User login
Phase III dupilumab data show significant improvements in atopic dermatitis
Treatment with dupilumab resulted in significant clinical improvements in adults with inadequately controlled moderate to-severe atopic dermatitis, in two phase III studies evaluating the biologic agent, according to Regeneron and Sanofi.
The phase III results of the two 16-week studies, SOLO 1 and SOLO 2, in nearly 1,400 adults with baseline Investigator’s Global Assessment (IGA) scores of 3 (moderate disease) or 4 (severe), were announced by Regeneron and Sanofi. The companies are codeveloping dupilumab, which inhibits signaling of interleukin-4 and IL-13, “two key cytokines required for the T helper 2 (Th2) immune response,” according to Regeneron.
In the studies, patients were randomized to treatment with 300 mg subcutaneously of dupilumab once a week or every 2 weeks (after a 600-mg loading dose) or placebo, for 16 weeks.
At 16 weeks, significantly more of those in the two treatment groups achieved clearing or near clearing of skin lesions – a primary endpoint – compared with placebo: In SOLO 1 and SOLO 2, respectively, an IGA score of 0 (clear) or 1 (almost clear) was achieved by 37% and 36% of those treated with 300 mg weekly, and 38% and 36% of those treated every 2 weeks, compared with 10% and 8.5% of those on placebo (P less than .0001).
Improvement from baseline in the Eczema Area and Severity Index (EASI) score in the SOLO 1 and SOLO 2 studies, respectively, were 72% and 69% of those treated with 300 mg weekly and 72% and 67% of those treated every 2 weeks, compared with 38% and 31% of those on placebo (P less than .0001).
The rates of adverse events ranged from 65% to 73% for those on dupilumab, and from 65% to 72% for those on placebo. The rates of serious adverse events were 1%-3% among those on dupilumab and 5%-6% for placebo; serious and severe infections were more common among those on placebo. Compared with placebo, injection site reactions were higher among those on dupilumab (10%-20% vs. 7%-8%). Conjunctivitis was more common among dupilumab-treated patients (7%-12% vs. 2% for placebo). One patient stopped treatment because of conjunctivitis.
The phase III results, which were announced in an April 1 press release, will be presented at a future medical meeting, and the companies plan to file for approval with the Food and Drug Administration in the third quarter of 2016.
Treatment with dupilumab resulted in significant clinical improvements in adults with inadequately controlled moderate to-severe atopic dermatitis, in two phase III studies evaluating the biologic agent, according to Regeneron and Sanofi.
The phase III results of the two 16-week studies, SOLO 1 and SOLO 2, in nearly 1,400 adults with baseline Investigator’s Global Assessment (IGA) scores of 3 (moderate disease) or 4 (severe), were announced by Regeneron and Sanofi. The companies are codeveloping dupilumab, which inhibits signaling of interleukin-4 and IL-13, “two key cytokines required for the T helper 2 (Th2) immune response,” according to Regeneron.
In the studies, patients were randomized to treatment with 300 mg subcutaneously of dupilumab once a week or every 2 weeks (after a 600-mg loading dose) or placebo, for 16 weeks.
At 16 weeks, significantly more of those in the two treatment groups achieved clearing or near clearing of skin lesions – a primary endpoint – compared with placebo: In SOLO 1 and SOLO 2, respectively, an IGA score of 0 (clear) or 1 (almost clear) was achieved by 37% and 36% of those treated with 300 mg weekly, and 38% and 36% of those treated every 2 weeks, compared with 10% and 8.5% of those on placebo (P less than .0001).
Improvement from baseline in the Eczema Area and Severity Index (EASI) score in the SOLO 1 and SOLO 2 studies, respectively, were 72% and 69% of those treated with 300 mg weekly and 72% and 67% of those treated every 2 weeks, compared with 38% and 31% of those on placebo (P less than .0001).
The rates of adverse events ranged from 65% to 73% for those on dupilumab, and from 65% to 72% for those on placebo. The rates of serious adverse events were 1%-3% among those on dupilumab and 5%-6% for placebo; serious and severe infections were more common among those on placebo. Compared with placebo, injection site reactions were higher among those on dupilumab (10%-20% vs. 7%-8%). Conjunctivitis was more common among dupilumab-treated patients (7%-12% vs. 2% for placebo). One patient stopped treatment because of conjunctivitis.
The phase III results, which were announced in an April 1 press release, will be presented at a future medical meeting, and the companies plan to file for approval with the Food and Drug Administration in the third quarter of 2016.
Treatment with dupilumab resulted in significant clinical improvements in adults with inadequately controlled moderate to-severe atopic dermatitis, in two phase III studies evaluating the biologic agent, according to Regeneron and Sanofi.
The phase III results of the two 16-week studies, SOLO 1 and SOLO 2, in nearly 1,400 adults with baseline Investigator’s Global Assessment (IGA) scores of 3 (moderate disease) or 4 (severe), were announced by Regeneron and Sanofi. The companies are codeveloping dupilumab, which inhibits signaling of interleukin-4 and IL-13, “two key cytokines required for the T helper 2 (Th2) immune response,” according to Regeneron.
In the studies, patients were randomized to treatment with 300 mg subcutaneously of dupilumab once a week or every 2 weeks (after a 600-mg loading dose) or placebo, for 16 weeks.
At 16 weeks, significantly more of those in the two treatment groups achieved clearing or near clearing of skin lesions – a primary endpoint – compared with placebo: In SOLO 1 and SOLO 2, respectively, an IGA score of 0 (clear) or 1 (almost clear) was achieved by 37% and 36% of those treated with 300 mg weekly, and 38% and 36% of those treated every 2 weeks, compared with 10% and 8.5% of those on placebo (P less than .0001).
Improvement from baseline in the Eczema Area and Severity Index (EASI) score in the SOLO 1 and SOLO 2 studies, respectively, were 72% and 69% of those treated with 300 mg weekly and 72% and 67% of those treated every 2 weeks, compared with 38% and 31% of those on placebo (P less than .0001).
The rates of adverse events ranged from 65% to 73% for those on dupilumab, and from 65% to 72% for those on placebo. The rates of serious adverse events were 1%-3% among those on dupilumab and 5%-6% for placebo; serious and severe infections were more common among those on placebo. Compared with placebo, injection site reactions were higher among those on dupilumab (10%-20% vs. 7%-8%). Conjunctivitis was more common among dupilumab-treated patients (7%-12% vs. 2% for placebo). One patient stopped treatment because of conjunctivitis.
The phase III results, which were announced in an April 1 press release, will be presented at a future medical meeting, and the companies plan to file for approval with the Food and Drug Administration in the third quarter of 2016.
FDA Approves First Generic Form of Oxiconazole Nitrate Cream
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
FDA approves first generic form of oxiconazole nitrate cream
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
A generic formulation of oxiconazole nitrate cream, 1% has been approved by the Food and Drug Administration, for the treatment of tinea pedis, tinea cruris, tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum) and tinea (pityriasis) versicolor due to Malassezia furfur.
This is the first generic version of Oxistat to be approved, according to the FDA’s statement announcing the approval.
The label for the generic, manufactured by Taro Pharmaceuticals U.S.A. is available here.
AAD: Transgender patients: Isotretinoin regs can be a challenge
WASHINGTON – Enrolling a transgender man in the iPLEDGE pregnancy prevention program is among the issues dermatologists can face when caring for transgender patients, Dr. Brian Ginsberg said at the annual meeting of the American Academy of Dermatology.*
iPLEDGE requirements are based on an individual’s gender, not only on their potential childbearing potential, “making it a challenge to appropriately classify our transgender patients,” said Dr. Ginsberg, a dermatologist in private practice in New York. Enrolling a transgender man who is experiencing severe acne vulgaris as a result of hormone therapy is one of the hurdles in caring for this patient population, he added.
Dr. Ginsberg provided advice on how to manage adverse effects of hormone therapy on skin and hair, and other dermatologic issues that transgender individuals may experience. Because there is not much information on this topic in the medical literature, he pointed out that his recommendations are based largely on personal and anecdotal experiences.
Transgender men taking testosterone experience significant increases in sebum production, and there are many reports of transgender men with severe acne vulgaris, he noted.
Transgender men may still be of childbearing potential, even if they are on testosterone, and he typically keeps his transgender male patients classified as females of childbearing potential, for the sake of iPLEDGE, and has “a very important and honest conversation with them” about having to register as females.
“It’s unfortunate that for now, we have to have that conversation,” but it must be done, he added.
A member of the audience said he has a transgender male patient who is preparing for reduction mammoplasty, is on testosterone, has severe acne, and was previously registered in the iPLEDGE program as a female. “So what’s my next step?” he asked.
Dr. Ginsberg said he has had patients in the same situation, and after having an honest conversation with the patient, “we realized the priority was getting the patient on isotretinoin and the patient was comfortable in maintaining the registration as a female of childbearing potential.”
This is not an issue for female transgender patients, who do not have a uterus and are not of childbearing potential. These patients are, however, at an increased risk of hormone-associated dermatoses.
“Transgender women taking estrogens experience rapid and prolonged low sebum production, resulting in xerosis and asteatotic eczema,” he said.
Another issue is when to prescribe finasteride for transgender men on testosterone who experience male pattern hair loss. He advised avoiding finasteride until body hair and other desired secondary sex characteristics are fully developed, which could be up to 2 years, Dr. Ginsberg said.
To make transgender patients more comfortable in the office, Dr. Ginsberg recommended the following:
• Modify intake forms. Allow for patients to write in their gender, instead of offering the option of male or female.
• Respect the use of correct pronouns. “If you have a transgender woman sitting in front of you, don’t refer to her as him,” he commented. Consider asking the patient which pronoun is preferred.
• Make no assumptions. “Gender identity has nothing to do with sexual orientation,” he said. “A person’s gender is however they self-identify, period.” It has nothing to do with clothing, hormones, surgery “or any other aspect of transitioning,” he explained. “If a patient sitting in front of you says that they are a man, it doesn’t matter what they look like or what they’ve had done, that person is a man.”
• Be comfortable about being uncomfortable. “The community is coming to understand that we don’t know everything. … and we may have questions, we may not understand the details of the surgery or medications that they’re on. So rather than ignore the issue altogether, ask them about it,” Dr. Ginsberg said. “They will be more excited that you care … and want to help, rather than ignore the issue altogether.” Dr. Ginsberg reported no relevant disclosures.
emechcatie@frontlinemedcom.com
This article was updated March 8, 2016.
*Correction, 03/15/2016: An earlier version of this article misstated Dr. Brian Ginsberg's name.
WASHINGTON – Enrolling a transgender man in the iPLEDGE pregnancy prevention program is among the issues dermatologists can face when caring for transgender patients, Dr. Brian Ginsberg said at the annual meeting of the American Academy of Dermatology.*
iPLEDGE requirements are based on an individual’s gender, not only on their potential childbearing potential, “making it a challenge to appropriately classify our transgender patients,” said Dr. Ginsberg, a dermatologist in private practice in New York. Enrolling a transgender man who is experiencing severe acne vulgaris as a result of hormone therapy is one of the hurdles in caring for this patient population, he added.
Dr. Ginsberg provided advice on how to manage adverse effects of hormone therapy on skin and hair, and other dermatologic issues that transgender individuals may experience. Because there is not much information on this topic in the medical literature, he pointed out that his recommendations are based largely on personal and anecdotal experiences.
Transgender men taking testosterone experience significant increases in sebum production, and there are many reports of transgender men with severe acne vulgaris, he noted.
Transgender men may still be of childbearing potential, even if they are on testosterone, and he typically keeps his transgender male patients classified as females of childbearing potential, for the sake of iPLEDGE, and has “a very important and honest conversation with them” about having to register as females.
“It’s unfortunate that for now, we have to have that conversation,” but it must be done, he added.
A member of the audience said he has a transgender male patient who is preparing for reduction mammoplasty, is on testosterone, has severe acne, and was previously registered in the iPLEDGE program as a female. “So what’s my next step?” he asked.
Dr. Ginsberg said he has had patients in the same situation, and after having an honest conversation with the patient, “we realized the priority was getting the patient on isotretinoin and the patient was comfortable in maintaining the registration as a female of childbearing potential.”
This is not an issue for female transgender patients, who do not have a uterus and are not of childbearing potential. These patients are, however, at an increased risk of hormone-associated dermatoses.
“Transgender women taking estrogens experience rapid and prolonged low sebum production, resulting in xerosis and asteatotic eczema,” he said.
Another issue is when to prescribe finasteride for transgender men on testosterone who experience male pattern hair loss. He advised avoiding finasteride until body hair and other desired secondary sex characteristics are fully developed, which could be up to 2 years, Dr. Ginsberg said.
To make transgender patients more comfortable in the office, Dr. Ginsberg recommended the following:
• Modify intake forms. Allow for patients to write in their gender, instead of offering the option of male or female.
• Respect the use of correct pronouns. “If you have a transgender woman sitting in front of you, don’t refer to her as him,” he commented. Consider asking the patient which pronoun is preferred.
• Make no assumptions. “Gender identity has nothing to do with sexual orientation,” he said. “A person’s gender is however they self-identify, period.” It has nothing to do with clothing, hormones, surgery “or any other aspect of transitioning,” he explained. “If a patient sitting in front of you says that they are a man, it doesn’t matter what they look like or what they’ve had done, that person is a man.”
• Be comfortable about being uncomfortable. “The community is coming to understand that we don’t know everything. … and we may have questions, we may not understand the details of the surgery or medications that they’re on. So rather than ignore the issue altogether, ask them about it,” Dr. Ginsberg said. “They will be more excited that you care … and want to help, rather than ignore the issue altogether.” Dr. Ginsberg reported no relevant disclosures.
emechcatie@frontlinemedcom.com
This article was updated March 8, 2016.
*Correction, 03/15/2016: An earlier version of this article misstated Dr. Brian Ginsberg's name.
WASHINGTON – Enrolling a transgender man in the iPLEDGE pregnancy prevention program is among the issues dermatologists can face when caring for transgender patients, Dr. Brian Ginsberg said at the annual meeting of the American Academy of Dermatology.*
iPLEDGE requirements are based on an individual’s gender, not only on their potential childbearing potential, “making it a challenge to appropriately classify our transgender patients,” said Dr. Ginsberg, a dermatologist in private practice in New York. Enrolling a transgender man who is experiencing severe acne vulgaris as a result of hormone therapy is one of the hurdles in caring for this patient population, he added.
Dr. Ginsberg provided advice on how to manage adverse effects of hormone therapy on skin and hair, and other dermatologic issues that transgender individuals may experience. Because there is not much information on this topic in the medical literature, he pointed out that his recommendations are based largely on personal and anecdotal experiences.
Transgender men taking testosterone experience significant increases in sebum production, and there are many reports of transgender men with severe acne vulgaris, he noted.
Transgender men may still be of childbearing potential, even if they are on testosterone, and he typically keeps his transgender male patients classified as females of childbearing potential, for the sake of iPLEDGE, and has “a very important and honest conversation with them” about having to register as females.
“It’s unfortunate that for now, we have to have that conversation,” but it must be done, he added.
A member of the audience said he has a transgender male patient who is preparing for reduction mammoplasty, is on testosterone, has severe acne, and was previously registered in the iPLEDGE program as a female. “So what’s my next step?” he asked.
Dr. Ginsberg said he has had patients in the same situation, and after having an honest conversation with the patient, “we realized the priority was getting the patient on isotretinoin and the patient was comfortable in maintaining the registration as a female of childbearing potential.”
This is not an issue for female transgender patients, who do not have a uterus and are not of childbearing potential. These patients are, however, at an increased risk of hormone-associated dermatoses.
“Transgender women taking estrogens experience rapid and prolonged low sebum production, resulting in xerosis and asteatotic eczema,” he said.
Another issue is when to prescribe finasteride for transgender men on testosterone who experience male pattern hair loss. He advised avoiding finasteride until body hair and other desired secondary sex characteristics are fully developed, which could be up to 2 years, Dr. Ginsberg said.
To make transgender patients more comfortable in the office, Dr. Ginsberg recommended the following:
• Modify intake forms. Allow for patients to write in their gender, instead of offering the option of male or female.
• Respect the use of correct pronouns. “If you have a transgender woman sitting in front of you, don’t refer to her as him,” he commented. Consider asking the patient which pronoun is preferred.
• Make no assumptions. “Gender identity has nothing to do with sexual orientation,” he said. “A person’s gender is however they self-identify, period.” It has nothing to do with clothing, hormones, surgery “or any other aspect of transitioning,” he explained. “If a patient sitting in front of you says that they are a man, it doesn’t matter what they look like or what they’ve had done, that person is a man.”
• Be comfortable about being uncomfortable. “The community is coming to understand that we don’t know everything. … and we may have questions, we may not understand the details of the surgery or medications that they’re on. So rather than ignore the issue altogether, ask them about it,” Dr. Ginsberg said. “They will be more excited that you care … and want to help, rather than ignore the issue altogether.” Dr. Ginsberg reported no relevant disclosures.
emechcatie@frontlinemedcom.com
This article was updated March 8, 2016.
*Correction, 03/15/2016: An earlier version of this article misstated Dr. Brian Ginsberg's name.
EXPERT ANALYSIS AT AAD 16
Children who have stem cell transplants need skin exams, sun protection
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
AT AAD 16
Key clinical point: Children who have had a hematopoietic stem cell transplant need to have routine skin exams and be educated about sun protection needs.
Major finding: 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Data source: A single-center study of 85 posttransplant patients and 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type.
Disclosures: The study was not sponsored and Dr. Sheu had no disclosures.
VIDEO: Study links hair loss in black women with genetics
WASHINGTON – Almost 41% of black women surveyed described hair loss that was consistent with central centrifugal cicatricial alopecia (CCCA), but only about 9% said they had been diagnosed with the condition, Dr. Yolanda Lenzy reported at the annual meeting of the American Academy of Dermatology.
In a video interview at the meeting, Dr. Lenzy of the University of Connecticut, Farmington, discussed the results of a hair survey she conducted with the Black Women’s Health Study at Boston University’s Slone Epidemiology Center. Nearly 6,000 women have completed the survey to date.
“For many years, it was thought to be due to hair styling practices,” but there are new data showing that genetics can be an important cause, she said, referring to research from South Africa indicating that CCCA can be inherited in an autosomal dominant fashion.
Dr. Lenzy, who practices dermatology in Chicopee, Mass., used a central hair loss photographic scale in the study, which also can be helpful in the office to monitor hair loss and “to quantify how much hair loss a person has … in terms of: Are they getting worse? Do they go from stage 3 to stage 5 or stage 1 to stage 3?”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Almost 41% of black women surveyed described hair loss that was consistent with central centrifugal cicatricial alopecia (CCCA), but only about 9% said they had been diagnosed with the condition, Dr. Yolanda Lenzy reported at the annual meeting of the American Academy of Dermatology.
In a video interview at the meeting, Dr. Lenzy of the University of Connecticut, Farmington, discussed the results of a hair survey she conducted with the Black Women’s Health Study at Boston University’s Slone Epidemiology Center. Nearly 6,000 women have completed the survey to date.
“For many years, it was thought to be due to hair styling practices,” but there are new data showing that genetics can be an important cause, she said, referring to research from South Africa indicating that CCCA can be inherited in an autosomal dominant fashion.
Dr. Lenzy, who practices dermatology in Chicopee, Mass., used a central hair loss photographic scale in the study, which also can be helpful in the office to monitor hair loss and “to quantify how much hair loss a person has … in terms of: Are they getting worse? Do they go from stage 3 to stage 5 or stage 1 to stage 3?”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Almost 41% of black women surveyed described hair loss that was consistent with central centrifugal cicatricial alopecia (CCCA), but only about 9% said they had been diagnosed with the condition, Dr. Yolanda Lenzy reported at the annual meeting of the American Academy of Dermatology.
In a video interview at the meeting, Dr. Lenzy of the University of Connecticut, Farmington, discussed the results of a hair survey she conducted with the Black Women’s Health Study at Boston University’s Slone Epidemiology Center. Nearly 6,000 women have completed the survey to date.
“For many years, it was thought to be due to hair styling practices,” but there are new data showing that genetics can be an important cause, she said, referring to research from South Africa indicating that CCCA can be inherited in an autosomal dominant fashion.
Dr. Lenzy, who practices dermatology in Chicopee, Mass., used a central hair loss photographic scale in the study, which also can be helpful in the office to monitor hair loss and “to quantify how much hair loss a person has … in terms of: Are they getting worse? Do they go from stage 3 to stage 5 or stage 1 to stage 3?”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AAD 16
VIDEO: Herpes gladiatorum may be misdiagnosed as impetigo
WAIKOLOA, HAWAII – Athletes who wrestle, play rugby, or engage in any sport “where there’s a grinding of skin on skin” are at risk for herpes simplex gladiatorum, according to Dr. Andrew Krakowski.
The risk is exacerbated by the occlusive uniforms worn by athletes, which maintain a high moisture content – creating an environment hospitable to the spread of viruses, explained Dr. Krakowski, chief medical officer at DermOne, West Conshohocken, Pa.
In an interview at the Hawaii Dermatology Seminar, the dermatologist explained how HSV gladiatorum differs in appearance from classic vesicular herpes simplex, and how dermatologists and primary care physicians may misdiagnose these rashes as impetigo – or miss the diagnosis altogether.
He also provides recommendations on how to treat this condition when it is diagnosed and outlines measures to reduce the spread of the virus.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. Krakowski is a consultant to Galderma and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Athletes who wrestle, play rugby, or engage in any sport “where there’s a grinding of skin on skin” are at risk for herpes simplex gladiatorum, according to Dr. Andrew Krakowski.
The risk is exacerbated by the occlusive uniforms worn by athletes, which maintain a high moisture content – creating an environment hospitable to the spread of viruses, explained Dr. Krakowski, chief medical officer at DermOne, West Conshohocken, Pa.
In an interview at the Hawaii Dermatology Seminar, the dermatologist explained how HSV gladiatorum differs in appearance from classic vesicular herpes simplex, and how dermatologists and primary care physicians may misdiagnose these rashes as impetigo – or miss the diagnosis altogether.
He also provides recommendations on how to treat this condition when it is diagnosed and outlines measures to reduce the spread of the virus.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. Krakowski is a consultant to Galderma and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Athletes who wrestle, play rugby, or engage in any sport “where there’s a grinding of skin on skin” are at risk for herpes simplex gladiatorum, according to Dr. Andrew Krakowski.
The risk is exacerbated by the occlusive uniforms worn by athletes, which maintain a high moisture content – creating an environment hospitable to the spread of viruses, explained Dr. Krakowski, chief medical officer at DermOne, West Conshohocken, Pa.
In an interview at the Hawaii Dermatology Seminar, the dermatologist explained how HSV gladiatorum differs in appearance from classic vesicular herpes simplex, and how dermatologists and primary care physicians may misdiagnose these rashes as impetigo – or miss the diagnosis altogether.
He also provides recommendations on how to treat this condition when it is diagnosed and outlines measures to reduce the spread of the virus.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. Krakowski is a consultant to Galderma and Valeant.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Genetic tests, clinical data sharpen pigmented lesion diagnosis
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: New microscopy tools improve melanoma detection
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Dermatologic features may help distinguish Zika infection
Dermatologists may be seeing patients who have recently traveled to an area affected by the current Zika outbreaks, who present with a rash and possibly a fever.
Before serology and possibly virology confirms the diagnosis, there are certain distinguishing characteristics that may help distinguish Zika initially from dengue and Chikungunya, according to Dr. Stephen K. Tyring.
Dr. Tyring, clinical professor of dermatology at the University of Texas, Houston, said in an interview that serology is required to confirm the diagnosis, and should be obtained via state and local health departments, which are increasingly being provided with test kits. Virology via polymerase chain reaction also may be needed to diagnose the infection.
About 20% of people infected with Zika virus develop symptoms. In Texas, by early February, 10 cases had been diagnosed statewide.
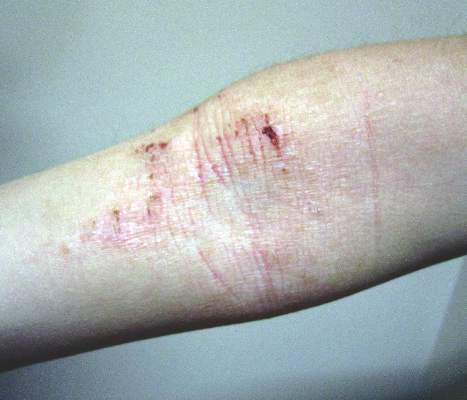
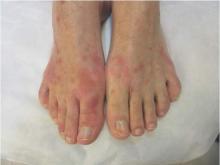
The Zika rash is characterized by blanchable macules and papules, which may start on the face or trunk 3-5 days after the febrile phase, and become more diffuse, said Dr. Tyring.
The erythematous macules with areas of sparing is similar to the rash seen with Chikungunya and dengue, two other viral infections that have cutaneous manifestations. With a Zika rash, macules are more likely than papules, but papules are certainly possible, he said.
In addition, someone with a Zika infection is more likely to have conjunctivitis than someone with dengue or Chikungunya, and may have red sclera, he noted. But all the other symptoms associated with dengue and Chikungunya, such as the arthralgias, headaches, and myalgias, could certainly be present with Zika as well, as the three diseases have similar clinical features.
Dr. Tyring referred to a study published in 2009 describing 31 cases in a 2007 Zika outbreak in Micronesia, which reported that 90% (28 patients) had a macular or papular rash. In addition, 20 (65%) had a mild fever, 20 (65%) had arthralgia of the small joints, and 17 (55%) had nonpurulent conjunctivitis (N Engl J Med. 2009 Jun 11;360[24]:2536-43).
In September, when Dr. Tyring was attending the Brazilian Society of Dermatology meeting in Sao Paolo, he visited some clinics and saw some of the first patients diagnosed with Zika virus – before the connection with the microcephaly or Guillain-Barre had been made. Serologic testing had confirmed that the cases were Zika infections, not Chikungunya or dengue.
At that time, cases were being viewed as a mild versions of Chikungunya or dengue, “in other words, nothing that they were fearing any more than all the other arboviruses that are so common,” Dr. Tyring said in an interview.
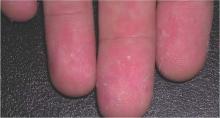
With some of the patients, “we saw a bit of desquamation of the extremities, such as the fingertips,” he said. (See photos.) “But generally, it’s not very distinguishable between dengue and Chikungunya.”
Health care providers are encourage to report suspected cases to their state health departments.
Dermatologists may be seeing patients who have recently traveled to an area affected by the current Zika outbreaks, who present with a rash and possibly a fever.
Before serology and possibly virology confirms the diagnosis, there are certain distinguishing characteristics that may help distinguish Zika initially from dengue and Chikungunya, according to Dr. Stephen K. Tyring.
Dr. Tyring, clinical professor of dermatology at the University of Texas, Houston, said in an interview that serology is required to confirm the diagnosis, and should be obtained via state and local health departments, which are increasingly being provided with test kits. Virology via polymerase chain reaction also may be needed to diagnose the infection.
About 20% of people infected with Zika virus develop symptoms. In Texas, by early February, 10 cases had been diagnosed statewide.
The Zika rash is characterized by blanchable macules and papules, which may start on the face or trunk 3-5 days after the febrile phase, and become more diffuse, said Dr. Tyring.
The erythematous macules with areas of sparing is similar to the rash seen with Chikungunya and dengue, two other viral infections that have cutaneous manifestations. With a Zika rash, macules are more likely than papules, but papules are certainly possible, he said.
In addition, someone with a Zika infection is more likely to have conjunctivitis than someone with dengue or Chikungunya, and may have red sclera, he noted. But all the other symptoms associated with dengue and Chikungunya, such as the arthralgias, headaches, and myalgias, could certainly be present with Zika as well, as the three diseases have similar clinical features.
Dr. Tyring referred to a study published in 2009 describing 31 cases in a 2007 Zika outbreak in Micronesia, which reported that 90% (28 patients) had a macular or papular rash. In addition, 20 (65%) had a mild fever, 20 (65%) had arthralgia of the small joints, and 17 (55%) had nonpurulent conjunctivitis (N Engl J Med. 2009 Jun 11;360[24]:2536-43).
In September, when Dr. Tyring was attending the Brazilian Society of Dermatology meeting in Sao Paolo, he visited some clinics and saw some of the first patients diagnosed with Zika virus – before the connection with the microcephaly or Guillain-Barre had been made. Serologic testing had confirmed that the cases were Zika infections, not Chikungunya or dengue.
At that time, cases were being viewed as a mild versions of Chikungunya or dengue, “in other words, nothing that they were fearing any more than all the other arboviruses that are so common,” Dr. Tyring said in an interview.
With some of the patients, “we saw a bit of desquamation of the extremities, such as the fingertips,” he said. (See photos.) “But generally, it’s not very distinguishable between dengue and Chikungunya.”
Health care providers are encourage to report suspected cases to their state health departments.
Dermatologists may be seeing patients who have recently traveled to an area affected by the current Zika outbreaks, who present with a rash and possibly a fever.
Before serology and possibly virology confirms the diagnosis, there are certain distinguishing characteristics that may help distinguish Zika initially from dengue and Chikungunya, according to Dr. Stephen K. Tyring.
Dr. Tyring, clinical professor of dermatology at the University of Texas, Houston, said in an interview that serology is required to confirm the diagnosis, and should be obtained via state and local health departments, which are increasingly being provided with test kits. Virology via polymerase chain reaction also may be needed to diagnose the infection.
About 20% of people infected with Zika virus develop symptoms. In Texas, by early February, 10 cases had been diagnosed statewide.
The Zika rash is characterized by blanchable macules and papules, which may start on the face or trunk 3-5 days after the febrile phase, and become more diffuse, said Dr. Tyring.
The erythematous macules with areas of sparing is similar to the rash seen with Chikungunya and dengue, two other viral infections that have cutaneous manifestations. With a Zika rash, macules are more likely than papules, but papules are certainly possible, he said.
In addition, someone with a Zika infection is more likely to have conjunctivitis than someone with dengue or Chikungunya, and may have red sclera, he noted. But all the other symptoms associated with dengue and Chikungunya, such as the arthralgias, headaches, and myalgias, could certainly be present with Zika as well, as the three diseases have similar clinical features.
Dr. Tyring referred to a study published in 2009 describing 31 cases in a 2007 Zika outbreak in Micronesia, which reported that 90% (28 patients) had a macular or papular rash. In addition, 20 (65%) had a mild fever, 20 (65%) had arthralgia of the small joints, and 17 (55%) had nonpurulent conjunctivitis (N Engl J Med. 2009 Jun 11;360[24]:2536-43).
In September, when Dr. Tyring was attending the Brazilian Society of Dermatology meeting in Sao Paolo, he visited some clinics and saw some of the first patients diagnosed with Zika virus – before the connection with the microcephaly or Guillain-Barre had been made. Serologic testing had confirmed that the cases were Zika infections, not Chikungunya or dengue.
At that time, cases were being viewed as a mild versions of Chikungunya or dengue, “in other words, nothing that they were fearing any more than all the other arboviruses that are so common,” Dr. Tyring said in an interview.
With some of the patients, “we saw a bit of desquamation of the extremities, such as the fingertips,” he said. (See photos.) “But generally, it’s not very distinguishable between dengue and Chikungunya.”
Health care providers are encourage to report suspected cases to their state health departments.