User login
What to make of the new ACC/AHA high blood pressure guidelines
Resources
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2017:S0735-S1097.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults. Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
Resources
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2017:S0735-S1097.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults. Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
Resources
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2017:S0735-S1097.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults. Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
The new shingles vaccine: What PCPs need to know
Resources
- US Food and Drug Administration. Shingrix. Available at: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm581491.htm. Accessed November 17, 2017.
- Hales CM, Harpaz R, Ortego-Sanchez I, et al. Update on recommendations for the use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731.
- Centers for Disease Control and Prevention. Herpes Zoster Work Group Activity Update. June 21, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/zoster-01-belongia.pdf. Accessed October 27, 2017.
- Centers for Disease Control and Prevention. Vaccination. Available at: https://www.cdc.gov/shingles/vaccination.html. Accessed November 17, 2017.
- Meeting of the Advisory Committee on Immunization Practices (ACIP). Atlanta, Ga; October 25-26, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/agenda-archive/agenda-2017-10.pdf. Accessed November 20, 2017.
Resources
- US Food and Drug Administration. Shingrix. Available at: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm581491.htm. Accessed November 17, 2017.
- Hales CM, Harpaz R, Ortego-Sanchez I, et al. Update on recommendations for the use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731.
- Centers for Disease Control and Prevention. Herpes Zoster Work Group Activity Update. June 21, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/zoster-01-belongia.pdf. Accessed October 27, 2017.
- Centers for Disease Control and Prevention. Vaccination. Available at: https://www.cdc.gov/shingles/vaccination.html. Accessed November 17, 2017.
- Meeting of the Advisory Committee on Immunization Practices (ACIP). Atlanta, Ga; October 25-26, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/agenda-archive/agenda-2017-10.pdf. Accessed November 20, 2017.
Resources
- US Food and Drug Administration. Shingrix. Available at: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm581491.htm. Accessed November 17, 2017.
- Hales CM, Harpaz R, Ortego-Sanchez I, et al. Update on recommendations for the use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731.
- Centers for Disease Control and Prevention. Herpes Zoster Work Group Activity Update. June 21, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/zoster-01-belongia.pdf. Accessed October 27, 2017.
- Centers for Disease Control and Prevention. Vaccination. Available at: https://www.cdc.gov/shingles/vaccination.html. Accessed November 17, 2017.
- Meeting of the Advisory Committee on Immunization Practices (ACIP). Atlanta, Ga; October 25-26, 2017. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/agenda-archive/agenda-2017-10.pdf. Accessed November 20, 2017.
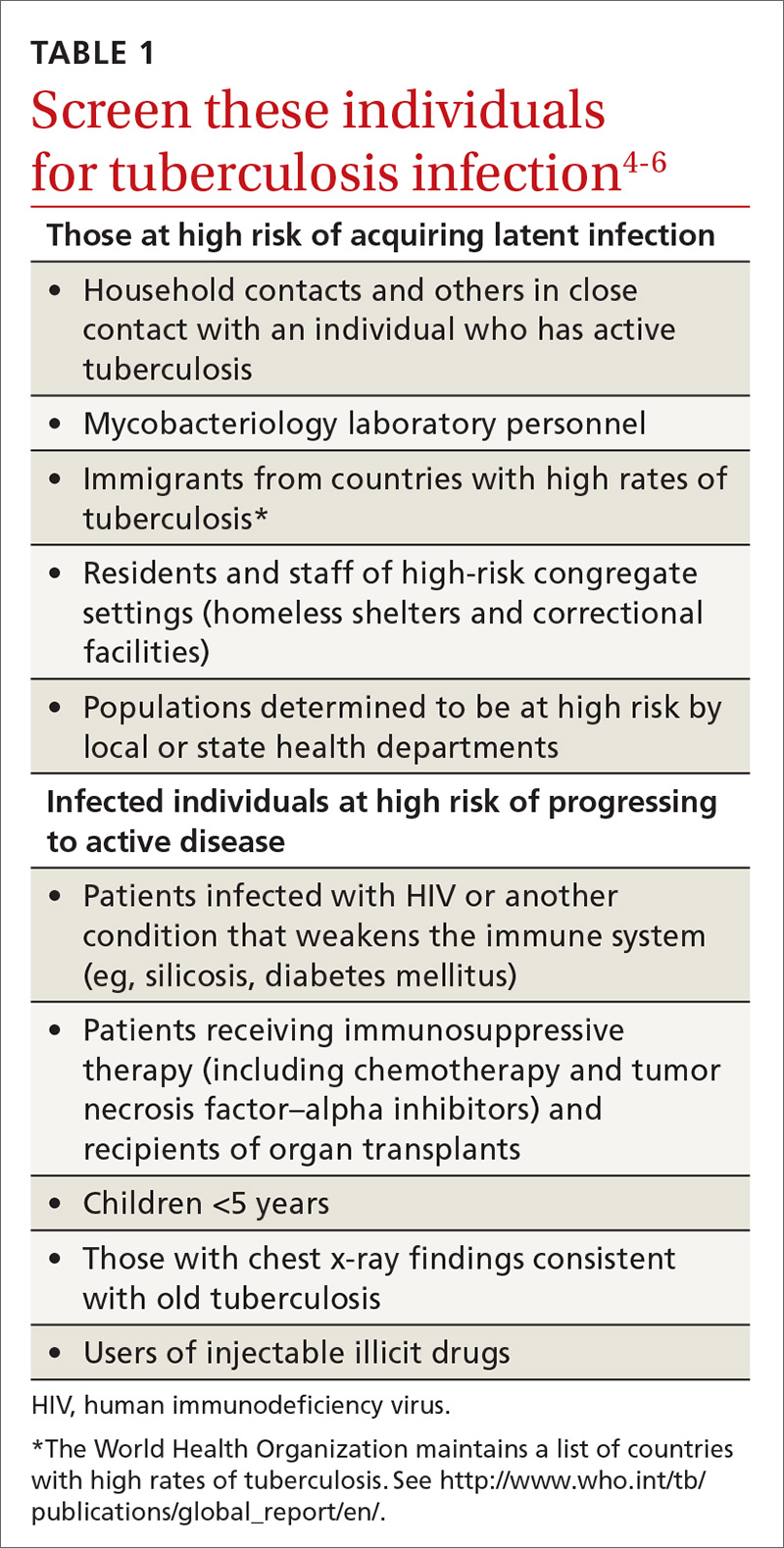
Screening for tuberculosis: Updated recommendations
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
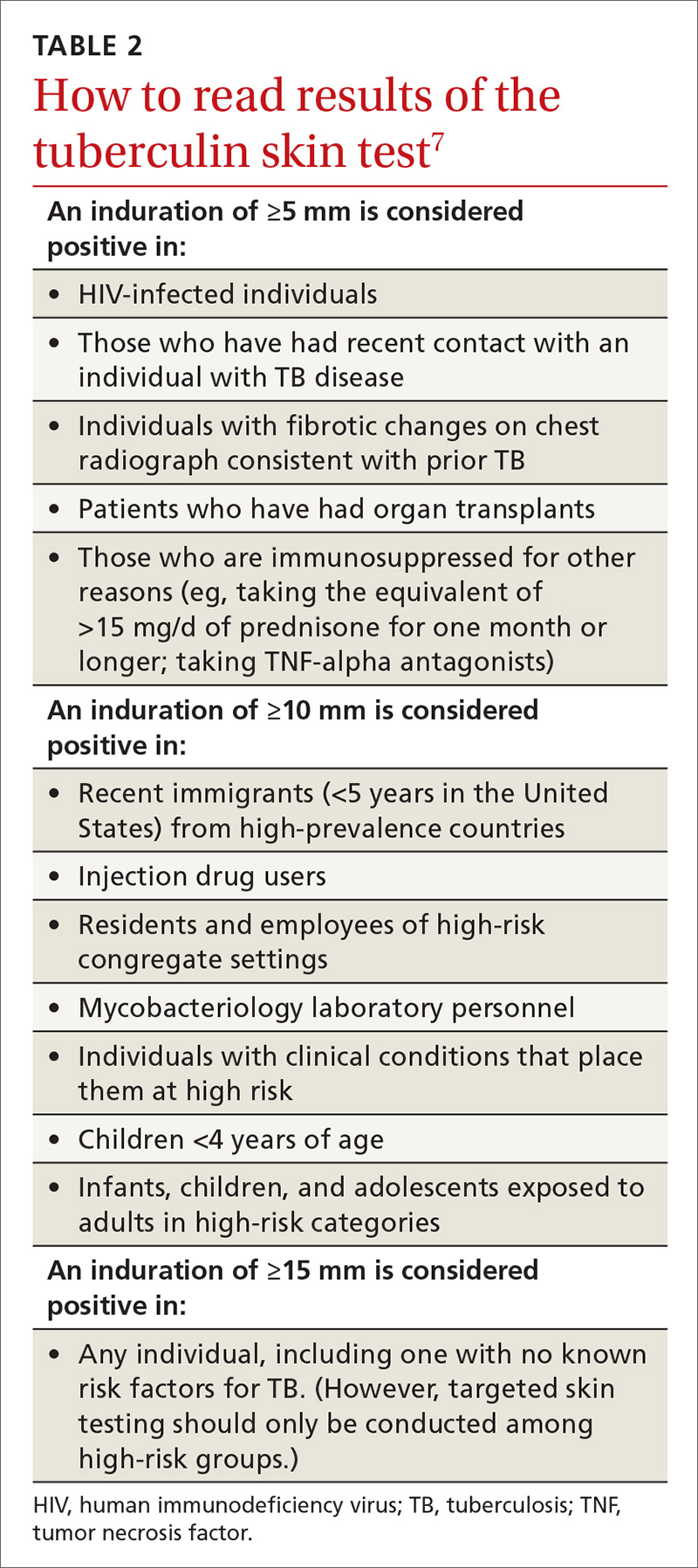
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
From The Journal of Family Practice | 2017;66(12):755-757.
Treat gun violence like the public health crisis it is
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
Last month’s mass shooting in Las Vegas, which killed 59 people and wounded 500, was committed by a single individual who legally purchased an arsenal that allowed him to fire hundreds of high-caliber bullets within minutes into a large crowd. This is just the latest in a series of high-profile mass killings that appear to be increasing in frequency.1
As terrifying as mass murders are, they account for only a small fraction of gun-related mortality. Everyday about 80 people in the United States are killed by a gun, usually by someone they know or by themselves (almost two-thirds of gun-related mortality involves suicide).2 No other developed country even comes close to our rate of gun-related violence.2
What to do? Recall anti-smoking efforts. Gun violence is a public health issue that should be addressed with tried and proven public health methods. A couple of examples from history hold valuable lessons. While tobacco-related mortality and morbidity remain public health concerns, we have made marked improvements and saved many lives through a series of public health interventions including increasing the price of tobacco products, restricting advertising and sales to minors, and prohibiting smoking in public areas, to name a few.3
These interventions occurred because the public recognized the threat of tobacco and was willing to adopt them. This was not always the case. During the first half of my life, smoking in public, including indoors at public events and even on airplanes, was accepted, and the “rights of smokers” were respected. This now seems inconceivable. Public health interventions work, and public perceptions and attitudes can change.
Consider inroads made in driver safety, too. We have also made marked improvements in motor vehicle crash-related deaths and injuries.4 For decades, we have recorded hundreds of data points on every car crash resulting in a death in a comprehensive database—the Fatality Analysis Reporting System (FARS). These data have been used by researchers to identify causes of crashes and crash-related deaths and have led to improvements in car design and road safety. Additional factors leading to improved road safety include restrictions on the age at which one can drive and on drinking alcohol and driving.
We can achieve similar improvements in gun-related mortality if we establish and maintain a comprehensive database, encourage and fund research, and are willing to adopt some commonsense product improvements and ownership restrictions that, nevertheless, preserve the right for most to responsibly own a firearm.
Don’t you think it’s time?
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
1. Blair JP, Schweit KW. A study of active shooter incidents in the United States between 2000 and 2013. Texas State University and the Federal Bureau of Investigation, US Department of Justice, Washington, DC. 2014. Available at: https://www.fbi.gov/file-repository/active-shooter-study-2000-2013-1.pdf. Accessed October 16, 2017.
2. Wintemute GJ. The epidemiology of firearm violence in the twenty-first century United States. Annu Rev Public Health. 2015;36:5-19.
3. Centers for Disease Control and Prevention. Tobacco use—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:986-993.
4. Centers for Disease Control and Prevention. Achievements in public health, 1900-1999 motor-vehicle safety: a 20th century public health achievement. MMWR Morb Mortal Wkly Rep. 1999;48:369-374.
Statins for primary prevention of CVD: To start or not to start?
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Resources
- Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420. Available at: https://jamanetwork.com/journals/jama/fullarticle/2645741.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol. 2014;63:2889-2934. Available at: http://www.sciencedirect.com/science/article/pii/S0735109713060282?via%3Dihub.
- Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. Available at: https://jamanetwork.com/journals/jama/fullarticle/2584058.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999-3058. Available at: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw272.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282. Available at: http://www.onlinecjc.ca/article/S0828-282X(16)30732-2/fulltext.
- Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA. 2017;317:1563-1567. Available at: https://jamanetwork.com/journals/jama/fullarticle/2618621.
Latest recommendations for the 2017-2018 flu season
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.
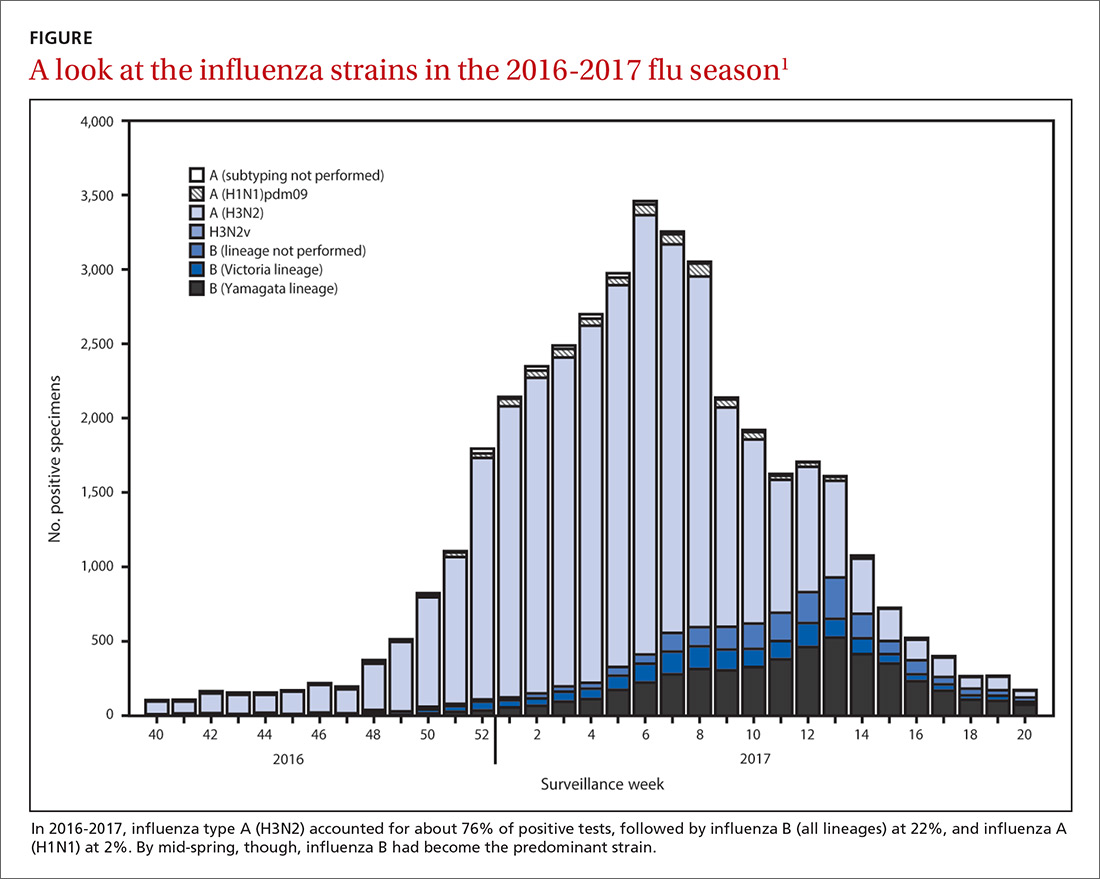
Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.
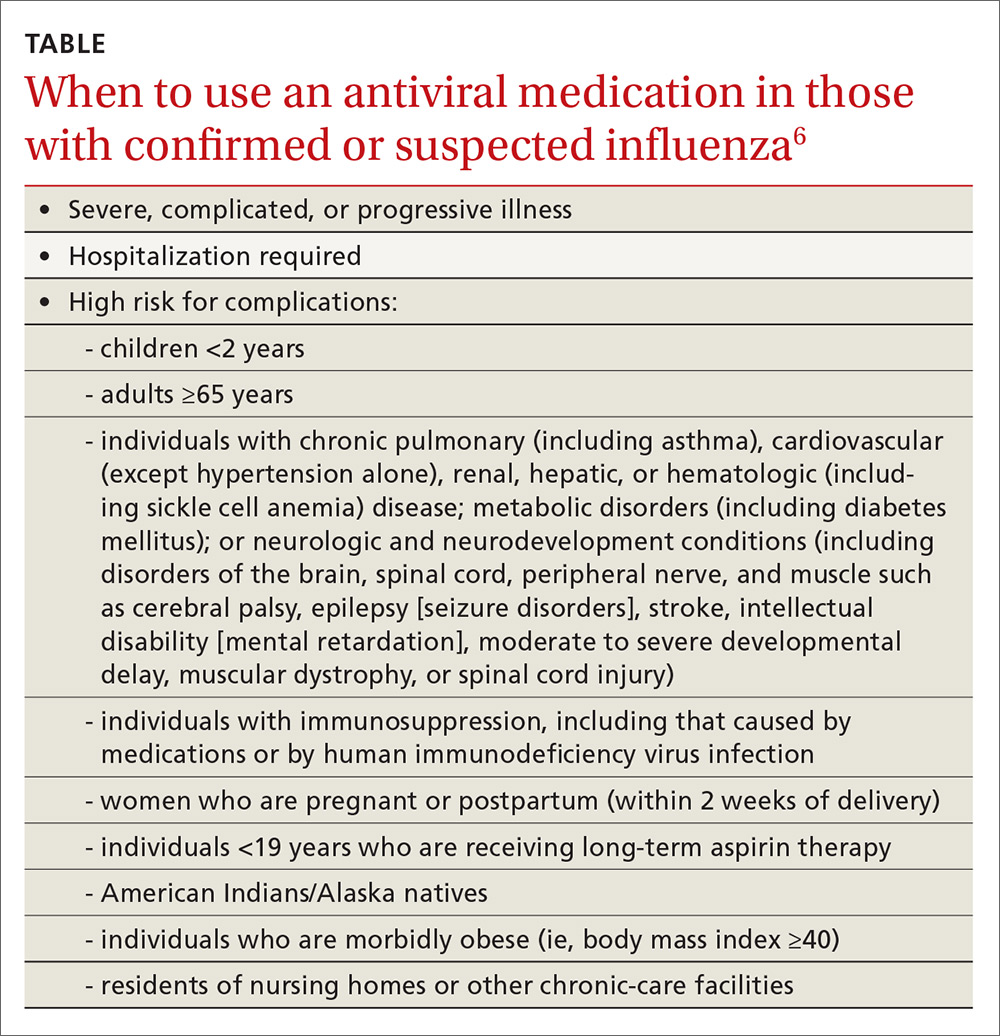
Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
The Centers for Disease Control and Prevention (CDC) recently reported details of the 2016-2017 influenza season in Morbidity and Mortality Weekly Report1 and at the June meeting of the Advisory Committee on Immunization Practices. The CDC monitors influenza activity using several systems, and last flu season was shown to be moderately severe, starting in December in the Western United States, moving east, and peaking in February.
During the peak, 5.1% of outpatient visits were attributed to influenza-like illnesses, and 8.2% of reported deaths were due to pneumonia and influenza. For the whole influenza season, there were more than 18,000 confirmed influenza-related hospitalizations, with 60% of these occurring among those ≥65 years.1 Confirmed influenza-associated pediatric deaths totaled 98.1
The predominant influenza strain last year was type A (H3N2), accounting for about 76% of positive tests in public health laboratories (FIGURE).1 This was followed by influenza B (all lineages) at 22%, and influenza A (H1N1), accounting for only 2%. However, in early April, the predominant strain changed from A (H3N2) to influenza B. Importantly, all viruses tested last year were sensitive to oseltamivir, zanamivir, and peramivir. No antiviral resistance was detected to these neuraminidase inhibitors.

Good news and bad news on vaccine effectiveness. The good news: Circulating viruses were a close match to those contained in the vaccine. The bad news: Vaccine effectiveness at preventing illness was estimated to be just 34% against A (H3N2) and 56% against influenza B viruses.1 There has been no analysis of the relative effectiveness of different vaccines and vaccine types.
The past 6 influenza seasons have revealed a pattern of lower vaccine effectiveness against A (H3N2) compared with effectiveness against A (H1N1) and influenza B viruses. While vaccine effectiveness is not optimal, routine universal use still prevents a great deal of mortality and morbidity. It’s estimated that in 2012-2013, vaccine effectiveness (comparable to that in 2016-2017) prevented 5.6 million illnesses, 2.7 million medical visits, 61,500 hospitalizations, and 1800 deaths.1
More good news: Vaccine safety studies are reassuring
The CDC monitors influenza vaccine safety by using several sources, including the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.2
Changes for the 2017-2018 influenza season
The composition of influenza vaccine products for the 2017-2018 season will differ slightly from last year’s formulation in the H1N1 component. Viral antigens to be included in the trivalent products are A/Michigan (H1N1), A/Hong Kong (H3N2), and B/Brisbane.3 Quadrivalent products will add B/Phuket to the other 3 antigens.3 A wide array of influenza vaccine products is available. Each one is described on the CDC Web site.4
Two minor changes in the recommendations were made at the June ACIP meeting.5 Afluria is approved by the FDA for use in children starting at age 5 years. ACIP had recommended that its use be reserved for children 9 years and older because previous influenza seasons had raised concerns about increased rates of febrile seizures in children younger than age 9. These concerns have been resolved, however, and the ACIP recommendations are now in concert with those of the FDA for this product.
Influenza immunization with an inactivated influenza vaccine product has been recommended for all pregnant women. Safety data are increasingly available for other product options as well, and ACIP now recommends vaccination in pregnancy with any age-appropriate product except for live attenuated influenza vaccine. 5
Antivirals: Give as needed, even before lab confirmation
The CDC recommends antiviral medication for individuals with confirmed or suspected influenza who have severe, complicated, or progressive illness, who require hospitalization, or who are at high risk of complications from influenza (TABLE6). Start treatment without waiting for laboratory confirmation for those with suspected influenza who are seriously ill. Outcomes are best when antivirals are started within 48 hours of illness onset, but they can be started even after this “window” has passed.

Once antiviral treatment has begun, make sure the full 5-day course is completed regardless of culture or rapid-test results.6 Use only neuraminidase inhibitors, as there is widespread resistance to adamantanes among influenza A viruses.
Influenza can occur year round
Rates of influenza infection are low in the summer, but cases do occur. Be especially alert if patients with influenza-like illness have been exposed to swine or poultry; they may have contracted a novel influenza A virus. Report such cases to the state or local health department so that staff can facilitate laboratory testing of viral subtypes. Follow the same protocol for patients with influenza symptoms who have traveled to areas where avian influenza viruses have been detected. The CDC is interested in detecting novel influenza viruses, which can start a pandemic.
Prepare for the 2017-2018 influenza season
Family physicians can help prevent influenza and its associated morbidity and mortality in several ways. Offer immunization to all patients, and immunize all health care personnel in your offices and clinics. Treat with antivirals those for whom they are recommended. Prepare office triage policies that prevent patients with flu symptoms from mixing with other patients, ensure that clinic infection control practices are enforced, and advise ill patients to avoid exposing others.7 Finally, stay current on influenza epidemiology and changes in recommendations for treatment and vaccination.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
1. Blanton L, Alabi N, Mustaquim D, et al. Update: Influenza activity in the United States during the 2016-2017 season and composition of the 2017-2018 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:668-676.
2. Shimabukuro T. End-of-season update: 2016-2017 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-04-shimabukuro.pdf. Accessed August 1, 2017.
3. CDC. Frequently asked flu questions 2017-2018 influenza season. Available at: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed July 17, 2017.
4. CDC. Influenza vaccines — United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Accessed July 17, 2017.
5. Grohskopf L. Influenza WG considerations and proposed recommendations. Presented at: meeting of the Advisory Committee on Immunization Practices; June 21, 2017; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/flu-06-grohskopf.pdf. Accessed August 1, 2017.
6. CDC. Use of antivirals. Available at: https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#Box. Accessed July 17, 2017.
7. CDC. Prevention strategies for seasonal influenza in healthcare settings. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed July 17, 2017.
Need an edge with T2DM? The case for team-based care
CDC: 4 conception strategies for HIV-discordant couples
Measles: Why it’s still a threat
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
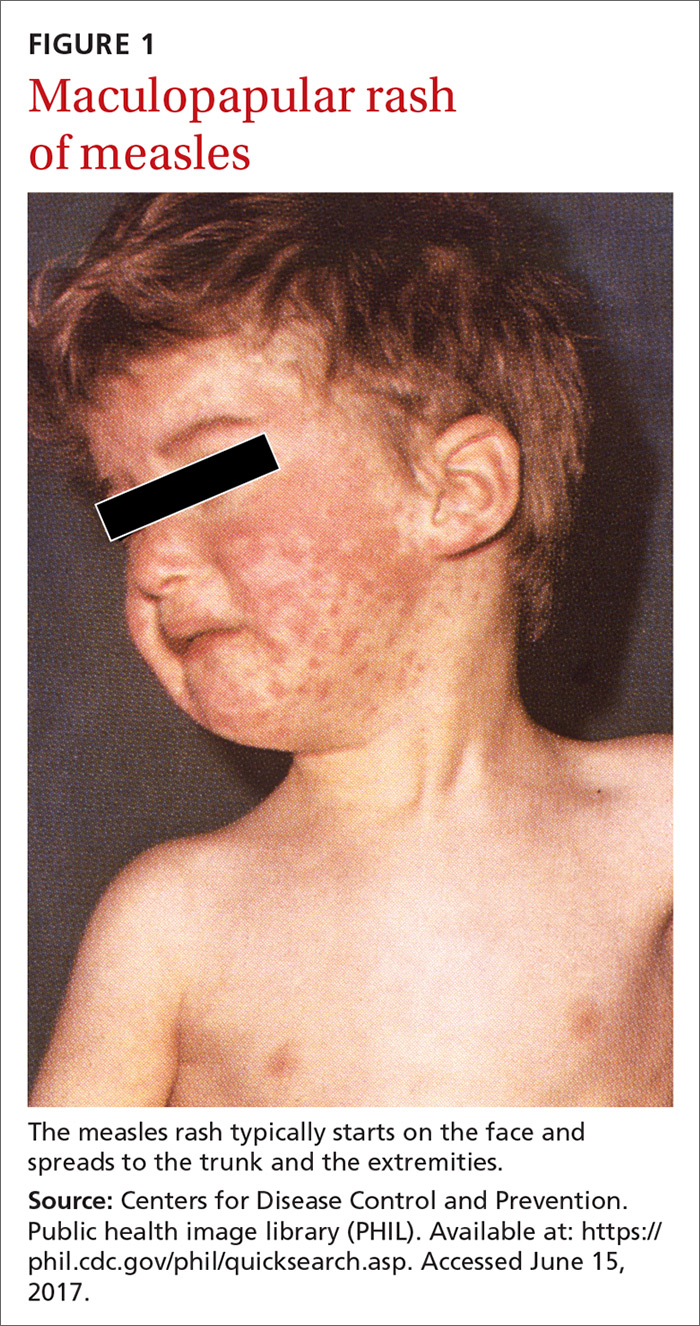
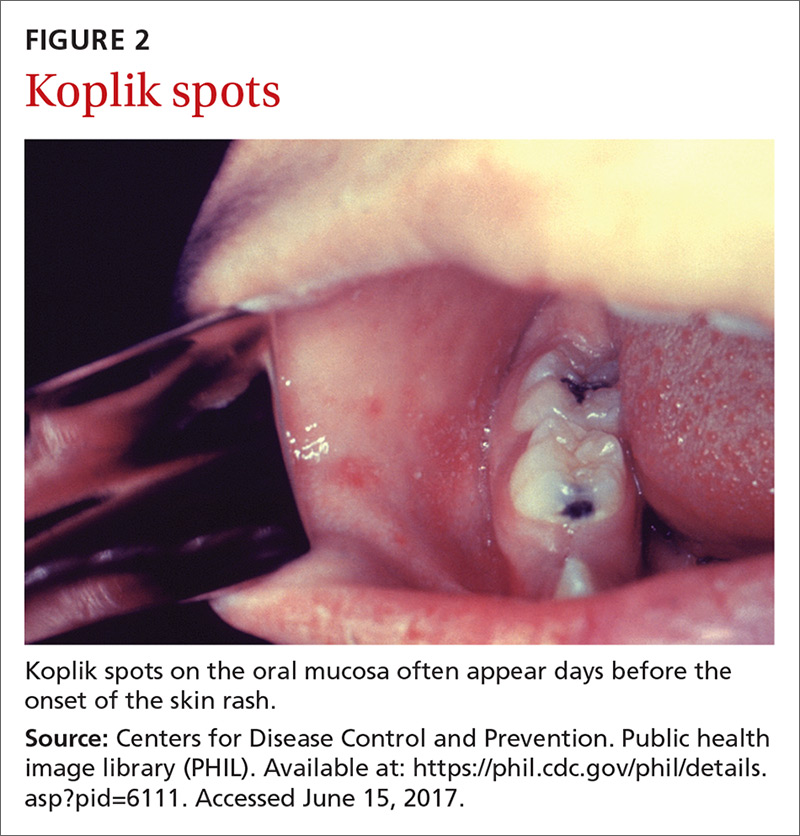
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.