 points in the usual care group (95% confidence interval, 1.5-5.7) and 7.9 points in the exercise group (95% CI, 6.0-9.9) as measured by the Michigan Hand Outcomes Questionnaire (Lancet 2014 Oct. 10.[doi:10.1016/S0140-6736 (14)60998-3]).
The cost of the exercise program was $250 USD per patient and cost per quality-adjusted life-year was $15,316 USD.
“A tailored hand exercise program is a worthwhile, low-cost intervention as an adjunct to a range of drug regimens,” the researchers concluded.
In an accompanying editorial, Christina H. Opava, Ph.D., of the Karolinska Institute in Stockholm, and Mathilda Björk, Ph.D., of Linköping University and Jönköping University, both in Sweden, wrote that successful interventions that were also cost effective were important, particularly because RA drugs are so expensive (Lancet 2014 Oct. 10 [doi:10.1016/S0140-6736(14)61285-X]).
“The use of hand exercise also has the potential to be integrated into e-health, via a web-based solution or interactive monitoring, which could further increase cost effectiveness and adherence,” they wrote.
However the positive results of the study might be jeopardized by bias introduced by selecting patients who were motivated to travel to hospital and follow a daily exercise program at home, they said.
Before the exercise program was implemented into clinical practice, it was also important to train therapists to ensure appropriate delivery of the intervention. This was especially important as the program included the use of extensive behavioral change support techniques that are not yet self-evident parts of physical therapy and occupational therapy practice, Dr. Opava and Dr. Björk said.
The trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. None of the study or editorial authors had anything to disclose.
FROM THE LANCET
Key clinical point: A tailored hand exercise program for RA patients is a cost-effective intervention for restoring hand function.
Major finding: Hand function at 12 months improved by 3.6 points in the usual care group (95% CI, 1.5-5.7) and 7.9 points in the exercise group (95% CI, 6.0-9.9) as measured by the Michigan Hand Outcomes Questionnaire
Data source: A multicenter, randomized trial of 490 RA patients assigned to usual care or a hand exercise program.
Disclosures: The trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. None of the authors had anything to disclose.
Biomarkers identify RA patients at risk of lung disease
An international team of researchers has identified biomarkers that may help pinpoint rheumatoid arthritis patients at risk of interstitial lung disease.
Dr. Juan Chen of the First Hospital of Xiamen (China) University and Dr. Tracy Doyle of Brigham and Women’s Hospital, Boston, classified Chinese rheumatoid arthritis (RA) patients into different clinical and radiographic stages of interstitial lung disease (ILD) – RA with no ILD, RA with mild ILD, and RA with advanced ILD (Arthritis Rheumatol. 2014 [doi:10.1002/art.38904]).
They used multiplex ELISA (enzyme-linked immunosorbent assay) to identify strong correlations between average serum levels of MMP-7 and IP10 and the grade of interstitial lung abnormalities. The average serum concentration of MMP-7 increased from 3.06 ng/mL in the RA patients with no ILD to 5.35 ng/mL in RA patients with ILD (P = .005). Levels of IP10 increased from 173.8 pg/mL in RA patients with no lung disease to 308.6 pg/mL in patients with ILD (P = .0004).
The researchers also found statistically significant elevations of both biomarkers in the patients with mild disease, “strengthening the apparent dose response relationship between these biomarkers and severity of radiographically defined interstitial lung abnormalities.”
Using a replication cohort from two academic centers in the United States, the researchers confirmed their findings by identifying statistically significant correlations between the two biomarkers and the presence of lung disease. The findings held strong even after adjustment for age, sex, smoking history, and 28-joint Disease Activity Score.
“[The results] demonstrate that elevated levels of IP10 and MMP-7 strongly correlate with the presence of RA-ILD, effectively supporting the hypothesis that RA-ILD represents a spectrum of pathology involving parenchymal lung inflammation and dysregulated tissue remodeling,” the study authors concluded.
Longitudinal studies are needed to determine the prognostic value of MMP-7 and IP10 in RA patients with clinically/radiographically established disease, “potentially enabling clinicians to distinguish those individuals most likely to develop progressive fibrosis and an IPF-like clinical course,” they added.
The research was supported in part by the Harvard KL2/Catalyst Medical Research Investigator Training Program as well as grants from the National Institutes of Health, Veterans Affairs Merit Review Program, and the *Rheumatology Research Foundation’s Within Our Reach program. No conflicts of interest were declared.
*Correction 10/30/2014: The article previously listed the Rheumatology Research Foundation under its old name, the American College of Rheumatology Research and Education Foundation.
An international team of researchers has identified biomarkers that may help pinpoint rheumatoid arthritis patients at risk of interstitial lung disease.
Dr. Juan Chen of the First Hospital of Xiamen (China) University and Dr. Tracy Doyle of Brigham and Women’s Hospital, Boston, classified Chinese rheumatoid arthritis (RA) patients into different clinical and radiographic stages of interstitial lung disease (ILD) – RA with no ILD, RA with mild ILD, and RA with advanced ILD (Arthritis Rheumatol. 2014 [doi:10.1002/art.38904]).
They used multiplex ELISA (enzyme-linked immunosorbent assay) to identify strong correlations between average serum levels of MMP-7 and IP10 and the grade of interstitial lung abnormalities. The average serum concentration of MMP-7 increased from 3.06 ng/mL in the RA patients with no ILD to 5.35 ng/mL in RA patients with ILD (P = .005). Levels of IP10 increased from 173.8 pg/mL in RA patients with no lung disease to 308.6 pg/mL in patients with ILD (P = .0004).
The researchers also found statistically significant elevations of both biomarkers in the patients with mild disease, “strengthening the apparent dose response relationship between these biomarkers and severity of radiographically defined interstitial lung abnormalities.”
Using a replication cohort from two academic centers in the United States, the researchers confirmed their findings by identifying statistically significant correlations between the two biomarkers and the presence of lung disease. The findings held strong even after adjustment for age, sex, smoking history, and 28-joint Disease Activity Score.
“[The results] demonstrate that elevated levels of IP10 and MMP-7 strongly correlate with the presence of RA-ILD, effectively supporting the hypothesis that RA-ILD represents a spectrum of pathology involving parenchymal lung inflammation and dysregulated tissue remodeling,” the study authors concluded.
Longitudinal studies are needed to determine the prognostic value of MMP-7 and IP10 in RA patients with clinically/radiographically established disease, “potentially enabling clinicians to distinguish those individuals most likely to develop progressive fibrosis and an IPF-like clinical course,” they added.
The research was supported in part by the Harvard KL2/Catalyst Medical Research Investigator Training Program as well as grants from the National Institutes of Health, Veterans Affairs Merit Review Program, and the *Rheumatology Research Foundation’s Within Our Reach program. No conflicts of interest were declared.
*Correction 10/30/2014: The article previously listed the Rheumatology Research Foundation under its old name, the American College of Rheumatology Research and Education Foundation.
An international team of researchers has identified biomarkers that may help pinpoint rheumatoid arthritis patients at risk of interstitial lung disease.
Dr. Juan Chen of the First Hospital of Xiamen (China) University and Dr. Tracy Doyle of Brigham and Women’s Hospital, Boston, classified Chinese rheumatoid arthritis (RA) patients into different clinical and radiographic stages of interstitial lung disease (ILD) – RA with no ILD, RA with mild ILD, and RA with advanced ILD (Arthritis Rheumatol. 2014 [doi:10.1002/art.38904]).
They used multiplex ELISA (enzyme-linked immunosorbent assay) to identify strong correlations between average serum levels of MMP-7 and IP10 and the grade of interstitial lung abnormalities. The average serum concentration of MMP-7 increased from 3.06 ng/mL in the RA patients with no ILD to 5.35 ng/mL in RA patients with ILD (P = .005). Levels of IP10 increased from 173.8 pg/mL in RA patients with no lung disease to 308.6 pg/mL in patients with ILD (P = .0004).
The researchers also found statistically significant elevations of both biomarkers in the patients with mild disease, “strengthening the apparent dose response relationship between these biomarkers and severity of radiographically defined interstitial lung abnormalities.”
Using a replication cohort from two academic centers in the United States, the researchers confirmed their findings by identifying statistically significant correlations between the two biomarkers and the presence of lung disease. The findings held strong even after adjustment for age, sex, smoking history, and 28-joint Disease Activity Score.
“[The results] demonstrate that elevated levels of IP10 and MMP-7 strongly correlate with the presence of RA-ILD, effectively supporting the hypothesis that RA-ILD represents a spectrum of pathology involving parenchymal lung inflammation and dysregulated tissue remodeling,” the study authors concluded.
Longitudinal studies are needed to determine the prognostic value of MMP-7 and IP10 in RA patients with clinically/radiographically established disease, “potentially enabling clinicians to distinguish those individuals most likely to develop progressive fibrosis and an IPF-like clinical course,” they added.
The research was supported in part by the Harvard KL2/Catalyst Medical Research Investigator Training Program as well as grants from the National Institutes of Health, Veterans Affairs Merit Review Program, and the *Rheumatology Research Foundation’s Within Our Reach program. No conflicts of interest were declared.
*Correction 10/30/2014: The article previously listed the Rheumatology Research Foundation under its old name, the American College of Rheumatology Research and Education Foundation.
FROM ARTHRITIS AND RHEUMATOLOGY
Key clinical point: The discovery of blood biomarkers may lead to early identification of lung complications in RA patients.
Major finding: MMP-7 and IP10 were elevated in the blood of patients with different stages of RA associated interstitial lung disease.
Data source: A Chinese identification cohort (n = 133) and a U.S. replication cohort (n = 86) of RA patients with or without ILD.
Disclosures: The research was supported in part by the Harvard KL2/Catalyst Medical Research Investigator Training Program as well as grants from the National Institutes of Health, Veterans Affairs Merit Review Program, and the *Rheumatology Research Foundation’s Within Our Reach program. No conflicts of interest were declared.
RA patients’ readmission rates after joint replacement are rising
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
Rheumatoid arthritis patients who have undergone a hip or knee replacement are more likely to be readmitted to a hospital than are patients with osteoarthritis, according to findings from a large prospective registry study.
The analysis revealed an increasing trend in the incidence of 90-day readmissions in the rheumatoid arthritis (RA) patients by year, at 5.8%, 8.9%, and 10.6% for 2009, 2010, and 2011, respectively, Dr. Jasvinder Singh of the Birmingham (Ala.) VA Medical Center and his colleagues reported (Arthritis Care Res. 2014 Oct. 9 [doi:10.1002/acr.22497]).
For osteoarthritis (OA) patients, the 90-day readmission rates were similar by year at 6.7%, 6.7%, and 6.8%, respectively. After accounting for differences, including the risk by year, the adjusted risk for 90-day readmission in RA patients was 0.89 (95% confidence interval, 0.46-1.71) in 2009, 1.34 (95% CI, 0.69-2.61) in 2010, and 1.74 (95% CI, 1.16-2.60) in 2011, compared with OA patients.
Readmission after an elective hip or knee replacement is a problem of significant public health proportions, the study authors noted. A 90-day readmission rate of 6.8% translates to more than 70,000 admissions annually in the United States, they said.
The investigators analyzed 34,311 joint replacement procedures during the 3-year period – 33,815 performed in OA patients and 496 in patients with RA.
Overall, 42 RA patients were readmitted over the 3-year period, and the two most common reasons for readmission were joint prosthesis infection (10.2%) and septicemia (10.2%). For the 2,277 OA patients who were readmitted, the most common reasons were joint prosthesis infection (5.7%) and other postoperative infections.
The finding of an increasing 90-day readmission over a 3-year period in RA patients was a particular concern. “We considered several patient, procedure, surgeon, and hospital variables as important covariates and adjusted for those that were significant (age, gender, American Society of Anesthesiologists category, and iron deficiency anemia) in our multivariable-adjusted model, indicating that the increasing readmission rate in RA patients is not explained by these variables,” they wrote.
The effects of medications and pre- and postoperative rehabilitation programs could have played a role in readmission rates in RA patients, but the authors did not have the information to analyze the impact of these factors.
No conflicts of interest were declared.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The 90-day risk of readmission after hip or knee replacement surgery is higher in patients with RA, compared with patients with OA.
Major finding: Readmissions for RA patients climbed significantly each year studied, compared with OA patients.
Data source: A prospective analysis of data from a total joint replacement registry of adults with OA and RA.
Disclosures: No conflicts of interest were declared.
Phone-based intervention helped breast cancer patients lose weight, but key question remains
A telephone-based lifestyle intervention can have a significant effect on weight loss in overweight breast cancer survivors, according to a study that also highlights the dilemmas of funding large lifestyle trials with definitive mortality endpoints.
Mean weight loss at 6 months was greater in women who received telephone-based coaching and were mailed health information vs. women who received mailed information only (5.3% vs. 0.7%; P less than .001), investigators reported online June 16 in the Journal of Clinical Oncology. The study was part of the multicenter LISA(Lifestyle Intervention Study in Adjuvant Treatment of Early Breast Cancer) trial.
At 24 months the weight loss still compared favorably in the intervention group (3.6% loss vs. 0.4% in the mail-only group; P less than .001), reported Dr. Pamela J. Goodwin of the Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, and her associates (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2013.53.1517].
But will losing weight improve breast cancer outcomes?
The original aim of LISA was to examine the effect of weight loss on disease-free survival, but patient accrual was terminated early (at 338 of the 2,150 planned participants), because of a loss of funding from sponsor Novartis Pharmaceuticals, leaving that question unanswered.
Patients were eligible to participate in the study if they had been diagnosed in the last 36 months, had a body mass index of 24 kg/m2 or higher, and were receiving letrozole for hormone receptor–positive breast cancer. Study participants were randomly assigned to receive general health information by mail, or to a lifestyle intervention where they received weight loss advice by telephone in addition to the health information by mail.
The telephone-based lifestyle intervention included a dietary goal (500-1,000 kcal per day deficit) and a physical activity goal (150-200 minutes of moderate-intensity physical activity per week) to achieve weight loss.
"Our results support the use of telephone-based delivery of weight-loss interventions in patients with breast cancer, and they suggest that our approach will be generally effective in postmenopausal patients receiving an aromatase inhibitor," the investigators concluded.
The results, combined with the recognition that obesity is associated with poor breast cancer outcomes, provide support for a randomized trial using a telephone-based weight-loss intervention that is adequately powered to detect clinically important effects on breast cancer outcomes, they said.
While the findings add to a growing body of evidence supporting the benefits of weight-loss interventions in overweight breast cancer survivors, questions remain regarding the effect of weight loss on breast cancer recurrence and mortality, said Melinda L. Irwin, Ph.D., M.P.H., in an accompanying editorial (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2014.56.4583]).
"How can important lifestyle trials with definitive mortality end points best be funded?" asked Dr. Irwin of Yale University, New Haven, Conn. She suggested that pharmaceutical companies be required to include a lifestyle intervention arm in drug trials.
"Given that pharmaceutical companies primarily fund therapeutic trials, with little incentive to fund lifestyle interventions, and given the scarcity of funding from government agencies for large-scale long-term trials of lifestyle interventions with disease-free survival endpoints, another option may be to require pharmaceutical companies to include lifestyle interventions as an active comparison arm to drug trials, especially in cases when drug options provide modest benefit, or uptake or adherence to particular medications [is] low," she said.
The study was funded by Novartis Pharmaceuticals. Dr. Goodwin and Dr. Irwin reported no disclosures. Two coauthors disclosed honoraria or research funding from Novartis and other companies.
A telephone-based lifestyle intervention can have a significant effect on weight loss in overweight breast cancer survivors, according to a study that also highlights the dilemmas of funding large lifestyle trials with definitive mortality endpoints.
Mean weight loss at 6 months was greater in women who received telephone-based coaching and were mailed health information vs. women who received mailed information only (5.3% vs. 0.7%; P less than .001), investigators reported online June 16 in the Journal of Clinical Oncology. The study was part of the multicenter LISA(Lifestyle Intervention Study in Adjuvant Treatment of Early Breast Cancer) trial.
At 24 months the weight loss still compared favorably in the intervention group (3.6% loss vs. 0.4% in the mail-only group; P less than .001), reported Dr. Pamela J. Goodwin of the Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, and her associates (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2013.53.1517].
But will losing weight improve breast cancer outcomes?
The original aim of LISA was to examine the effect of weight loss on disease-free survival, but patient accrual was terminated early (at 338 of the 2,150 planned participants), because of a loss of funding from sponsor Novartis Pharmaceuticals, leaving that question unanswered.
Patients were eligible to participate in the study if they had been diagnosed in the last 36 months, had a body mass index of 24 kg/m2 or higher, and were receiving letrozole for hormone receptor–positive breast cancer. Study participants were randomly assigned to receive general health information by mail, or to a lifestyle intervention where they received weight loss advice by telephone in addition to the health information by mail.
The telephone-based lifestyle intervention included a dietary goal (500-1,000 kcal per day deficit) and a physical activity goal (150-200 minutes of moderate-intensity physical activity per week) to achieve weight loss.
"Our results support the use of telephone-based delivery of weight-loss interventions in patients with breast cancer, and they suggest that our approach will be generally effective in postmenopausal patients receiving an aromatase inhibitor," the investigators concluded.
The results, combined with the recognition that obesity is associated with poor breast cancer outcomes, provide support for a randomized trial using a telephone-based weight-loss intervention that is adequately powered to detect clinically important effects on breast cancer outcomes, they said.
While the findings add to a growing body of evidence supporting the benefits of weight-loss interventions in overweight breast cancer survivors, questions remain regarding the effect of weight loss on breast cancer recurrence and mortality, said Melinda L. Irwin, Ph.D., M.P.H., in an accompanying editorial (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2014.56.4583]).
"How can important lifestyle trials with definitive mortality end points best be funded?" asked Dr. Irwin of Yale University, New Haven, Conn. She suggested that pharmaceutical companies be required to include a lifestyle intervention arm in drug trials.
"Given that pharmaceutical companies primarily fund therapeutic trials, with little incentive to fund lifestyle interventions, and given the scarcity of funding from government agencies for large-scale long-term trials of lifestyle interventions with disease-free survival endpoints, another option may be to require pharmaceutical companies to include lifestyle interventions as an active comparison arm to drug trials, especially in cases when drug options provide modest benefit, or uptake or adherence to particular medications [is] low," she said.
The study was funded by Novartis Pharmaceuticals. Dr. Goodwin and Dr. Irwin reported no disclosures. Two coauthors disclosed honoraria or research funding from Novartis and other companies.
A telephone-based lifestyle intervention can have a significant effect on weight loss in overweight breast cancer survivors, according to a study that also highlights the dilemmas of funding large lifestyle trials with definitive mortality endpoints.
Mean weight loss at 6 months was greater in women who received telephone-based coaching and were mailed health information vs. women who received mailed information only (5.3% vs. 0.7%; P less than .001), investigators reported online June 16 in the Journal of Clinical Oncology. The study was part of the multicenter LISA(Lifestyle Intervention Study in Adjuvant Treatment of Early Breast Cancer) trial.
At 24 months the weight loss still compared favorably in the intervention group (3.6% loss vs. 0.4% in the mail-only group; P less than .001), reported Dr. Pamela J. Goodwin of the Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, and her associates (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2013.53.1517].
But will losing weight improve breast cancer outcomes?
The original aim of LISA was to examine the effect of weight loss on disease-free survival, but patient accrual was terminated early (at 338 of the 2,150 planned participants), because of a loss of funding from sponsor Novartis Pharmaceuticals, leaving that question unanswered.
Patients were eligible to participate in the study if they had been diagnosed in the last 36 months, had a body mass index of 24 kg/m2 or higher, and were receiving letrozole for hormone receptor–positive breast cancer. Study participants were randomly assigned to receive general health information by mail, or to a lifestyle intervention where they received weight loss advice by telephone in addition to the health information by mail.
The telephone-based lifestyle intervention included a dietary goal (500-1,000 kcal per day deficit) and a physical activity goal (150-200 minutes of moderate-intensity physical activity per week) to achieve weight loss.
"Our results support the use of telephone-based delivery of weight-loss interventions in patients with breast cancer, and they suggest that our approach will be generally effective in postmenopausal patients receiving an aromatase inhibitor," the investigators concluded.
The results, combined with the recognition that obesity is associated with poor breast cancer outcomes, provide support for a randomized trial using a telephone-based weight-loss intervention that is adequately powered to detect clinically important effects on breast cancer outcomes, they said.
While the findings add to a growing body of evidence supporting the benefits of weight-loss interventions in overweight breast cancer survivors, questions remain regarding the effect of weight loss on breast cancer recurrence and mortality, said Melinda L. Irwin, Ph.D., M.P.H., in an accompanying editorial (J. Clin. Oncol. 2014 June 16 [doi: 10.1200/JCO.2014.56.4583]).
"How can important lifestyle trials with definitive mortality end points best be funded?" asked Dr. Irwin of Yale University, New Haven, Conn. She suggested that pharmaceutical companies be required to include a lifestyle intervention arm in drug trials.
"Given that pharmaceutical companies primarily fund therapeutic trials, with little incentive to fund lifestyle interventions, and given the scarcity of funding from government agencies for large-scale long-term trials of lifestyle interventions with disease-free survival endpoints, another option may be to require pharmaceutical companies to include lifestyle interventions as an active comparison arm to drug trials, especially in cases when drug options provide modest benefit, or uptake or adherence to particular medications [is] low," she said.
The study was funded by Novartis Pharmaceuticals. Dr. Goodwin and Dr. Irwin reported no disclosures. Two coauthors disclosed honoraria or research funding from Novartis and other companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A telephone-based lifestyle intervention was effective in helping overweight breast cancer patients who were receiving an aromatase inhibitor, achieve weight loss. But the original question of the intervention’s effect on breast cancer mortality was left unanswered because of withdrawal of funding.
Major finding: Women receiving a telephone-based lifestyle intervention in addition to mailed health information lost significantly more weight at 6 months than women who received the mailed information only (5.3% vs. 0.7%; P less than .001).
Data source: A multicenter, randomized trail of overweight patients with early breast cancer who were receiving adjuvant letrozole; patient accrual was terminated early (at 338 of the 2,150 planned participants) because of withdrawal of funding.
Disclosures: The study was funded by Novartis Pharmaceuticals. Dr. Goodwin reported no disclosures. Two coauthors disclosed honoraria or research funding from Novartis and other companies.
New tool measures ‘financial toxicity’ of cancer treatment
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
FROM CANCER
International RA risk tool for CVD comes closer to reality
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.
The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
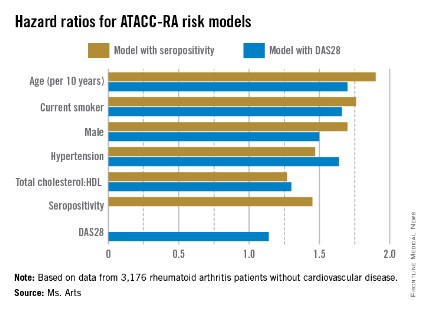
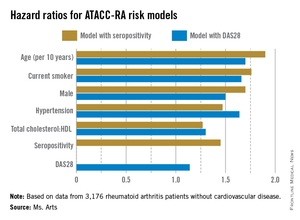
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.

The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.

The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
FROM THE EULAR CONGRESS 2014
Key clinical point: An RA-specific calculator for 10-year CVD risk may soon be available.
Major finding: The risk factors in models that used seropositivity or DAS28 had similar hazard ratios contributing to CVD risk.
Data source: Pooled data from 3,176 RA patients without CVD who were followed for the development of CVD for a mean of nearly 8 years.
Disclosures: The investigators had no conflicts of interest to declare.