User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
Pertussis: Comparison studies show DTwP more durable
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
FROM VACCINE
Pentavalent DTaP-Hb-Hib vaccine is found noninferior to comparator
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
FROM VACCINE
FDA approves rituximab biosimilar for cancer, autoimmune disorders
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
Adjuvanted influenza vaccine appears safe for at-risk children
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
New scale could measure vaccine hesitancy in developing countries
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Universal adolescent education on healthy relationships needed
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
Sexually active adolescent girls face reproductive coercion (RC) and adolescent relationship abuse (ARA), but there seems to be no statistically significant demographic factors, so education should be universally provided, wrote Amber L. Hill, MSPH, and colleagues in Obstetrics & Gynecology.
Ms. Hill of the University of Pittsburgh and colleagues conducted a secondary analysis of data from a cross-sectional baseline survey that had been used in a cluster-randomized trial. The SHARP (School Health Center Healthy Adolescent Relationship Program) trial, investigated an educational intervention regarding healthy relationships. Their analysis included survey data for 550 sexually active girls aged 14-19 years who’d received services from any of eight student health centers across Northern California during the 2012-2013 school year.
The investigators explained that ARA includes physical, sexual, and emotional abuse among adolescents in a romantic relationship; they further described RC as a form of ARA that increases risks of unintended pregnancy, such as contraceptive sabotage, condom manipulation, and pregnancy coercion. RC was defined as a positive response on a 10-item validated measure, and ARA was defined by positive response to at least one of three items that had been derived from Conflict Tactics Scale 2 and the Sexual Experiences Survey.
Among all females in the analysis, 12% reported reproductive coercion, and 17% reported relationship abuse . Black and Hispanic girls were the most likely to report RC, each at 15%; white girls were the most likely to report ARA at 22%. However, none of the demographic differences evaluated in this analysis, including these, were statistically significant, the authors cautioned.
One of the limitations of this study is that its sample was limited to school health centers in Northern California so it may not be generalizable. Furthermore, its cross-sectional design limits causal inference.
“By highlighting the relevance of reproductive coercion in adolescence, this study substantiates the urgent need for developmentally appropriate interventions,” Ms. Hill and associates concluded.
The authors did not report any potential conflicts of interest. Grants from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice and the National Center for Advancing Translational Sciences of the National Institutes of Health supported the study.
SOURCE: Hill AL et al. Obstet Gynecol. 2019;134(2):351-9.
FROM OBSTETRICS & GYNECOLOGY
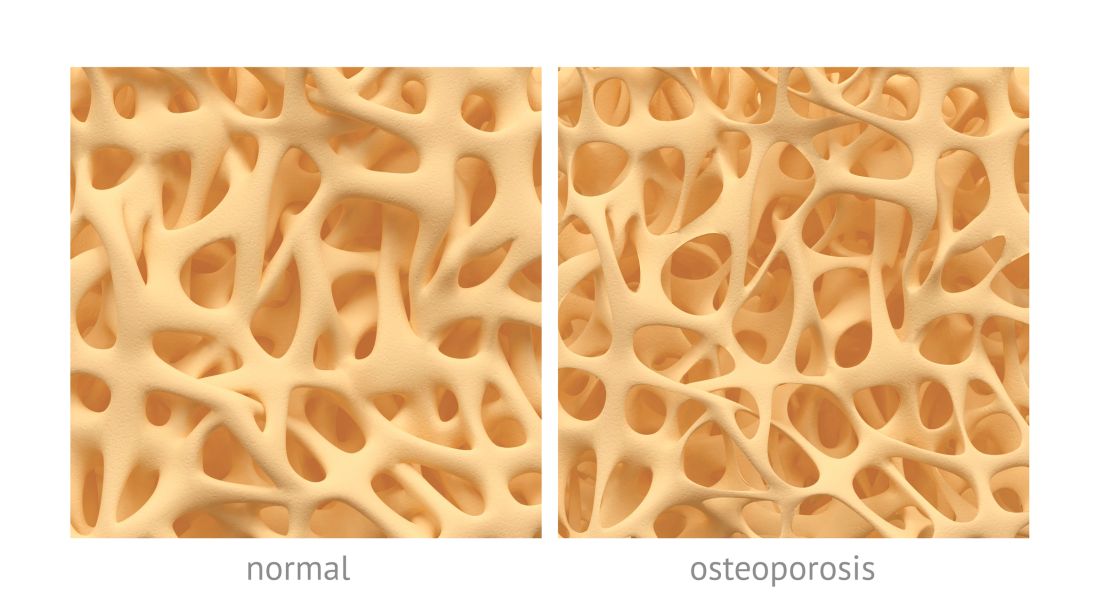
Osteoporosis, osteoarthritis risk high among cerebral palsy patients
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
FROM BONE
CBT intervention tied to Internet addiction remission in men
A manualized cognitive-behavioral therapy (CBT) program that combines group and individual interventions might effectively treat Internet and computer game addiction in men, results of a multicenter randomized trial suggest.
In the study, Klaus Wölfling, PhD, and colleagues randomized 143 men (mean age, 26.2 years) with Internet addiction (IA), which was defined as a score greater than 13 on the Assessment of Internet and Computer Game Addiction Self-Report (AICA-S). The men were recruited at four outpatient clinics in Germany and Austria from Jan. 24, 2012, to June 14, 2017. The primary endpoint of remission, defined as a score of less than 7 on the AICA-S, was achieved by 50 patients (69.4%) in the treatment arm, compared with 17 patients (23.9%) of the wait-list control arm. The greatest declines in AICA-S scores were seen by midtreatment, but mean scores continued at similar levels through follow-up. The study was published in JAMA Psychiatry (2019 Jul 10. doi: 10.1001/jamapsychiatry.2019.1676).
Dr. Wölfling and colleagues chose to limit the scope of the study to male participants because they represent most of those affected by IA; however, the authors admitted this limits the results’ generalizability. They also noted that many of the patients were ambivalent toward treatment, which is a core characteristic of patients affected by IA. Recruitment was slow, so the investigators had to evaluate a smaller number of participants than planned, although they felt their analyses were still statistically powerful enough to detect difference in endpoints. The authors also noted that, although they tried to control for comorbidities, which are often associated with IA, they had to define exclusion criteria for certain conditions such as major depression and some personality disorders.
IA was included in the DSM-5 in 2013 as a condition warranting further research, the authors of this study noted. More recently, it was “introduced as a new diagnosis in the International Classification of Diseases, 11th Revision, in the section Disorders Due to Substance Use or Addictive Behaviors.”
A manualized cognitive-behavioral therapy (CBT) program that combines group and individual interventions might effectively treat Internet and computer game addiction in men, results of a multicenter randomized trial suggest.
In the study, Klaus Wölfling, PhD, and colleagues randomized 143 men (mean age, 26.2 years) with Internet addiction (IA), which was defined as a score greater than 13 on the Assessment of Internet and Computer Game Addiction Self-Report (AICA-S). The men were recruited at four outpatient clinics in Germany and Austria from Jan. 24, 2012, to June 14, 2017. The primary endpoint of remission, defined as a score of less than 7 on the AICA-S, was achieved by 50 patients (69.4%) in the treatment arm, compared with 17 patients (23.9%) of the wait-list control arm. The greatest declines in AICA-S scores were seen by midtreatment, but mean scores continued at similar levels through follow-up. The study was published in JAMA Psychiatry (2019 Jul 10. doi: 10.1001/jamapsychiatry.2019.1676).
Dr. Wölfling and colleagues chose to limit the scope of the study to male participants because they represent most of those affected by IA; however, the authors admitted this limits the results’ generalizability. They also noted that many of the patients were ambivalent toward treatment, which is a core characteristic of patients affected by IA. Recruitment was slow, so the investigators had to evaluate a smaller number of participants than planned, although they felt their analyses were still statistically powerful enough to detect difference in endpoints. The authors also noted that, although they tried to control for comorbidities, which are often associated with IA, they had to define exclusion criteria for certain conditions such as major depression and some personality disorders.
IA was included in the DSM-5 in 2013 as a condition warranting further research, the authors of this study noted. More recently, it was “introduced as a new diagnosis in the International Classification of Diseases, 11th Revision, in the section Disorders Due to Substance Use or Addictive Behaviors.”
A manualized cognitive-behavioral therapy (CBT) program that combines group and individual interventions might effectively treat Internet and computer game addiction in men, results of a multicenter randomized trial suggest.
In the study, Klaus Wölfling, PhD, and colleagues randomized 143 men (mean age, 26.2 years) with Internet addiction (IA), which was defined as a score greater than 13 on the Assessment of Internet and Computer Game Addiction Self-Report (AICA-S). The men were recruited at four outpatient clinics in Germany and Austria from Jan. 24, 2012, to June 14, 2017. The primary endpoint of remission, defined as a score of less than 7 on the AICA-S, was achieved by 50 patients (69.4%) in the treatment arm, compared with 17 patients (23.9%) of the wait-list control arm. The greatest declines in AICA-S scores were seen by midtreatment, but mean scores continued at similar levels through follow-up. The study was published in JAMA Psychiatry (2019 Jul 10. doi: 10.1001/jamapsychiatry.2019.1676).
Dr. Wölfling and colleagues chose to limit the scope of the study to male participants because they represent most of those affected by IA; however, the authors admitted this limits the results’ generalizability. They also noted that many of the patients were ambivalent toward treatment, which is a core characteristic of patients affected by IA. Recruitment was slow, so the investigators had to evaluate a smaller number of participants than planned, although they felt their analyses were still statistically powerful enough to detect difference in endpoints. The authors also noted that, although they tried to control for comorbidities, which are often associated with IA, they had to define exclusion criteria for certain conditions such as major depression and some personality disorders.
IA was included in the DSM-5 in 2013 as a condition warranting further research, the authors of this study noted. More recently, it was “introduced as a new diagnosis in the International Classification of Diseases, 11th Revision, in the section Disorders Due to Substance Use or Addictive Behaviors.”
FROM JAMA PSYCHIATRY
Parent education improves pediatric influenza vaccination rates
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
FROM PEDIATRICS
Acquired MMR immunity doesn’t last to age 1 year
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
FROM VACCINE