User login
ACIP votes to drop preference for LAIV in healthy children aged 2-8 years
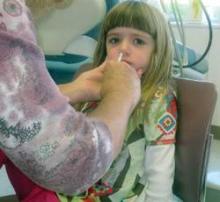
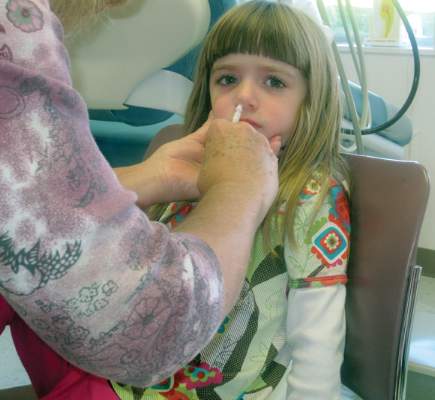
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
FROM AN ACIP MEETING
FDA panel backs approval of dermal filler for hand augmentation
SILVER SPRING, MD. – Expanding the approval of the Radiesse dermal filler to include cosmetic improvement of the hands was supported by the majority of a Food and Drug Administration Advisory Panel, whose confidence in the risk-benefit profile was bolstered in part by wide off-label use in the United States and approval of this indication in other countries.
At a meeting of the FDA’s General and Plastic Surgery Devices Panel on Feb. 27, the panel voted 9-4, with one abstention, that the benefits of the dermal filler, marketed as Radiesse by Merz North America , outweighed its risks for the proposed indication, “hand augmentation to correct volume deficit in the hands.” Despite some issues with the US pivotal study, and the unavailability of data some panelists said would have been helpful, such as X-rays of treated hands to determine if the filler obscured imaging of bone, most of the panel also voted that there was “reasonable assurance” that it was effective (12-2) and that it was safe (11-3) for this use.
Issues raised by panelists included the need to better define patients who should not receive this treatment, further evaluation of possible adverse effects of treatment on hand function and strength, and evaluation of the filler in patients with severe volume deficit in the hands, who were not included in the study.
Voting in favor of approval, Dr. Karen Burke, a dermatologist at Mt. Sinai Medical Center, New York City, cited the long history of Radiesse use for the hands in many countries and in many patients in the United States. “I believe it is safe and that efficacy was demonstrated,” and that FDA approval is necessary so this use can be properly regulated and “we can teach people openly how to use it,” she said.
If approved, Radiesse – a semisolid dermal filler made of synthetic calcium hyroxyapatite suspended in a gel – would be the first dermal filler approved for hand augmentation. Merz describes it as a “durable” filler that is not permanent, and is broken down over several months.
Radiesse was approved in 2006 for subdermal implantation for correction of moderate to severe facial wrinkles and folds, and for restoration and/or correction of facial lipoatrophy that occurs in people with HIV. It has been approved for hand augmentation in 52 countries, including in Europe and Canada, and like other fillers, has been used off-label in the United States for this use. Fillers are regulated by the FDA as devices.
The US study, a prospective, 12-month study at 6 sites, randomized 85 patients to Radiesse (who received a maximum of 3 cc injected per hand), and 29 initially to no treatment, who served as controls. Responses were evaluated by clinicians who could not see the patient, using the Merz Hand Grading Scale (MGHS), a 5-point scale ranging from 0 (no loss of fatty tissue) to 5 (very severe loss of fatty tissue, marked visibility of veins and tendons), with an improvement of at least 1 considered clinically meaningful, according to Merz, which validated the scale before the pivotal study. Most hands at baseline were rated as 2 or 3 on the MGHS scale (moderate or severe loss of fatty tissue with mild or moderate visibility of veins or tendons); most were women, their mean age was 53-54 years, and most were white.
At three months, 75% in the Radiesse group had at least a 1 point improvement on the MGHS scale, the primary effectiveness endpoint, vs. 3.4% of controls, a statistically significant difference. Based on the Global Aesthetic Improvement Scale (GAIS) at three months, a secondary endpoint, 76% of treated hands were rated as “much “ or “very much improved” in appearance; none were rated as worse and 2% were rated as having not changed. Among those who did not have a second treatment, the effect was durable through 12 months
Patients recorded adverse events in a diary. The most common adverse event among treated patients, which included controls who opted for treatment after the three month period, was swelling, in 99%. Other common adverse events included pain (92%), redness and bruising (74%), difficulty performing activities (46%), and loss of sensation (18%); 6% had nodules.
Most of the adverse events were mild (61%) or moderate (35%); and most resolved without sequelae. Severe events included swelling, pain, and redness. No intervention was reported for 98% of adverse events, which lasted a mean of about 8 days, with 89% resolving within 14 days, according to Merz. There were no unanticipated events. (Over 12 years of experience with the use in the hand, swelling has been the most common adverse effect of treatment, according to Merz.)
In the study, 13 patients developed lumps, bumps and nodules, most were mild and none were severe and they resolved without intervention or sequelae. At the end of the study, no patients had a persistent granuloma. In three studies of 234 patients evaluating Radiesse in the hands, no granulomas have been reported, according to the company.
There were 8 severe recurrent adverse events in 7 patients: 5 reports of swelling, 1 report pf pain, 1 report of difficulty in performing activities, and 1 report of bruising.
FDA reviewers agreed that the clinical study appeared to meet the primary effectiveness endpoint, but pointed out that the amount of improvement varied between study sites. Among other issues they raised was the increased rate, severity, and duration of adverse events associated with treatment, particularly pain and swelling, which seemed to be higher and lasted longer than when Radiesse was used for the two facial indications. In addition, they indicated that the results of hand function tests were difficult to interpret.
Panelist Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery, Northwestern University, Chicago, said there were some significant issues with the pivotal study, “but they do not undermine the overall conclusions” that treatment appeared to be safe and effective. The significant off-label use and experience elsewhere “was sufficient to sway me,” he added.
One of the panelists voting no on the risk-benefit question, Dr. Clayton Peimer, an orthopedic surgeon at Michigan State University, said he simply did not have enough information to support approval. He added that there were still unanswered questions, such as what would happen if the filler ends up next to synovial tissue, or if it was used in patients with systemic sclerosis, or calcinosis cutis. “I respect that board-certified plastic surgeons, dermatologists, and hand surgeons are using this safely” in the hands, he said, but added that he was concerned that FDA approval could lead to widespread use by cosmetologists and mid-level practitioners.
FDA usually follows the recommendations of its advisory panels. The panelists had no disclosures.
If approved, Merz has plans for a postapproval, open-label study that would follow at least 100 patients after they receive hand augmentation with Radiesse for three years after their initial treatment, monitoring for adverse events and satisfaction with treatment.
SILVER SPRING, MD. – Expanding the approval of the Radiesse dermal filler to include cosmetic improvement of the hands was supported by the majority of a Food and Drug Administration Advisory Panel, whose confidence in the risk-benefit profile was bolstered in part by wide off-label use in the United States and approval of this indication in other countries.
At a meeting of the FDA’s General and Plastic Surgery Devices Panel on Feb. 27, the panel voted 9-4, with one abstention, that the benefits of the dermal filler, marketed as Radiesse by Merz North America , outweighed its risks for the proposed indication, “hand augmentation to correct volume deficit in the hands.” Despite some issues with the US pivotal study, and the unavailability of data some panelists said would have been helpful, such as X-rays of treated hands to determine if the filler obscured imaging of bone, most of the panel also voted that there was “reasonable assurance” that it was effective (12-2) and that it was safe (11-3) for this use.
Issues raised by panelists included the need to better define patients who should not receive this treatment, further evaluation of possible adverse effects of treatment on hand function and strength, and evaluation of the filler in patients with severe volume deficit in the hands, who were not included in the study.
Voting in favor of approval, Dr. Karen Burke, a dermatologist at Mt. Sinai Medical Center, New York City, cited the long history of Radiesse use for the hands in many countries and in many patients in the United States. “I believe it is safe and that efficacy was demonstrated,” and that FDA approval is necessary so this use can be properly regulated and “we can teach people openly how to use it,” she said.
If approved, Radiesse – a semisolid dermal filler made of synthetic calcium hyroxyapatite suspended in a gel – would be the first dermal filler approved for hand augmentation. Merz describes it as a “durable” filler that is not permanent, and is broken down over several months.
Radiesse was approved in 2006 for subdermal implantation for correction of moderate to severe facial wrinkles and folds, and for restoration and/or correction of facial lipoatrophy that occurs in people with HIV. It has been approved for hand augmentation in 52 countries, including in Europe and Canada, and like other fillers, has been used off-label in the United States for this use. Fillers are regulated by the FDA as devices.
The US study, a prospective, 12-month study at 6 sites, randomized 85 patients to Radiesse (who received a maximum of 3 cc injected per hand), and 29 initially to no treatment, who served as controls. Responses were evaluated by clinicians who could not see the patient, using the Merz Hand Grading Scale (MGHS), a 5-point scale ranging from 0 (no loss of fatty tissue) to 5 (very severe loss of fatty tissue, marked visibility of veins and tendons), with an improvement of at least 1 considered clinically meaningful, according to Merz, which validated the scale before the pivotal study. Most hands at baseline were rated as 2 or 3 on the MGHS scale (moderate or severe loss of fatty tissue with mild or moderate visibility of veins or tendons); most were women, their mean age was 53-54 years, and most were white.
At three months, 75% in the Radiesse group had at least a 1 point improvement on the MGHS scale, the primary effectiveness endpoint, vs. 3.4% of controls, a statistically significant difference. Based on the Global Aesthetic Improvement Scale (GAIS) at three months, a secondary endpoint, 76% of treated hands were rated as “much “ or “very much improved” in appearance; none were rated as worse and 2% were rated as having not changed. Among those who did not have a second treatment, the effect was durable through 12 months
Patients recorded adverse events in a diary. The most common adverse event among treated patients, which included controls who opted for treatment after the three month period, was swelling, in 99%. Other common adverse events included pain (92%), redness and bruising (74%), difficulty performing activities (46%), and loss of sensation (18%); 6% had nodules.
Most of the adverse events were mild (61%) or moderate (35%); and most resolved without sequelae. Severe events included swelling, pain, and redness. No intervention was reported for 98% of adverse events, which lasted a mean of about 8 days, with 89% resolving within 14 days, according to Merz. There were no unanticipated events. (Over 12 years of experience with the use in the hand, swelling has been the most common adverse effect of treatment, according to Merz.)
In the study, 13 patients developed lumps, bumps and nodules, most were mild and none were severe and they resolved without intervention or sequelae. At the end of the study, no patients had a persistent granuloma. In three studies of 234 patients evaluating Radiesse in the hands, no granulomas have been reported, according to the company.
There were 8 severe recurrent adverse events in 7 patients: 5 reports of swelling, 1 report pf pain, 1 report of difficulty in performing activities, and 1 report of bruising.
FDA reviewers agreed that the clinical study appeared to meet the primary effectiveness endpoint, but pointed out that the amount of improvement varied between study sites. Among other issues they raised was the increased rate, severity, and duration of adverse events associated with treatment, particularly pain and swelling, which seemed to be higher and lasted longer than when Radiesse was used for the two facial indications. In addition, they indicated that the results of hand function tests were difficult to interpret.
Panelist Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery, Northwestern University, Chicago, said there were some significant issues with the pivotal study, “but they do not undermine the overall conclusions” that treatment appeared to be safe and effective. The significant off-label use and experience elsewhere “was sufficient to sway me,” he added.
One of the panelists voting no on the risk-benefit question, Dr. Clayton Peimer, an orthopedic surgeon at Michigan State University, said he simply did not have enough information to support approval. He added that there were still unanswered questions, such as what would happen if the filler ends up next to synovial tissue, or if it was used in patients with systemic sclerosis, or calcinosis cutis. “I respect that board-certified plastic surgeons, dermatologists, and hand surgeons are using this safely” in the hands, he said, but added that he was concerned that FDA approval could lead to widespread use by cosmetologists and mid-level practitioners.
FDA usually follows the recommendations of its advisory panels. The panelists had no disclosures.
If approved, Merz has plans for a postapproval, open-label study that would follow at least 100 patients after they receive hand augmentation with Radiesse for three years after their initial treatment, monitoring for adverse events and satisfaction with treatment.
SILVER SPRING, MD. – Expanding the approval of the Radiesse dermal filler to include cosmetic improvement of the hands was supported by the majority of a Food and Drug Administration Advisory Panel, whose confidence in the risk-benefit profile was bolstered in part by wide off-label use in the United States and approval of this indication in other countries.
At a meeting of the FDA’s General and Plastic Surgery Devices Panel on Feb. 27, the panel voted 9-4, with one abstention, that the benefits of the dermal filler, marketed as Radiesse by Merz North America , outweighed its risks for the proposed indication, “hand augmentation to correct volume deficit in the hands.” Despite some issues with the US pivotal study, and the unavailability of data some panelists said would have been helpful, such as X-rays of treated hands to determine if the filler obscured imaging of bone, most of the panel also voted that there was “reasonable assurance” that it was effective (12-2) and that it was safe (11-3) for this use.
Issues raised by panelists included the need to better define patients who should not receive this treatment, further evaluation of possible adverse effects of treatment on hand function and strength, and evaluation of the filler in patients with severe volume deficit in the hands, who were not included in the study.
Voting in favor of approval, Dr. Karen Burke, a dermatologist at Mt. Sinai Medical Center, New York City, cited the long history of Radiesse use for the hands in many countries and in many patients in the United States. “I believe it is safe and that efficacy was demonstrated,” and that FDA approval is necessary so this use can be properly regulated and “we can teach people openly how to use it,” she said.
If approved, Radiesse – a semisolid dermal filler made of synthetic calcium hyroxyapatite suspended in a gel – would be the first dermal filler approved for hand augmentation. Merz describes it as a “durable” filler that is not permanent, and is broken down over several months.
Radiesse was approved in 2006 for subdermal implantation for correction of moderate to severe facial wrinkles and folds, and for restoration and/or correction of facial lipoatrophy that occurs in people with HIV. It has been approved for hand augmentation in 52 countries, including in Europe and Canada, and like other fillers, has been used off-label in the United States for this use. Fillers are regulated by the FDA as devices.
The US study, a prospective, 12-month study at 6 sites, randomized 85 patients to Radiesse (who received a maximum of 3 cc injected per hand), and 29 initially to no treatment, who served as controls. Responses were evaluated by clinicians who could not see the patient, using the Merz Hand Grading Scale (MGHS), a 5-point scale ranging from 0 (no loss of fatty tissue) to 5 (very severe loss of fatty tissue, marked visibility of veins and tendons), with an improvement of at least 1 considered clinically meaningful, according to Merz, which validated the scale before the pivotal study. Most hands at baseline were rated as 2 or 3 on the MGHS scale (moderate or severe loss of fatty tissue with mild or moderate visibility of veins or tendons); most were women, their mean age was 53-54 years, and most were white.
At three months, 75% in the Radiesse group had at least a 1 point improvement on the MGHS scale, the primary effectiveness endpoint, vs. 3.4% of controls, a statistically significant difference. Based on the Global Aesthetic Improvement Scale (GAIS) at three months, a secondary endpoint, 76% of treated hands were rated as “much “ or “very much improved” in appearance; none were rated as worse and 2% were rated as having not changed. Among those who did not have a second treatment, the effect was durable through 12 months
Patients recorded adverse events in a diary. The most common adverse event among treated patients, which included controls who opted for treatment after the three month period, was swelling, in 99%. Other common adverse events included pain (92%), redness and bruising (74%), difficulty performing activities (46%), and loss of sensation (18%); 6% had nodules.
Most of the adverse events were mild (61%) or moderate (35%); and most resolved without sequelae. Severe events included swelling, pain, and redness. No intervention was reported for 98% of adverse events, which lasted a mean of about 8 days, with 89% resolving within 14 days, according to Merz. There were no unanticipated events. (Over 12 years of experience with the use in the hand, swelling has been the most common adverse effect of treatment, according to Merz.)
In the study, 13 patients developed lumps, bumps and nodules, most were mild and none were severe and they resolved without intervention or sequelae. At the end of the study, no patients had a persistent granuloma. In three studies of 234 patients evaluating Radiesse in the hands, no granulomas have been reported, according to the company.
There were 8 severe recurrent adverse events in 7 patients: 5 reports of swelling, 1 report pf pain, 1 report of difficulty in performing activities, and 1 report of bruising.
FDA reviewers agreed that the clinical study appeared to meet the primary effectiveness endpoint, but pointed out that the amount of improvement varied between study sites. Among other issues they raised was the increased rate, severity, and duration of adverse events associated with treatment, particularly pain and swelling, which seemed to be higher and lasted longer than when Radiesse was used for the two facial indications. In addition, they indicated that the results of hand function tests were difficult to interpret.
Panelist Dr. Murad Alam, professor of dermatology, otolaryngology, and surgery, Northwestern University, Chicago, said there were some significant issues with the pivotal study, “but they do not undermine the overall conclusions” that treatment appeared to be safe and effective. The significant off-label use and experience elsewhere “was sufficient to sway me,” he added.
One of the panelists voting no on the risk-benefit question, Dr. Clayton Peimer, an orthopedic surgeon at Michigan State University, said he simply did not have enough information to support approval. He added that there were still unanswered questions, such as what would happen if the filler ends up next to synovial tissue, or if it was used in patients with systemic sclerosis, or calcinosis cutis. “I respect that board-certified plastic surgeons, dermatologists, and hand surgeons are using this safely” in the hands, he said, but added that he was concerned that FDA approval could lead to widespread use by cosmetologists and mid-level practitioners.
FDA usually follows the recommendations of its advisory panels. The panelists had no disclosures.
If approved, Merz has plans for a postapproval, open-label study that would follow at least 100 patients after they receive hand augmentation with Radiesse for three years after their initial treatment, monitoring for adverse events and satisfaction with treatment.
AT AN FDA ADVISORY COMMITTEE MEETING
ACIP votes on incorporating 9-valent HPV vaccine into recommendations
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to support proposed recommendations for use of the recently licensed 9-valent human papillomavirus vaccine.
At a meeting on Feb. 26, members of ACIP voted 14-0 with one abstention, to support the draft wording for HPV vaccination, which has been modified to account for the availability of the 9-valent HPV vaccine (Gardasil-9), licensed by the Food and Drug Administration in December 2014. The five additional high-risk HPV types – 31, 33, 45, 52, and 58 – included in the 9-valent vaccine are associated with about 20% of cervical cancers, and are not included in the quadrivalent vaccine (Gardasil) that was approved in 2006.
The age groups, as proposed by ACIP’s HPV work group, are the same as currently recommended for HPV vaccination: females and males aged 11 or 12 years (but the series can be started at age 9 years); and females aged 13-26 years and males aged 13-21 years, who have not been vaccinated or have not completed the three-dose series. The statement that males aged 22-26 years may be vaccinated, as currently recommended, also is included.
For males over 15 years, this would be off-label use since the 9-valent vaccine is licensed in males aged 9-15 years, but immunogenicity data in males aged 16-26 years has been presented to ACIP and has been submitted to the FDA, Dr. Lauri Markowitz of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), said at the meeting.
The draft also adds to the previous recommendation that females can be vaccinated with the bivalent vaccine, the quadrivalent vaccine (as long as it available), or the 9-valent vaccine; and males can be vaccinated with the quadrivalent vaccine (as long as it is available) or the 9-valent vaccine.
Other proposed modifications include wording regarding interchangeability of the vaccines, which no longer includes the statement that ACIP recommends that females complete the HPV vaccination series with the same vaccine product, whenever possible.
Instead, it states that when providers do not know which vaccine the female patient received previously, or does not have it available, “or are in settings transitioning” to the 9-valent vaccine, “for protection against HPV 16 and 18, any HPV vaccine product may be used to continue or complete the series for females.” For males, the statement now says that the quadrivalent or the 9-valent vaccine “may be used to continue or complete the series.” (Previously, it said that only the quadrivalent vaccine was licensed in males.)
Dr. Markowitz said that no change in HPV vaccination during pregnancy has been proposed and that a new pregnancy registry for the 9-valent vaccine has been created. (HPV vaccination is not recommended during pregnancy.)
The HPV work group also is considering use of the 9-valent vaccine in people who have completed the HPV vaccination series, which will be presented at the next ACIP meeting in June 2015.
Gardasil 9 was licensed in December 2014, for females aged 9-26 years, and males aged 9-15 years for the prevention of cervical, vulvar, vaginal and anal cancers caused by HPV types 16, 18, 31, 33, 45, 52 and 58, and for the prevention of genital warts caused by HPV types 6 or 11. When it was licensed, the FDA said that the 9-valent vaccine has the potential to prevent approximately 90% of cervical, vulvar, vaginal, and anal cancers.
A representative of the manufacturer, Merck Sharpe & Dohme, said at the meeting that the 9-valent vaccine is commercially available and that the company is confident that there will be an adequate supply of both the 9-valent and the quadrivalent vaccine.
The bivalent HPV vaccine, Cervarix, was licensed in 2009 for females only and protects against HPV types 16 and 18. More than 98% of the HPV vaccine used in the United States has been with the quadrivalent vaccine, according to Dr. Markowitz.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to support proposed recommendations for use of the recently licensed 9-valent human papillomavirus vaccine.
At a meeting on Feb. 26, members of ACIP voted 14-0 with one abstention, to support the draft wording for HPV vaccination, which has been modified to account for the availability of the 9-valent HPV vaccine (Gardasil-9), licensed by the Food and Drug Administration in December 2014. The five additional high-risk HPV types – 31, 33, 45, 52, and 58 – included in the 9-valent vaccine are associated with about 20% of cervical cancers, and are not included in the quadrivalent vaccine (Gardasil) that was approved in 2006.
The age groups, as proposed by ACIP’s HPV work group, are the same as currently recommended for HPV vaccination: females and males aged 11 or 12 years (but the series can be started at age 9 years); and females aged 13-26 years and males aged 13-21 years, who have not been vaccinated or have not completed the three-dose series. The statement that males aged 22-26 years may be vaccinated, as currently recommended, also is included.
For males over 15 years, this would be off-label use since the 9-valent vaccine is licensed in males aged 9-15 years, but immunogenicity data in males aged 16-26 years has been presented to ACIP and has been submitted to the FDA, Dr. Lauri Markowitz of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), said at the meeting.
The draft also adds to the previous recommendation that females can be vaccinated with the bivalent vaccine, the quadrivalent vaccine (as long as it available), or the 9-valent vaccine; and males can be vaccinated with the quadrivalent vaccine (as long as it is available) or the 9-valent vaccine.
Other proposed modifications include wording regarding interchangeability of the vaccines, which no longer includes the statement that ACIP recommends that females complete the HPV vaccination series with the same vaccine product, whenever possible.
Instead, it states that when providers do not know which vaccine the female patient received previously, or does not have it available, “or are in settings transitioning” to the 9-valent vaccine, “for protection against HPV 16 and 18, any HPV vaccine product may be used to continue or complete the series for females.” For males, the statement now says that the quadrivalent or the 9-valent vaccine “may be used to continue or complete the series.” (Previously, it said that only the quadrivalent vaccine was licensed in males.)
Dr. Markowitz said that no change in HPV vaccination during pregnancy has been proposed and that a new pregnancy registry for the 9-valent vaccine has been created. (HPV vaccination is not recommended during pregnancy.)
The HPV work group also is considering use of the 9-valent vaccine in people who have completed the HPV vaccination series, which will be presented at the next ACIP meeting in June 2015.
Gardasil 9 was licensed in December 2014, for females aged 9-26 years, and males aged 9-15 years for the prevention of cervical, vulvar, vaginal and anal cancers caused by HPV types 16, 18, 31, 33, 45, 52 and 58, and for the prevention of genital warts caused by HPV types 6 or 11. When it was licensed, the FDA said that the 9-valent vaccine has the potential to prevent approximately 90% of cervical, vulvar, vaginal, and anal cancers.
A representative of the manufacturer, Merck Sharpe & Dohme, said at the meeting that the 9-valent vaccine is commercially available and that the company is confident that there will be an adequate supply of both the 9-valent and the quadrivalent vaccine.
The bivalent HPV vaccine, Cervarix, was licensed in 2009 for females only and protects against HPV types 16 and 18. More than 98% of the HPV vaccine used in the United States has been with the quadrivalent vaccine, according to Dr. Markowitz.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to support proposed recommendations for use of the recently licensed 9-valent human papillomavirus vaccine.
At a meeting on Feb. 26, members of ACIP voted 14-0 with one abstention, to support the draft wording for HPV vaccination, which has been modified to account for the availability of the 9-valent HPV vaccine (Gardasil-9), licensed by the Food and Drug Administration in December 2014. The five additional high-risk HPV types – 31, 33, 45, 52, and 58 – included in the 9-valent vaccine are associated with about 20% of cervical cancers, and are not included in the quadrivalent vaccine (Gardasil) that was approved in 2006.
The age groups, as proposed by ACIP’s HPV work group, are the same as currently recommended for HPV vaccination: females and males aged 11 or 12 years (but the series can be started at age 9 years); and females aged 13-26 years and males aged 13-21 years, who have not been vaccinated or have not completed the three-dose series. The statement that males aged 22-26 years may be vaccinated, as currently recommended, also is included.
For males over 15 years, this would be off-label use since the 9-valent vaccine is licensed in males aged 9-15 years, but immunogenicity data in males aged 16-26 years has been presented to ACIP and has been submitted to the FDA, Dr. Lauri Markowitz of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), said at the meeting.
The draft also adds to the previous recommendation that females can be vaccinated with the bivalent vaccine, the quadrivalent vaccine (as long as it available), or the 9-valent vaccine; and males can be vaccinated with the quadrivalent vaccine (as long as it is available) or the 9-valent vaccine.
Other proposed modifications include wording regarding interchangeability of the vaccines, which no longer includes the statement that ACIP recommends that females complete the HPV vaccination series with the same vaccine product, whenever possible.
Instead, it states that when providers do not know which vaccine the female patient received previously, or does not have it available, “or are in settings transitioning” to the 9-valent vaccine, “for protection against HPV 16 and 18, any HPV vaccine product may be used to continue or complete the series for females.” For males, the statement now says that the quadrivalent or the 9-valent vaccine “may be used to continue or complete the series.” (Previously, it said that only the quadrivalent vaccine was licensed in males.)
Dr. Markowitz said that no change in HPV vaccination during pregnancy has been proposed and that a new pregnancy registry for the 9-valent vaccine has been created. (HPV vaccination is not recommended during pregnancy.)
The HPV work group also is considering use of the 9-valent vaccine in people who have completed the HPV vaccination series, which will be presented at the next ACIP meeting in June 2015.
Gardasil 9 was licensed in December 2014, for females aged 9-26 years, and males aged 9-15 years for the prevention of cervical, vulvar, vaginal and anal cancers caused by HPV types 16, 18, 31, 33, 45, 52 and 58, and for the prevention of genital warts caused by HPV types 6 or 11. When it was licensed, the FDA said that the 9-valent vaccine has the potential to prevent approximately 90% of cervical, vulvar, vaginal, and anal cancers.
A representative of the manufacturer, Merck Sharpe & Dohme, said at the meeting that the 9-valent vaccine is commercially available and that the company is confident that there will be an adequate supply of both the 9-valent and the quadrivalent vaccine.
The bivalent HPV vaccine, Cervarix, was licensed in 2009 for females only and protects against HPV types 16 and 18. More than 98% of the HPV vaccine used in the United States has been with the quadrivalent vaccine, according to Dr. Markowitz.
FROM AN ACIP MEETING
ACIP Recommends Meningococcal B Vaccine During College Outbreaks
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
FROM AN ACIP MEETING
ACIP recommends meningococcal B vaccine during college outbreaks
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
FROM AN ACIP MEETING
C. difficile burden in U.S. documented in 2011 estimates of infections, deaths
The estimated 453,000 infections and 29,300 deaths caused by Clostridium difficile in the United States in 2011 underline the importance of appropriate use of antibiotics and rigorous infection control measures in health care settings, Dr. Michael Bell, an official at the Centers for Disease Control and Prevention, said during a CDC telebriefing.
“To reduce the majority of C. difficile infections, we will need to improve how antibiotics are being prescribed in hospitals and throughout health care,” said Dr. Bell, deputy director of the division of health care quality promotion, at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Early diagnosis is also critical to prevent spread of C. difficile. Many infections are community acquired, and “it is essential that patients and their clinicians be aware that they need to take any diarrhea following antibiotic use very seriously,” he added.
The briefing was held to discuss the results and implications of the study published in the New England Journal of Medicine, which was supported by the CDC and the Emerging Infections Program (EIP) Cooperative Agreement between the 10 EIP sites and the CDC. In the study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100).
Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Fernanda Lessa of the CDC, and her associates. Future efforts should focus on antibiotic use, the proper management of which may be effective in decreasing infection rates, the authors said in the report.
“Antibiotics clearly are driving this whole epidemic,” one of the study authors, Dr. Clifford McDonald of the CDC, said during the briefing. The epidemic strain in the United States, which emerged in 2000 in Pittsburgh and Montreal, is now spread globally, and accounted for about 30% of cases in this study, he added. It is transmitted more easily than other strains and causes more severe disease.
Dr. Bell said that to reduce the rate of these infections, antibiotics should be used only when needed and for as long as necessary, “and to ensure rigorous infection control in all health care settings.” The CDC’s National Strategy to Combat Antibiotic Resistant Bacteria has the potential to reduce C. difficile infections by 50%, he added.
“There’s no room for error” when infection control is considered, he added, pointing out that hand sanitizers do not kill C. difficile spores, which spread easily and are durable, “so that any breach in correct glove use, hand hygiene, or cleaning protocol can allow the spores to spread.”
The study also estimated that more than 150,000 infections were community acquired, with no documentation of inpatient exposure in the hospital. “Nonetheless, as we showed in another recent CDC study, 80% of patients with community-associated C. difficile infections did, in fact, have contact with a health care setting like a doctor’s office or a dental clinic,” generally during the 3-month period before being diagnosed, and most of the patients had also been treated with antibiotics, Dr. Bell said.
Future efforts should focus on antibiotic use, which may be effective in decreasing infection rates, Dr. Fernanda C. Lessa and her coauthors at the Centers for Disease Control and Prevention reported in The New England Journal of Medicine.
In a study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100). Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Lessa and associates.
National efforts to address the increase in C. difficile infections include the requirement since 2013 that hospitals participating in the Centers for Medicare & Medicaid Services’ Hospital Inpatient Quality Reporting Program data on C. difficile infections to the CDC’s National Healthcare Safety Network, which has shown at least a 10% drop since 2013, Dr Bell said. Targets for reducing C. difficile infections in the United States by 2020 are being established in the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination.
Madhu Rajaraman contributed to this report.
The estimated 453,000 infections and 29,300 deaths caused by Clostridium difficile in the United States in 2011 underline the importance of appropriate use of antibiotics and rigorous infection control measures in health care settings, Dr. Michael Bell, an official at the Centers for Disease Control and Prevention, said during a CDC telebriefing.
“To reduce the majority of C. difficile infections, we will need to improve how antibiotics are being prescribed in hospitals and throughout health care,” said Dr. Bell, deputy director of the division of health care quality promotion, at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Early diagnosis is also critical to prevent spread of C. difficile. Many infections are community acquired, and “it is essential that patients and their clinicians be aware that they need to take any diarrhea following antibiotic use very seriously,” he added.
The briefing was held to discuss the results and implications of the study published in the New England Journal of Medicine, which was supported by the CDC and the Emerging Infections Program (EIP) Cooperative Agreement between the 10 EIP sites and the CDC. In the study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100).
Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Fernanda Lessa of the CDC, and her associates. Future efforts should focus on antibiotic use, the proper management of which may be effective in decreasing infection rates, the authors said in the report.
“Antibiotics clearly are driving this whole epidemic,” one of the study authors, Dr. Clifford McDonald of the CDC, said during the briefing. The epidemic strain in the United States, which emerged in 2000 in Pittsburgh and Montreal, is now spread globally, and accounted for about 30% of cases in this study, he added. It is transmitted more easily than other strains and causes more severe disease.
Dr. Bell said that to reduce the rate of these infections, antibiotics should be used only when needed and for as long as necessary, “and to ensure rigorous infection control in all health care settings.” The CDC’s National Strategy to Combat Antibiotic Resistant Bacteria has the potential to reduce C. difficile infections by 50%, he added.
“There’s no room for error” when infection control is considered, he added, pointing out that hand sanitizers do not kill C. difficile spores, which spread easily and are durable, “so that any breach in correct glove use, hand hygiene, or cleaning protocol can allow the spores to spread.”
The study also estimated that more than 150,000 infections were community acquired, with no documentation of inpatient exposure in the hospital. “Nonetheless, as we showed in another recent CDC study, 80% of patients with community-associated C. difficile infections did, in fact, have contact with a health care setting like a doctor’s office or a dental clinic,” generally during the 3-month period before being diagnosed, and most of the patients had also been treated with antibiotics, Dr. Bell said.
Future efforts should focus on antibiotic use, which may be effective in decreasing infection rates, Dr. Fernanda C. Lessa and her coauthors at the Centers for Disease Control and Prevention reported in The New England Journal of Medicine.
In a study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100). Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Lessa and associates.
National efforts to address the increase in C. difficile infections include the requirement since 2013 that hospitals participating in the Centers for Medicare & Medicaid Services’ Hospital Inpatient Quality Reporting Program data on C. difficile infections to the CDC’s National Healthcare Safety Network, which has shown at least a 10% drop since 2013, Dr Bell said. Targets for reducing C. difficile infections in the United States by 2020 are being established in the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination.
Madhu Rajaraman contributed to this report.
The estimated 453,000 infections and 29,300 deaths caused by Clostridium difficile in the United States in 2011 underline the importance of appropriate use of antibiotics and rigorous infection control measures in health care settings, Dr. Michael Bell, an official at the Centers for Disease Control and Prevention, said during a CDC telebriefing.
“To reduce the majority of C. difficile infections, we will need to improve how antibiotics are being prescribed in hospitals and throughout health care,” said Dr. Bell, deputy director of the division of health care quality promotion, at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Early diagnosis is also critical to prevent spread of C. difficile. Many infections are community acquired, and “it is essential that patients and their clinicians be aware that they need to take any diarrhea following antibiotic use very seriously,” he added.
The briefing was held to discuss the results and implications of the study published in the New England Journal of Medicine, which was supported by the CDC and the Emerging Infections Program (EIP) Cooperative Agreement between the 10 EIP sites and the CDC. In the study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100).
Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Fernanda Lessa of the CDC, and her associates. Future efforts should focus on antibiotic use, the proper management of which may be effective in decreasing infection rates, the authors said in the report.
“Antibiotics clearly are driving this whole epidemic,” one of the study authors, Dr. Clifford McDonald of the CDC, said during the briefing. The epidemic strain in the United States, which emerged in 2000 in Pittsburgh and Montreal, is now spread globally, and accounted for about 30% of cases in this study, he added. It is transmitted more easily than other strains and causes more severe disease.
Dr. Bell said that to reduce the rate of these infections, antibiotics should be used only when needed and for as long as necessary, “and to ensure rigorous infection control in all health care settings.” The CDC’s National Strategy to Combat Antibiotic Resistant Bacteria has the potential to reduce C. difficile infections by 50%, he added.
“There’s no room for error” when infection control is considered, he added, pointing out that hand sanitizers do not kill C. difficile spores, which spread easily and are durable, “so that any breach in correct glove use, hand hygiene, or cleaning protocol can allow the spores to spread.”
The study also estimated that more than 150,000 infections were community acquired, with no documentation of inpatient exposure in the hospital. “Nonetheless, as we showed in another recent CDC study, 80% of patients with community-associated C. difficile infections did, in fact, have contact with a health care setting like a doctor’s office or a dental clinic,” generally during the 3-month period before being diagnosed, and most of the patients had also been treated with antibiotics, Dr. Bell said.
Future efforts should focus on antibiotic use, which may be effective in decreasing infection rates, Dr. Fernanda C. Lessa and her coauthors at the Centers for Disease Control and Prevention reported in The New England Journal of Medicine.
In a study of 10 geographic regions in the United States in 2011, 15,461 cases were confirmed, with the estimated incidence of the infection being 453,000 (95% confidence interval, 397,100-508,500) after predictors of incidence were adjusted for, the investigators found. The estimated number of deaths from C. difficile was 29,300 (95% CI, 16,500-42,100). Estimates for disease incidence were higher among women, whites, and patients 65 years of age or older, wrote Dr. Lessa and associates.
National efforts to address the increase in C. difficile infections include the requirement since 2013 that hospitals participating in the Centers for Medicare & Medicaid Services’ Hospital Inpatient Quality Reporting Program data on C. difficile infections to the CDC’s National Healthcare Safety Network, which has shown at least a 10% drop since 2013, Dr Bell said. Targets for reducing C. difficile infections in the United States by 2020 are being established in the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination.
Madhu Rajaraman contributed to this report.
FROM A CDC TELEBRIEFING
Mitochondrial transfer approved in U.K.; debate begins in U.S.
A type of gene therapy to prevent maternal transmission of mitochondrial diseases will soon be available in the United Kingdom, while in the United States, a government-sponsored study on the bioethical and social policy implications of the controversial technology is just getting underway.
On Feb. 24, the U.K.’s House of Lords voted to authorize “mitochondrial donation” to be used with in vitro fertilization when it is determined that a woman’s eggs may contain mitochondrial DNA abnormalities, and that an individual with those abnormalities is at risk of having or developing a serious mitochondrial disease. The House of Commons had approved the use of the procedure earlier in February.
With this landmark vote, the U.K. becomes the first country to allow the use of the technique, although it is unlikely to be used widely, since specialists will be required to receive approval from the Human Fertilisation and Embryology Authority (HFEA) each time they wish to use the technique.
Mitochondrial donation involves removal of the nuclear DNA from the egg or fertilized egg of a woman with abnormal mitochondrial DNA, which is then transferred into an egg or fertilized egg from a healthy donor, after the nuclear material has been removed from the donor egg. This technique is also referred to as “mitochondrial transfer” or “mitochondrial replacement therapy.”
Critics of the procedure have labeled it “three parent” IVF and said it will produce “designer babies.” They warn that, if allowed, the technology would mark the beginning of a slippery slope toward enabling modification of nuclear DNA and germ line modification.
And that debate is starting to heat up in the United States.
In the U.S., the use of this technology is under the purview of the Food and Drug Administration, which has the authority to regulate human cells used in therapy “involving the transfer of genetic material by means other than the union of gamete nuclei,” an agency spokesperson said.
For clinical trials of this technique to proceed, the FDA must review and approve an Investigational New Drug (IND) application, submitted by an investigator. Permission does not indicate approval, only that the FDA is allowing the trial to proceed.
In 2014, the FDA held a meeting of its Cellular, Tissue, and Gene Therapies Advisory Committee to discuss scientific and clinical issues related to the technology, including the types of women who should be enrolled in the first trials. The agency requested that the Institute of Medicine study the ethical and social policy issues related to new techniques that involve genetically modifying eggs and zygotes for the purpose of preventing transmission of mitochondrial diseases.
The IOM committee met for the first time in January; the final report is expected to be available in March 2016.
Animal trials underway
Researchers at Oregon Health & Science University (OHSU) in Portland have tested mitochondrial DNA transfer in macaque monkeys, using unfertilized eggs, and are now ready to start clinical trials in humans using this technique.
“We welcome this development” in the United Kingdom, where the regulatory and ethical issues were thoroughly considered, Shoukhrat Mitalipov, Ph.D., the lead investigator, said in an interview. His group provided regulators in the U.K. with animal and in vitro data.
At the FDA meeting last year, Dr. Mitalipov, the head of OHSU’s Center for Embryonic Cell and Gene Therapy, described the successful results of mitochondrial transfer in four macaque monkeys and said they were ready to start trials in humans. At the time, the monkeys were almost adult age and were healthy and had normal blood results, which showed that mitochondrial DNA in oocytes could be replaced, he told the panel.
An important issue is whether the offspring of these monkeys are normal, since one criticism of the technology is that a permanent change is being made to the germline and would be passed along to future generations.
The monkeys are now a year older, and based on discussions at the FDA panel meeting, the Oregon researchers are extending their nonhuman primate research.
Dr. Mitalipov said they had not submitted a formal IND to the FDA, but have approached the agency about what kind of additional animal and in vitro studies using human cells are needed before clinical trials in humans can begin.
If they gained clearance for human trials, Dr. Mitalipov said their plan would be to limit the study to 5-10 families known to be carriers of mitochondrial DNA mutations, who have already had a child diagnosed with the mitochondrial disease, which could include mutations that cause severe conditions early in life, such as Leigh’s syndrome or MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes).
Debating the ethics
Dr. Mitalipov acknowledged the ethical concerns, but said that unlike other types of gene therapy, mitochondrial replacement therapy aims to eliminate the cause of the condition, “before it is actually passed to the child.”
While there are concerns that the technology could eventually lead to nonmedical genetic enhancements, he said, “it is up to society to regulate those issues.”
One of the most vocal critics of mitochondrial replacement techniques is Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society in Berkeley, Calif.
“All of us should be concerned about the safety risks to any resulting children,” as well as social policy and the availability of the technique “breaking a widespread global agreement that we shouldn’t make genetic changes that will enter the human germline,” she said in an interview.
Moreover, a woman at risk of transmitting a mitochondrial disease who wants to have a healthy child is both emotionally and physically vulnerable. “Physicians treating these women have an elevated responsibility to make sure they are giving them the full picture,” she said.
In a statement issued shortly after the U.K. House of Lords approved the use of the procedure, Dr. Darnovsky said that “unlike experimental gene therapies where risks are taken on by consenting individuals, these techniques turn children into our biological experiments and forever alter the human germline in unknowable ways.”
But Josephine Johnston, director of research at the Hastings Center, a bioethics research institute in Garrison, N.Y., who studies ethical considerations related to assisted reproductive technology, said the U.K. approach “seems like a very reasonable plan.”
And she predicts that because of the way ART is regulated in the U.K., mitochondrial donation would be tightly restricted and would likely result in about 10 of these procedures being performed over the next few years.
While germline modification – manipulating the genetics of an embryo to make a change that will be inherited to the next generation – has been considered by many to be an ethical line not to be crossed, Ms. Johnston said she hopes people can move past the slippery slope objections.
“I hope we as a human community are sophisticated enough to understand that just because we said yes to one thing, doesn’t necessarily mean we have to say yes to everything that might come after it,” she said.
A type of gene therapy to prevent maternal transmission of mitochondrial diseases will soon be available in the United Kingdom, while in the United States, a government-sponsored study on the bioethical and social policy implications of the controversial technology is just getting underway.
On Feb. 24, the U.K.’s House of Lords voted to authorize “mitochondrial donation” to be used with in vitro fertilization when it is determined that a woman’s eggs may contain mitochondrial DNA abnormalities, and that an individual with those abnormalities is at risk of having or developing a serious mitochondrial disease. The House of Commons had approved the use of the procedure earlier in February.
With this landmark vote, the U.K. becomes the first country to allow the use of the technique, although it is unlikely to be used widely, since specialists will be required to receive approval from the Human Fertilisation and Embryology Authority (HFEA) each time they wish to use the technique.
Mitochondrial donation involves removal of the nuclear DNA from the egg or fertilized egg of a woman with abnormal mitochondrial DNA, which is then transferred into an egg or fertilized egg from a healthy donor, after the nuclear material has been removed from the donor egg. This technique is also referred to as “mitochondrial transfer” or “mitochondrial replacement therapy.”
Critics of the procedure have labeled it “three parent” IVF and said it will produce “designer babies.” They warn that, if allowed, the technology would mark the beginning of a slippery slope toward enabling modification of nuclear DNA and germ line modification.
And that debate is starting to heat up in the United States.
In the U.S., the use of this technology is under the purview of the Food and Drug Administration, which has the authority to regulate human cells used in therapy “involving the transfer of genetic material by means other than the union of gamete nuclei,” an agency spokesperson said.
For clinical trials of this technique to proceed, the FDA must review and approve an Investigational New Drug (IND) application, submitted by an investigator. Permission does not indicate approval, only that the FDA is allowing the trial to proceed.
In 2014, the FDA held a meeting of its Cellular, Tissue, and Gene Therapies Advisory Committee to discuss scientific and clinical issues related to the technology, including the types of women who should be enrolled in the first trials. The agency requested that the Institute of Medicine study the ethical and social policy issues related to new techniques that involve genetically modifying eggs and zygotes for the purpose of preventing transmission of mitochondrial diseases.
The IOM committee met for the first time in January; the final report is expected to be available in March 2016.
Animal trials underway
Researchers at Oregon Health & Science University (OHSU) in Portland have tested mitochondrial DNA transfer in macaque monkeys, using unfertilized eggs, and are now ready to start clinical trials in humans using this technique.
“We welcome this development” in the United Kingdom, where the regulatory and ethical issues were thoroughly considered, Shoukhrat Mitalipov, Ph.D., the lead investigator, said in an interview. His group provided regulators in the U.K. with animal and in vitro data.
At the FDA meeting last year, Dr. Mitalipov, the head of OHSU’s Center for Embryonic Cell and Gene Therapy, described the successful results of mitochondrial transfer in four macaque monkeys and said they were ready to start trials in humans. At the time, the monkeys were almost adult age and were healthy and had normal blood results, which showed that mitochondrial DNA in oocytes could be replaced, he told the panel.
An important issue is whether the offspring of these monkeys are normal, since one criticism of the technology is that a permanent change is being made to the germline and would be passed along to future generations.
The monkeys are now a year older, and based on discussions at the FDA panel meeting, the Oregon researchers are extending their nonhuman primate research.
Dr. Mitalipov said they had not submitted a formal IND to the FDA, but have approached the agency about what kind of additional animal and in vitro studies using human cells are needed before clinical trials in humans can begin.
If they gained clearance for human trials, Dr. Mitalipov said their plan would be to limit the study to 5-10 families known to be carriers of mitochondrial DNA mutations, who have already had a child diagnosed with the mitochondrial disease, which could include mutations that cause severe conditions early in life, such as Leigh’s syndrome or MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes).
Debating the ethics
Dr. Mitalipov acknowledged the ethical concerns, but said that unlike other types of gene therapy, mitochondrial replacement therapy aims to eliminate the cause of the condition, “before it is actually passed to the child.”
While there are concerns that the technology could eventually lead to nonmedical genetic enhancements, he said, “it is up to society to regulate those issues.”
One of the most vocal critics of mitochondrial replacement techniques is Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society in Berkeley, Calif.
“All of us should be concerned about the safety risks to any resulting children,” as well as social policy and the availability of the technique “breaking a widespread global agreement that we shouldn’t make genetic changes that will enter the human germline,” she said in an interview.
Moreover, a woman at risk of transmitting a mitochondrial disease who wants to have a healthy child is both emotionally and physically vulnerable. “Physicians treating these women have an elevated responsibility to make sure they are giving them the full picture,” she said.
In a statement issued shortly after the U.K. House of Lords approved the use of the procedure, Dr. Darnovsky said that “unlike experimental gene therapies where risks are taken on by consenting individuals, these techniques turn children into our biological experiments and forever alter the human germline in unknowable ways.”
But Josephine Johnston, director of research at the Hastings Center, a bioethics research institute in Garrison, N.Y., who studies ethical considerations related to assisted reproductive technology, said the U.K. approach “seems like a very reasonable plan.”
And she predicts that because of the way ART is regulated in the U.K., mitochondrial donation would be tightly restricted and would likely result in about 10 of these procedures being performed over the next few years.
While germline modification – manipulating the genetics of an embryo to make a change that will be inherited to the next generation – has been considered by many to be an ethical line not to be crossed, Ms. Johnston said she hopes people can move past the slippery slope objections.
“I hope we as a human community are sophisticated enough to understand that just because we said yes to one thing, doesn’t necessarily mean we have to say yes to everything that might come after it,” she said.
A type of gene therapy to prevent maternal transmission of mitochondrial diseases will soon be available in the United Kingdom, while in the United States, a government-sponsored study on the bioethical and social policy implications of the controversial technology is just getting underway.
On Feb. 24, the U.K.’s House of Lords voted to authorize “mitochondrial donation” to be used with in vitro fertilization when it is determined that a woman’s eggs may contain mitochondrial DNA abnormalities, and that an individual with those abnormalities is at risk of having or developing a serious mitochondrial disease. The House of Commons had approved the use of the procedure earlier in February.
With this landmark vote, the U.K. becomes the first country to allow the use of the technique, although it is unlikely to be used widely, since specialists will be required to receive approval from the Human Fertilisation and Embryology Authority (HFEA) each time they wish to use the technique.
Mitochondrial donation involves removal of the nuclear DNA from the egg or fertilized egg of a woman with abnormal mitochondrial DNA, which is then transferred into an egg or fertilized egg from a healthy donor, after the nuclear material has been removed from the donor egg. This technique is also referred to as “mitochondrial transfer” or “mitochondrial replacement therapy.”
Critics of the procedure have labeled it “three parent” IVF and said it will produce “designer babies.” They warn that, if allowed, the technology would mark the beginning of a slippery slope toward enabling modification of nuclear DNA and germ line modification.
And that debate is starting to heat up in the United States.
In the U.S., the use of this technology is under the purview of the Food and Drug Administration, which has the authority to regulate human cells used in therapy “involving the transfer of genetic material by means other than the union of gamete nuclei,” an agency spokesperson said.
For clinical trials of this technique to proceed, the FDA must review and approve an Investigational New Drug (IND) application, submitted by an investigator. Permission does not indicate approval, only that the FDA is allowing the trial to proceed.
In 2014, the FDA held a meeting of its Cellular, Tissue, and Gene Therapies Advisory Committee to discuss scientific and clinical issues related to the technology, including the types of women who should be enrolled in the first trials. The agency requested that the Institute of Medicine study the ethical and social policy issues related to new techniques that involve genetically modifying eggs and zygotes for the purpose of preventing transmission of mitochondrial diseases.
The IOM committee met for the first time in January; the final report is expected to be available in March 2016.
Animal trials underway
Researchers at Oregon Health & Science University (OHSU) in Portland have tested mitochondrial DNA transfer in macaque monkeys, using unfertilized eggs, and are now ready to start clinical trials in humans using this technique.
“We welcome this development” in the United Kingdom, where the regulatory and ethical issues were thoroughly considered, Shoukhrat Mitalipov, Ph.D., the lead investigator, said in an interview. His group provided regulators in the U.K. with animal and in vitro data.
At the FDA meeting last year, Dr. Mitalipov, the head of OHSU’s Center for Embryonic Cell and Gene Therapy, described the successful results of mitochondrial transfer in four macaque monkeys and said they were ready to start trials in humans. At the time, the monkeys were almost adult age and were healthy and had normal blood results, which showed that mitochondrial DNA in oocytes could be replaced, he told the panel.
An important issue is whether the offspring of these monkeys are normal, since one criticism of the technology is that a permanent change is being made to the germline and would be passed along to future generations.
The monkeys are now a year older, and based on discussions at the FDA panel meeting, the Oregon researchers are extending their nonhuman primate research.
Dr. Mitalipov said they had not submitted a formal IND to the FDA, but have approached the agency about what kind of additional animal and in vitro studies using human cells are needed before clinical trials in humans can begin.
If they gained clearance for human trials, Dr. Mitalipov said their plan would be to limit the study to 5-10 families known to be carriers of mitochondrial DNA mutations, who have already had a child diagnosed with the mitochondrial disease, which could include mutations that cause severe conditions early in life, such as Leigh’s syndrome or MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes).
Debating the ethics
Dr. Mitalipov acknowledged the ethical concerns, but said that unlike other types of gene therapy, mitochondrial replacement therapy aims to eliminate the cause of the condition, “before it is actually passed to the child.”
While there are concerns that the technology could eventually lead to nonmedical genetic enhancements, he said, “it is up to society to regulate those issues.”
One of the most vocal critics of mitochondrial replacement techniques is Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society in Berkeley, Calif.
“All of us should be concerned about the safety risks to any resulting children,” as well as social policy and the availability of the technique “breaking a widespread global agreement that we shouldn’t make genetic changes that will enter the human germline,” she said in an interview.
Moreover, a woman at risk of transmitting a mitochondrial disease who wants to have a healthy child is both emotionally and physically vulnerable. “Physicians treating these women have an elevated responsibility to make sure they are giving them the full picture,” she said.
In a statement issued shortly after the U.K. House of Lords approved the use of the procedure, Dr. Darnovsky said that “unlike experimental gene therapies where risks are taken on by consenting individuals, these techniques turn children into our biological experiments and forever alter the human germline in unknowable ways.”
But Josephine Johnston, director of research at the Hastings Center, a bioethics research institute in Garrison, N.Y., who studies ethical considerations related to assisted reproductive technology, said the U.K. approach “seems like a very reasonable plan.”
And she predicts that because of the way ART is regulated in the U.K., mitochondrial donation would be tightly restricted and would likely result in about 10 of these procedures being performed over the next few years.
While germline modification – manipulating the genetics of an embryo to make a change that will be inherited to the next generation – has been considered by many to be an ethical line not to be crossed, Ms. Johnston said she hopes people can move past the slippery slope objections.
“I hope we as a human community are sophisticated enough to understand that just because we said yes to one thing, doesn’t necessarily mean we have to say yes to everything that might come after it,” she said.
FDA approves first HDAC inhibitor for multiple myeloma
Panobinostat, an orally administered histone deacetylase inhibitor, has been approved as a treatment for multiple myeloma, the first treatment in this class to be approved for this type of malignancy, the Food and Drug Administration announced on Feb. 23.
The approval is for use in combination with bortezomib and dexamethasone for treating patients with multiple myeloma who have received at least two prior regimens, including bortezomib and an immunomodulatory agent. This is an accelerated approval, based on a surrogate endpoint considered “reasonably likely to predict clinical benefit to patients,” and the manufacturer is required by the FDA to conduct a confirmatory trial for full approval. The indications section of the prescribing information includes the following statement: “This indication is approved under accelerated approval based on progression-free survival (PFS). Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Originally, the manufacturer, Novartis Pharmaceuticals, filed for approval of panobinostat, combined with bortezomib (Velcade), a proteasome inhibitor, and dexamethasone, as a treatment for patients with multiple myeloma who had received at least one prior therapy, based on the results of the PANORAMA-1 (Panobinostat or Placebo With Bortezomib and Dexamethasone in Patients With Relapsed Multiple Myeloma) study. At a meeting in November 2014, the majority of the FDA’s Oncologic Drugs Advisory Committee voted that the benefits of treatment did not outweigh the risks for this indication, although panelists said that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on PFS.
After the meeting, Novartis submitted more data that supported approval in the patients who have received at least two prior standard therapies, including bortezomib and an immunomodulatory agent, according to the FDA statement. Among 193 patients in the study who had received at least two other treatments that included bortezomib and an immunomodulatory agent, the median PFS was about 10.6 months among those who were treated with the three agents, vs. 5.8 months among those on bortezomib and dexamethasone only. The response rate was 59% among panobinostat-treated patients, vs. 41% of those on bortezomib and dexamethasone only.
This approval is “particularly important because it has been shown to slow the progression of multiple myeloma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. He referred to the novel mechanism of action of panobinostat as “making it a potentially attractive candidate agent for the treatment of multiple myeloma.”
The prescribing information includes a boxed warning that says severe diarrhea affected 25% of treated patients, and that treated patients have developed severe and fatal cardiac ischemic events, severe arrhythmias, and ECG changes. The drug is approved with a Risk Evaluation and Mitigation Strategy (REMS) addressing these risks.
The most common adverse events associated with panobinostat included diarrhea, fatigue, nausea, swelling in the arms or legs, decreased appetite, fever, vomiting, and weakness; the most common laboratory abnormalities were hypophosphatemia, hypokalemia, hyponatremia, an increased creatinine level, thrombocytopenia, leukopenia, and anemia, according to the FDA statement.
Citing National Cancer Institute statistics, the FDA statement said that in the United States every year, about 21,700 people are diagnosed with multiple myeloma and 10,710 die from the disease.
Novartis is marketing the drug as Farydak. The company’s statement announcing approval said that ”as an HDAC [histone deacetylases] inhibitor, its epigenetic activity may help to restore cell function in multiple myeloma.”
Panobinostat, an orally administered histone deacetylase inhibitor, has been approved as a treatment for multiple myeloma, the first treatment in this class to be approved for this type of malignancy, the Food and Drug Administration announced on Feb. 23.
The approval is for use in combination with bortezomib and dexamethasone for treating patients with multiple myeloma who have received at least two prior regimens, including bortezomib and an immunomodulatory agent. This is an accelerated approval, based on a surrogate endpoint considered “reasonably likely to predict clinical benefit to patients,” and the manufacturer is required by the FDA to conduct a confirmatory trial for full approval. The indications section of the prescribing information includes the following statement: “This indication is approved under accelerated approval based on progression-free survival (PFS). Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Originally, the manufacturer, Novartis Pharmaceuticals, filed for approval of panobinostat, combined with bortezomib (Velcade), a proteasome inhibitor, and dexamethasone, as a treatment for patients with multiple myeloma who had received at least one prior therapy, based on the results of the PANORAMA-1 (Panobinostat or Placebo With Bortezomib and Dexamethasone in Patients With Relapsed Multiple Myeloma) study. At a meeting in November 2014, the majority of the FDA’s Oncologic Drugs Advisory Committee voted that the benefits of treatment did not outweigh the risks for this indication, although panelists said that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on PFS.
After the meeting, Novartis submitted more data that supported approval in the patients who have received at least two prior standard therapies, including bortezomib and an immunomodulatory agent, according to the FDA statement. Among 193 patients in the study who had received at least two other treatments that included bortezomib and an immunomodulatory agent, the median PFS was about 10.6 months among those who were treated with the three agents, vs. 5.8 months among those on bortezomib and dexamethasone only. The response rate was 59% among panobinostat-treated patients, vs. 41% of those on bortezomib and dexamethasone only.
This approval is “particularly important because it has been shown to slow the progression of multiple myeloma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. He referred to the novel mechanism of action of panobinostat as “making it a potentially attractive candidate agent for the treatment of multiple myeloma.”
The prescribing information includes a boxed warning that says severe diarrhea affected 25% of treated patients, and that treated patients have developed severe and fatal cardiac ischemic events, severe arrhythmias, and ECG changes. The drug is approved with a Risk Evaluation and Mitigation Strategy (REMS) addressing these risks.
The most common adverse events associated with panobinostat included diarrhea, fatigue, nausea, swelling in the arms or legs, decreased appetite, fever, vomiting, and weakness; the most common laboratory abnormalities were hypophosphatemia, hypokalemia, hyponatremia, an increased creatinine level, thrombocytopenia, leukopenia, and anemia, according to the FDA statement.
Citing National Cancer Institute statistics, the FDA statement said that in the United States every year, about 21,700 people are diagnosed with multiple myeloma and 10,710 die from the disease.
Novartis is marketing the drug as Farydak. The company’s statement announcing approval said that ”as an HDAC [histone deacetylases] inhibitor, its epigenetic activity may help to restore cell function in multiple myeloma.”
Panobinostat, an orally administered histone deacetylase inhibitor, has been approved as a treatment for multiple myeloma, the first treatment in this class to be approved for this type of malignancy, the Food and Drug Administration announced on Feb. 23.
The approval is for use in combination with bortezomib and dexamethasone for treating patients with multiple myeloma who have received at least two prior regimens, including bortezomib and an immunomodulatory agent. This is an accelerated approval, based on a surrogate endpoint considered “reasonably likely to predict clinical benefit to patients,” and the manufacturer is required by the FDA to conduct a confirmatory trial for full approval. The indications section of the prescribing information includes the following statement: “This indication is approved under accelerated approval based on progression-free survival (PFS). Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
Originally, the manufacturer, Novartis Pharmaceuticals, filed for approval of panobinostat, combined with bortezomib (Velcade), a proteasome inhibitor, and dexamethasone, as a treatment for patients with multiple myeloma who had received at least one prior therapy, based on the results of the PANORAMA-1 (Panobinostat or Placebo With Bortezomib and Dexamethasone in Patients With Relapsed Multiple Myeloma) study. At a meeting in November 2014, the majority of the FDA’s Oncologic Drugs Advisory Committee voted that the benefits of treatment did not outweigh the risks for this indication, although panelists said that there was evidence that panobinostat had biologic activity against multiple myeloma and had an effect on PFS.
After the meeting, Novartis submitted more data that supported approval in the patients who have received at least two prior standard therapies, including bortezomib and an immunomodulatory agent, according to the FDA statement. Among 193 patients in the study who had received at least two other treatments that included bortezomib and an immunomodulatory agent, the median PFS was about 10.6 months among those who were treated with the three agents, vs. 5.8 months among those on bortezomib and dexamethasone only. The response rate was 59% among panobinostat-treated patients, vs. 41% of those on bortezomib and dexamethasone only.
This approval is “particularly important because it has been shown to slow the progression of multiple myeloma,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. He referred to the novel mechanism of action of panobinostat as “making it a potentially attractive candidate agent for the treatment of multiple myeloma.”
The prescribing information includes a boxed warning that says severe diarrhea affected 25% of treated patients, and that treated patients have developed severe and fatal cardiac ischemic events, severe arrhythmias, and ECG changes. The drug is approved with a Risk Evaluation and Mitigation Strategy (REMS) addressing these risks.
The most common adverse events associated with panobinostat included diarrhea, fatigue, nausea, swelling in the arms or legs, decreased appetite, fever, vomiting, and weakness; the most common laboratory abnormalities were hypophosphatemia, hypokalemia, hyponatremia, an increased creatinine level, thrombocytopenia, leukopenia, and anemia, according to the FDA statement.
Citing National Cancer Institute statistics, the FDA statement said that in the United States every year, about 21,700 people are diagnosed with multiple myeloma and 10,710 die from the disease.
Novartis is marketing the drug as Farydak. The company’s statement announcing approval said that ”as an HDAC [histone deacetylases] inhibitor, its epigenetic activity may help to restore cell function in multiple myeloma.”
FDA approves adhesive treatment for superficial varicose veins
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
Difficult-to-clean ERCP duodenoscopes linked to serious infections
The Food and Drug Administration is alerting health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who have undergone endoscopic retrograde cholangiopancreatography (ERCP), despite proper cleaning and disinfection of the devices.
Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to a safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization are followed, which appears to be related to the design of the duodenoscope.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which points out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the FDA statement, which does not mention whether any of the reports were fatal.
But on Feb. 18, UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, patients, and health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Food and Drug Administration is alerting health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who have undergone endoscopic retrograde cholangiopancreatography (ERCP), despite proper cleaning and disinfection of the devices.
Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to a safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization are followed, which appears to be related to the design of the duodenoscope.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which points out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the FDA statement, which does not mention whether any of the reports were fatal.
But on Feb. 18, UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, patients, and health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Food and Drug Administration is alerting health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who have undergone endoscopic retrograde cholangiopancreatography (ERCP), despite proper cleaning and disinfection of the devices.
Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to a safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization are followed, which appears to be related to the design of the duodenoscope.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which points out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the FDA statement, which does not mention whether any of the reports were fatal.
But on Feb. 18, UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, patients, and health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.