User login
Dermatoporosis in Older Adults: A Condition That Requires Holistic, Creative Management
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
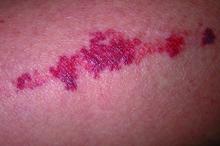
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Managing Atopic Dermatitis in Older Adults: A Common, Unique Challenge
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Rethinking Management of Skin Cancer in Older Patients
WASHINGTON — In 2013, Vishal A. Patel, MD, was completing a fellowship in Mohs surgery and cutaneous oncology at Columbia University Irving Medical Center, New York City, when a study was published showing that most nonmelanoma skin cancers (NMSCs) were treated with surgery, regardless of the patient’s life expectancy. Life expectancy “should enter into treatment decisions,” the authors concluded.
“ Dr. Patel recalled at the ElderDerm conference hosted by the Department of Dermatology at George Washington University, Washington, DC, and described as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Today, however, more than a decade later, guidelines still promote surgical therapy as the gold standard across the board, and questions raised by the study are still unaddressed, Dr. Patel, associate professor of dermatology and medicine/oncology at George Washington University, said at the meeting. These questions are becoming increasingly urgent as the incidence of skin cancer, especially NMSC, rises in the older adult population, especially in patients older than 85 years. “It’s a function of our training and our treatment guidelines that we reach for the most definitive treatment, which happens to be the most aggressive, in these patients,” added Dr. Patel, who is also director of the cutaneous oncology program at the GW Cancer Center.
“Sometimes we lose track of what ... we need to do” to provide care that reflects the best interests of the older patient, he continued. “Surgery may be the gold standard for treating the majority of NMSCs ... but is it the [best option] for what our older patients and patients with limited life expectancy need?”
Learning about what truly matters to the patient is a key element of the “age-friendly, whole-person care” that dermatologists must embrace as older adults become an increasingly large subset of their patient population, Christina Prather, MD, director and associate professor of geriatrics and palliative medicine at George Washington University, said at the meeting.
By 2040, projections are that the number of adults aged 85 years and older in the United States will be nearly quadruple the number in 2000, according to one estimate.
“We know that there are less than 6000 practicing geriatricians in the country ... [so the healthcare system] needs more of you who know how to bring an age-friendly approach to care,” Dr. Prather said. Dermatology is among the specialties that need to be “geriatricized.”
NMSC Increasing in the Older Population
The incidence of skin cancer is rising faster than that of any other cancer, Dr. Patel said. One window into the epidemiology, he said, comes from recently published data showing that an average of 6.1 million adults were treated each year for skin cancer during 2016-2018 (5.2 million of them for NMSC) — an increase from an average of 5.8 million annually in the 2012-2015 period. The data come from the Medical Expenditure Panel Survey (MEPS), which is conducted by the US Public Health Service through the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention.
As a frame of reference, the average number of adults treated each year for nonskin cancers during these periods rose from 10.8 to 11.9 million, according to the 2023 MEPS data. “Skin cancer is about one-third of all cancers combined,” Dr. Patel said.
Not only is the incidence of NMSC significantly higher than that of melanoma but it also shows a more prominent aging trend. This was documented recently in a long-term observational study from Japan, in which researchers looked at the change in the median age of patients with NMSC and melanoma, compared with cancers of other organs, from 1991 to 2020 and found that NMSC had by far the greatest rise in median age, to a median age of 80 years in 2021.
Even more notable, Dr. Patel said, was a particularly marked increase in the number of patients with skin cancer aged 90 years and older. In 2021, this group of older adults accounted for 17% of patients receiving treatment for skin cancer at the Japanese hospital where the data were collected.
The 2013 study that stirred Dr. Patel as a fellow was of 1536 consecutive patients diagnosed with NMSC at two dermatology clinics (a University of California San Francisco–based private clinic and a Veterans Affairs Medical Center clinic) and followed for 6 years. “What’s interesting and worth thinking about is that, regardless of patients’ life expectancy, NMSCs were treated aggressively and surgically, and the choice of surgery was not influenced by the patient’s poor prognosis in a multivariate model” adjusted for tumor and patient characteristics, he said at the meeting.
The researchers defined limited life expectancy as either 85 years or older or having a Charleston Comorbidity Index ≥ 3. Approximately half of the patients with limited life expectancy died within 5 years, none of NMSC. Most patients with limited life expectancy were not often bothered by their tumors, and approximately one in five reported a treatment complication within 2 years. The 5-year tumor recurrence rate was 3.7%.
A more recent study looked at 1181 patients older than 85 years with NMSC referred for Mohs surgery. Almost all patients in the multicenter, prospective cohort study (91.3%) were treated with Mohs.
Treated patients were more likely to have facial tumors and higher functional status than those not treated with Mohs surgery, and the most common reasons provided by surgeons for proceeding with the surgery were a patient desire for a high cure rate (66%), higher functional status for age (57%), and high-risk tumor type (40%). Almost 42% of the referred patients were 89 years or older.
“Granted, [the reasons] are justified indications for surgery,” Dr. Patel said. Yet the study brings up the question of “whether we need to do Mohs surgery this frequently in elderly patients?” In an email after the meeting, he added, “it’s a question we may need to reconsider as the elderly population continues to increase and median age of NMSC rises.”
Underutilized Management Options for NMSC
In his practice, discussions of treatment options are preceded by a thorough discussion of the disease itself. Many lesions are low risk, and helping patients understand risks, as well as understanding what is important to the patient — especially those with limited life expectancy — will guide shared decision-making to choose the best treatment, Dr. Patel said at the meeting.
The dermatologist’s risk assessment — both staging and stratifying risk as it relates to specific outcomes such as recurrence, metastases, or death — takes on added importance in the older patient, he emphasized. “I think we underutilize the risk assessment.”
Also underutilized is the option of shave removal for low-risk squamous cell carcinomas and basal cell carcinomas, Dr. Patel said, noting that, in the National Comprehensive Cancer Network guidelines, “there’s an option for shave removal and nothing more if you have clear margins.”
Alternatively, disc excision with the initial biopsy can often be considered. “Having that intent to treat at the time of biopsy may be all that needs to be done” in older patients with obvious or highly suspicious lesions, he said.
Systemic immunotherapy has joined the treatment armamentarium for advanced basal cell carcinoma and advanced cutaneous squamous cell carcinoma, and if early, ongoing research of intralesional programmed cell death protein 1 inhibitor treatment advances, this could be another option for older adults in the future, Dr. Patel said. Targeting drug delivery directly to the tumor would lower the total dose, decrease systemic exposure, and could be used to avoid surgery for some groups of patients, such as those with limited life expectancy.
A Personal Story, a Word on Melanoma
Dr. Prather recalled when her 97-year-old grandfather had a skin lesion on his forehead removed, and a conversation he had with her mother about whether he really needed to have the procedure because he had cognitive impairment and was on oral anticoagulants.
The clinician “said it absolutely had to go. ... I can’t tell you how much his doctors’ visits and wound care consumed my family’s life for the next few years — for this thing that never quite healed,” she said.
“Was it necessary? The more I’ve learned over time is that it wasn’t,” Dr. Prather added. “We have to take time [with our older patients] and think critically. What is feasible? What makes the most sense? What is the most important thing I need to know about the patient?”
Also important, Dr. Patel noted, is the big-picture consideration of skin cancer treatment costs. The MEPS survey data showing the rising prevalence of skin cancer treatment also documented the economic burden: A nearly 30% increase in the average annual cost of treating NMSC from $5 billion in 2012-2015 to $6.5 billion in 2016-2018. (The average annual costs of treating melanoma decreased slightly.) “Skin cancer is a big drain on our limited resources,” he said.
With melanoma as well, dermatologists must think critically and holistically about the individual patient — and not have “a single view lens of the disease and how we treat the disease,” said Dr. Patel, urging the audience to read a “Sounding Board” article published in The New England Journal of Medicine in 2021. The article argued that there is overdiagnosis of cutaneous melanoma stemming from increased screening, falling clinical thresholds for biopsy, and falling pathological thresholds for labeling morphologic changes as cancer.
“There’s a diagnostic disconnect and a problem of overdiagnosis ... because we’re afraid to miss or make a mistake,” he said. “It leads to the question, do all lesions denoted as skin cancers need aggressive treatment? What does it mean for the patient in front of you?”
Dr. Patel reported receiving honoraria from Regeneron, Almirall, Biofrontera, Sun Pharma, and SkylineDx and serving on the speaker bureau of Regeneron and Almirall. He is chief medical officer for Lazarus AI and is cofounder of the Skin Cancer Outcomes consortium. Dr. Prather disclosed relationships with the National Institutes of Health, AHRQ, The Washington Home Foundation, and the Alzheimer’s Association.
A version of this article appeared on Medscape.com.
WASHINGTON — In 2013, Vishal A. Patel, MD, was completing a fellowship in Mohs surgery and cutaneous oncology at Columbia University Irving Medical Center, New York City, when a study was published showing that most nonmelanoma skin cancers (NMSCs) were treated with surgery, regardless of the patient’s life expectancy. Life expectancy “should enter into treatment decisions,” the authors concluded.
“ Dr. Patel recalled at the ElderDerm conference hosted by the Department of Dermatology at George Washington University, Washington, DC, and described as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Today, however, more than a decade later, guidelines still promote surgical therapy as the gold standard across the board, and questions raised by the study are still unaddressed, Dr. Patel, associate professor of dermatology and medicine/oncology at George Washington University, said at the meeting. These questions are becoming increasingly urgent as the incidence of skin cancer, especially NMSC, rises in the older adult population, especially in patients older than 85 years. “It’s a function of our training and our treatment guidelines that we reach for the most definitive treatment, which happens to be the most aggressive, in these patients,” added Dr. Patel, who is also director of the cutaneous oncology program at the GW Cancer Center.
“Sometimes we lose track of what ... we need to do” to provide care that reflects the best interests of the older patient, he continued. “Surgery may be the gold standard for treating the majority of NMSCs ... but is it the [best option] for what our older patients and patients with limited life expectancy need?”
Learning about what truly matters to the patient is a key element of the “age-friendly, whole-person care” that dermatologists must embrace as older adults become an increasingly large subset of their patient population, Christina Prather, MD, director and associate professor of geriatrics and palliative medicine at George Washington University, said at the meeting.
By 2040, projections are that the number of adults aged 85 years and older in the United States will be nearly quadruple the number in 2000, according to one estimate.
“We know that there are less than 6000 practicing geriatricians in the country ... [so the healthcare system] needs more of you who know how to bring an age-friendly approach to care,” Dr. Prather said. Dermatology is among the specialties that need to be “geriatricized.”
NMSC Increasing in the Older Population
The incidence of skin cancer is rising faster than that of any other cancer, Dr. Patel said. One window into the epidemiology, he said, comes from recently published data showing that an average of 6.1 million adults were treated each year for skin cancer during 2016-2018 (5.2 million of them for NMSC) — an increase from an average of 5.8 million annually in the 2012-2015 period. The data come from the Medical Expenditure Panel Survey (MEPS), which is conducted by the US Public Health Service through the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention.
As a frame of reference, the average number of adults treated each year for nonskin cancers during these periods rose from 10.8 to 11.9 million, according to the 2023 MEPS data. “Skin cancer is about one-third of all cancers combined,” Dr. Patel said.
Not only is the incidence of NMSC significantly higher than that of melanoma but it also shows a more prominent aging trend. This was documented recently in a long-term observational study from Japan, in which researchers looked at the change in the median age of patients with NMSC and melanoma, compared with cancers of other organs, from 1991 to 2020 and found that NMSC had by far the greatest rise in median age, to a median age of 80 years in 2021.
Even more notable, Dr. Patel said, was a particularly marked increase in the number of patients with skin cancer aged 90 years and older. In 2021, this group of older adults accounted for 17% of patients receiving treatment for skin cancer at the Japanese hospital where the data were collected.
The 2013 study that stirred Dr. Patel as a fellow was of 1536 consecutive patients diagnosed with NMSC at two dermatology clinics (a University of California San Francisco–based private clinic and a Veterans Affairs Medical Center clinic) and followed for 6 years. “What’s interesting and worth thinking about is that, regardless of patients’ life expectancy, NMSCs were treated aggressively and surgically, and the choice of surgery was not influenced by the patient’s poor prognosis in a multivariate model” adjusted for tumor and patient characteristics, he said at the meeting.
The researchers defined limited life expectancy as either 85 years or older or having a Charleston Comorbidity Index ≥ 3. Approximately half of the patients with limited life expectancy died within 5 years, none of NMSC. Most patients with limited life expectancy were not often bothered by their tumors, and approximately one in five reported a treatment complication within 2 years. The 5-year tumor recurrence rate was 3.7%.
A more recent study looked at 1181 patients older than 85 years with NMSC referred for Mohs surgery. Almost all patients in the multicenter, prospective cohort study (91.3%) were treated with Mohs.
Treated patients were more likely to have facial tumors and higher functional status than those not treated with Mohs surgery, and the most common reasons provided by surgeons for proceeding with the surgery were a patient desire for a high cure rate (66%), higher functional status for age (57%), and high-risk tumor type (40%). Almost 42% of the referred patients were 89 years or older.
“Granted, [the reasons] are justified indications for surgery,” Dr. Patel said. Yet the study brings up the question of “whether we need to do Mohs surgery this frequently in elderly patients?” In an email after the meeting, he added, “it’s a question we may need to reconsider as the elderly population continues to increase and median age of NMSC rises.”
Underutilized Management Options for NMSC
In his practice, discussions of treatment options are preceded by a thorough discussion of the disease itself. Many lesions are low risk, and helping patients understand risks, as well as understanding what is important to the patient — especially those with limited life expectancy — will guide shared decision-making to choose the best treatment, Dr. Patel said at the meeting.
The dermatologist’s risk assessment — both staging and stratifying risk as it relates to specific outcomes such as recurrence, metastases, or death — takes on added importance in the older patient, he emphasized. “I think we underutilize the risk assessment.”
Also underutilized is the option of shave removal for low-risk squamous cell carcinomas and basal cell carcinomas, Dr. Patel said, noting that, in the National Comprehensive Cancer Network guidelines, “there’s an option for shave removal and nothing more if you have clear margins.”
Alternatively, disc excision with the initial biopsy can often be considered. “Having that intent to treat at the time of biopsy may be all that needs to be done” in older patients with obvious or highly suspicious lesions, he said.
Systemic immunotherapy has joined the treatment armamentarium for advanced basal cell carcinoma and advanced cutaneous squamous cell carcinoma, and if early, ongoing research of intralesional programmed cell death protein 1 inhibitor treatment advances, this could be another option for older adults in the future, Dr. Patel said. Targeting drug delivery directly to the tumor would lower the total dose, decrease systemic exposure, and could be used to avoid surgery for some groups of patients, such as those with limited life expectancy.
A Personal Story, a Word on Melanoma
Dr. Prather recalled when her 97-year-old grandfather had a skin lesion on his forehead removed, and a conversation he had with her mother about whether he really needed to have the procedure because he had cognitive impairment and was on oral anticoagulants.
The clinician “said it absolutely had to go. ... I can’t tell you how much his doctors’ visits and wound care consumed my family’s life for the next few years — for this thing that never quite healed,” she said.
“Was it necessary? The more I’ve learned over time is that it wasn’t,” Dr. Prather added. “We have to take time [with our older patients] and think critically. What is feasible? What makes the most sense? What is the most important thing I need to know about the patient?”
Also important, Dr. Patel noted, is the big-picture consideration of skin cancer treatment costs. The MEPS survey data showing the rising prevalence of skin cancer treatment also documented the economic burden: A nearly 30% increase in the average annual cost of treating NMSC from $5 billion in 2012-2015 to $6.5 billion in 2016-2018. (The average annual costs of treating melanoma decreased slightly.) “Skin cancer is a big drain on our limited resources,” he said.
With melanoma as well, dermatologists must think critically and holistically about the individual patient — and not have “a single view lens of the disease and how we treat the disease,” said Dr. Patel, urging the audience to read a “Sounding Board” article published in The New England Journal of Medicine in 2021. The article argued that there is overdiagnosis of cutaneous melanoma stemming from increased screening, falling clinical thresholds for biopsy, and falling pathological thresholds for labeling morphologic changes as cancer.
“There’s a diagnostic disconnect and a problem of overdiagnosis ... because we’re afraid to miss or make a mistake,” he said. “It leads to the question, do all lesions denoted as skin cancers need aggressive treatment? What does it mean for the patient in front of you?”
Dr. Patel reported receiving honoraria from Regeneron, Almirall, Biofrontera, Sun Pharma, and SkylineDx and serving on the speaker bureau of Regeneron and Almirall. He is chief medical officer for Lazarus AI and is cofounder of the Skin Cancer Outcomes consortium. Dr. Prather disclosed relationships with the National Institutes of Health, AHRQ, The Washington Home Foundation, and the Alzheimer’s Association.
A version of this article appeared on Medscape.com.
WASHINGTON — In 2013, Vishal A. Patel, MD, was completing a fellowship in Mohs surgery and cutaneous oncology at Columbia University Irving Medical Center, New York City, when a study was published showing that most nonmelanoma skin cancers (NMSCs) were treated with surgery, regardless of the patient’s life expectancy. Life expectancy “should enter into treatment decisions,” the authors concluded.
“ Dr. Patel recalled at the ElderDerm conference hosted by the Department of Dermatology at George Washington University, Washington, DC, and described as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Today, however, more than a decade later, guidelines still promote surgical therapy as the gold standard across the board, and questions raised by the study are still unaddressed, Dr. Patel, associate professor of dermatology and medicine/oncology at George Washington University, said at the meeting. These questions are becoming increasingly urgent as the incidence of skin cancer, especially NMSC, rises in the older adult population, especially in patients older than 85 years. “It’s a function of our training and our treatment guidelines that we reach for the most definitive treatment, which happens to be the most aggressive, in these patients,” added Dr. Patel, who is also director of the cutaneous oncology program at the GW Cancer Center.
“Sometimes we lose track of what ... we need to do” to provide care that reflects the best interests of the older patient, he continued. “Surgery may be the gold standard for treating the majority of NMSCs ... but is it the [best option] for what our older patients and patients with limited life expectancy need?”
Learning about what truly matters to the patient is a key element of the “age-friendly, whole-person care” that dermatologists must embrace as older adults become an increasingly large subset of their patient population, Christina Prather, MD, director and associate professor of geriatrics and palliative medicine at George Washington University, said at the meeting.
By 2040, projections are that the number of adults aged 85 years and older in the United States will be nearly quadruple the number in 2000, according to one estimate.
“We know that there are less than 6000 practicing geriatricians in the country ... [so the healthcare system] needs more of you who know how to bring an age-friendly approach to care,” Dr. Prather said. Dermatology is among the specialties that need to be “geriatricized.”
NMSC Increasing in the Older Population
The incidence of skin cancer is rising faster than that of any other cancer, Dr. Patel said. One window into the epidemiology, he said, comes from recently published data showing that an average of 6.1 million adults were treated each year for skin cancer during 2016-2018 (5.2 million of them for NMSC) — an increase from an average of 5.8 million annually in the 2012-2015 period. The data come from the Medical Expenditure Panel Survey (MEPS), which is conducted by the US Public Health Service through the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention.
As a frame of reference, the average number of adults treated each year for nonskin cancers during these periods rose from 10.8 to 11.9 million, according to the 2023 MEPS data. “Skin cancer is about one-third of all cancers combined,” Dr. Patel said.
Not only is the incidence of NMSC significantly higher than that of melanoma but it also shows a more prominent aging trend. This was documented recently in a long-term observational study from Japan, in which researchers looked at the change in the median age of patients with NMSC and melanoma, compared with cancers of other organs, from 1991 to 2020 and found that NMSC had by far the greatest rise in median age, to a median age of 80 years in 2021.
Even more notable, Dr. Patel said, was a particularly marked increase in the number of patients with skin cancer aged 90 years and older. In 2021, this group of older adults accounted for 17% of patients receiving treatment for skin cancer at the Japanese hospital where the data were collected.
The 2013 study that stirred Dr. Patel as a fellow was of 1536 consecutive patients diagnosed with NMSC at two dermatology clinics (a University of California San Francisco–based private clinic and a Veterans Affairs Medical Center clinic) and followed for 6 years. “What’s interesting and worth thinking about is that, regardless of patients’ life expectancy, NMSCs were treated aggressively and surgically, and the choice of surgery was not influenced by the patient’s poor prognosis in a multivariate model” adjusted for tumor and patient characteristics, he said at the meeting.
The researchers defined limited life expectancy as either 85 years or older or having a Charleston Comorbidity Index ≥ 3. Approximately half of the patients with limited life expectancy died within 5 years, none of NMSC. Most patients with limited life expectancy were not often bothered by their tumors, and approximately one in five reported a treatment complication within 2 years. The 5-year tumor recurrence rate was 3.7%.
A more recent study looked at 1181 patients older than 85 years with NMSC referred for Mohs surgery. Almost all patients in the multicenter, prospective cohort study (91.3%) were treated with Mohs.
Treated patients were more likely to have facial tumors and higher functional status than those not treated with Mohs surgery, and the most common reasons provided by surgeons for proceeding with the surgery were a patient desire for a high cure rate (66%), higher functional status for age (57%), and high-risk tumor type (40%). Almost 42% of the referred patients were 89 years or older.
“Granted, [the reasons] are justified indications for surgery,” Dr. Patel said. Yet the study brings up the question of “whether we need to do Mohs surgery this frequently in elderly patients?” In an email after the meeting, he added, “it’s a question we may need to reconsider as the elderly population continues to increase and median age of NMSC rises.”
Underutilized Management Options for NMSC
In his practice, discussions of treatment options are preceded by a thorough discussion of the disease itself. Many lesions are low risk, and helping patients understand risks, as well as understanding what is important to the patient — especially those with limited life expectancy — will guide shared decision-making to choose the best treatment, Dr. Patel said at the meeting.
The dermatologist’s risk assessment — both staging and stratifying risk as it relates to specific outcomes such as recurrence, metastases, or death — takes on added importance in the older patient, he emphasized. “I think we underutilize the risk assessment.”
Also underutilized is the option of shave removal for low-risk squamous cell carcinomas and basal cell carcinomas, Dr. Patel said, noting that, in the National Comprehensive Cancer Network guidelines, “there’s an option for shave removal and nothing more if you have clear margins.”
Alternatively, disc excision with the initial biopsy can often be considered. “Having that intent to treat at the time of biopsy may be all that needs to be done” in older patients with obvious or highly suspicious lesions, he said.
Systemic immunotherapy has joined the treatment armamentarium for advanced basal cell carcinoma and advanced cutaneous squamous cell carcinoma, and if early, ongoing research of intralesional programmed cell death protein 1 inhibitor treatment advances, this could be another option for older adults in the future, Dr. Patel said. Targeting drug delivery directly to the tumor would lower the total dose, decrease systemic exposure, and could be used to avoid surgery for some groups of patients, such as those with limited life expectancy.
A Personal Story, a Word on Melanoma
Dr. Prather recalled when her 97-year-old grandfather had a skin lesion on his forehead removed, and a conversation he had with her mother about whether he really needed to have the procedure because he had cognitive impairment and was on oral anticoagulants.
The clinician “said it absolutely had to go. ... I can’t tell you how much his doctors’ visits and wound care consumed my family’s life for the next few years — for this thing that never quite healed,” she said.
“Was it necessary? The more I’ve learned over time is that it wasn’t,” Dr. Prather added. “We have to take time [with our older patients] and think critically. What is feasible? What makes the most sense? What is the most important thing I need to know about the patient?”
Also important, Dr. Patel noted, is the big-picture consideration of skin cancer treatment costs. The MEPS survey data showing the rising prevalence of skin cancer treatment also documented the economic burden: A nearly 30% increase in the average annual cost of treating NMSC from $5 billion in 2012-2015 to $6.5 billion in 2016-2018. (The average annual costs of treating melanoma decreased slightly.) “Skin cancer is a big drain on our limited resources,” he said.
With melanoma as well, dermatologists must think critically and holistically about the individual patient — and not have “a single view lens of the disease and how we treat the disease,” said Dr. Patel, urging the audience to read a “Sounding Board” article published in The New England Journal of Medicine in 2021. The article argued that there is overdiagnosis of cutaneous melanoma stemming from increased screening, falling clinical thresholds for biopsy, and falling pathological thresholds for labeling morphologic changes as cancer.
“There’s a diagnostic disconnect and a problem of overdiagnosis ... because we’re afraid to miss or make a mistake,” he said. “It leads to the question, do all lesions denoted as skin cancers need aggressive treatment? What does it mean for the patient in front of you?”
Dr. Patel reported receiving honoraria from Regeneron, Almirall, Biofrontera, Sun Pharma, and SkylineDx and serving on the speaker bureau of Regeneron and Almirall. He is chief medical officer for Lazarus AI and is cofounder of the Skin Cancer Outcomes consortium. Dr. Prather disclosed relationships with the National Institutes of Health, AHRQ, The Washington Home Foundation, and the Alzheimer’s Association.
A version of this article appeared on Medscape.com.
Magnesium Sulfate’s Ability to Reduce Cerebral Palsy in Preterm Birth Reaffirmed
An updated Cochrane Systematic Review of magnesium sulfate administered before preterm birth for neuroprotection has reaffirmed that the compound significantly reduces the risk of cerebral palsy and has added the finding that it also may reduce the risk of severe neonatal intraventricular hemorrhage.
Still unknown, however, is whether the effects of magnesium sulfate vary according to patient characteristics such as gestational age, or by treatment characteristics such as timing and dose. “We need further research to determine exactly who to treat, and when and how, to ideally standardize clinical practice recommendations across the world,” said Emily S. Shepherd, PhD, lead author of the review.
Magnesium sulfate is widely used for preterm cerebral palsy prevention but variance in national and local recommendations for its use may impede its optimal uptake in some places, she and her co-investigators wrote in the review.
In the United States, the American College of Obstetricians and Gynecologists advises institutions to develop their own guidelines regarding inclusion criteria and treatment regimens “in accordance with one of the larger trials.” (ACOG’s Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection was originally published in 2010 and was reaffirmed in 2023.)
In a Master Class column on magnesium sulfate for neuroprotection published earlier this year in Ob.Gyn. News, Irina Burd, MD, PhD, wrote that most hospitals in the United States have chosen a higher dose of magnesium sulfate administered up to 31 weeks’ gestation (6-g bolus, followed by 2 g/hour), in keeping with the protocols used in the BEAM trial published by the National Institute of Child Health and Human Development (NICHD). Dr. Burd is the Sylvan Frieman, MD, Endowed Professor and chair of the department of obstetrics, gynecology and reproductive sciences at the University of Maryland School of Medicine, Baltimore, Maryland.
The new Cochrane review included six randomized controlled trials (including the NICHD trial) covering 5917 pregnant participants and 6759 fetuses. Eligibility criteria varied, but all the RCTs included patients in preterm labor or with expected or planned imminent preterm birth at less than 34 weeks’ gestation.
Treatment regimens varied: three trials administered a 4-g loading dose only, and three included a maintenance dose (a 4-6-g loading dose and a 1-2 g/hour maintenance dose). “Although we attempted to explore variation through subgroup analyses, the ability to do this was limited,” the researchers wrote.
Up to 2 years of corrected age, magnesium sulfate reduced the risk of cerebral palsy compared with placebo (relative risk, 0.71; 95% confidence interval (CI), 0.57-0.89) and death or cerebral palsy (RR, 0.87; 95% CI, 0.77-0.98), with a high-certainty grade of evidence. The number needed to treat to prevent one case of cerebral palsy was 60 and the number needed to treat death or cerebral palsy was 56. The impact on severe intracranial hemorrhage (RR, 0.76; 95% CI, 0.60-0.98), a secondary outcome, was backed by moderate-certainty evidence.
Compared with the 2009 Cochrane review, the new study includes two new randomized controlled trials. One of which, the MAGENTA trial, administered magnesium sulfate at 30-34 weeks gestation and included new school-age follow-up data from two previously included trials. While the available data suggest little to no difference in outcomes at school age, more follow-up data are needed to assess this with greater certainty, the reviewers wrote.
While severe adverse outcomes (death, cardiac or respiratory arrest) for pregnant individuals appear not to have increased in pregnant patients who received magnesium sulfate (low-certainty evidence), the compound “probably increased maternal adverse effects severe enough to stop treatment,” the reviewers report (average RR, 3.21; 95% CI, 1.88-5.48; moderate-certainty evidence).
Side effects that were more frequent among women receiving magnesium sulfate include hypotension, tachycardia, warmth over body/flushing, nausea or vomiting, sweating, and dizziness.
“Treatment cessation due to such side effects was in the context of trials being conducted to establish benefit,” noted Dr. Shepherd, of the University of Adelaide in Australia. “With benefit now shown, these side effects may be viewed as comparatively minor/generally tolerable considering the potential benefits for children.”
Proving the neuroprotective value of magnesium sulfate took many years, Dr. Burd explained in the Master Class, as none of the randomized controlled trials analyzed in eventual meta-analyses and systematic reviews had reached their primary endpoints. It wasn’t until researchers obtained unpublished data and conducted these analyses and reviews that a significant effect of magnesium sulfate on cerebral palsy could be seen. Dr. Burd and other researchers are now working to better understand its biologic plausibility and precise mechanisms of action.
Dr. Shepherd disclosed that she is a former editor for Cochrane Pregnancy and Childbirth and current sign-off editor for Cochrane Central Editorial Service but reported having no involvement in the editorial processing of the review. Other authors disclosed that they were investigators for included trials and/or have published opinions in medical journals related to magnesium sulfate to reduce cerebral palsy. Dr. Burd reported no disclosures.
An updated Cochrane Systematic Review of magnesium sulfate administered before preterm birth for neuroprotection has reaffirmed that the compound significantly reduces the risk of cerebral palsy and has added the finding that it also may reduce the risk of severe neonatal intraventricular hemorrhage.
Still unknown, however, is whether the effects of magnesium sulfate vary according to patient characteristics such as gestational age, or by treatment characteristics such as timing and dose. “We need further research to determine exactly who to treat, and when and how, to ideally standardize clinical practice recommendations across the world,” said Emily S. Shepherd, PhD, lead author of the review.
Magnesium sulfate is widely used for preterm cerebral palsy prevention but variance in national and local recommendations for its use may impede its optimal uptake in some places, she and her co-investigators wrote in the review.
In the United States, the American College of Obstetricians and Gynecologists advises institutions to develop their own guidelines regarding inclusion criteria and treatment regimens “in accordance with one of the larger trials.” (ACOG’s Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection was originally published in 2010 and was reaffirmed in 2023.)
In a Master Class column on magnesium sulfate for neuroprotection published earlier this year in Ob.Gyn. News, Irina Burd, MD, PhD, wrote that most hospitals in the United States have chosen a higher dose of magnesium sulfate administered up to 31 weeks’ gestation (6-g bolus, followed by 2 g/hour), in keeping with the protocols used in the BEAM trial published by the National Institute of Child Health and Human Development (NICHD). Dr. Burd is the Sylvan Frieman, MD, Endowed Professor and chair of the department of obstetrics, gynecology and reproductive sciences at the University of Maryland School of Medicine, Baltimore, Maryland.
The new Cochrane review included six randomized controlled trials (including the NICHD trial) covering 5917 pregnant participants and 6759 fetuses. Eligibility criteria varied, but all the RCTs included patients in preterm labor or with expected or planned imminent preterm birth at less than 34 weeks’ gestation.
Treatment regimens varied: three trials administered a 4-g loading dose only, and three included a maintenance dose (a 4-6-g loading dose and a 1-2 g/hour maintenance dose). “Although we attempted to explore variation through subgroup analyses, the ability to do this was limited,” the researchers wrote.
Up to 2 years of corrected age, magnesium sulfate reduced the risk of cerebral palsy compared with placebo (relative risk, 0.71; 95% confidence interval (CI), 0.57-0.89) and death or cerebral palsy (RR, 0.87; 95% CI, 0.77-0.98), with a high-certainty grade of evidence. The number needed to treat to prevent one case of cerebral palsy was 60 and the number needed to treat death or cerebral palsy was 56. The impact on severe intracranial hemorrhage (RR, 0.76; 95% CI, 0.60-0.98), a secondary outcome, was backed by moderate-certainty evidence.
Compared with the 2009 Cochrane review, the new study includes two new randomized controlled trials. One of which, the MAGENTA trial, administered magnesium sulfate at 30-34 weeks gestation and included new school-age follow-up data from two previously included trials. While the available data suggest little to no difference in outcomes at school age, more follow-up data are needed to assess this with greater certainty, the reviewers wrote.
While severe adverse outcomes (death, cardiac or respiratory arrest) for pregnant individuals appear not to have increased in pregnant patients who received magnesium sulfate (low-certainty evidence), the compound “probably increased maternal adverse effects severe enough to stop treatment,” the reviewers report (average RR, 3.21; 95% CI, 1.88-5.48; moderate-certainty evidence).
Side effects that were more frequent among women receiving magnesium sulfate include hypotension, tachycardia, warmth over body/flushing, nausea or vomiting, sweating, and dizziness.
“Treatment cessation due to such side effects was in the context of trials being conducted to establish benefit,” noted Dr. Shepherd, of the University of Adelaide in Australia. “With benefit now shown, these side effects may be viewed as comparatively minor/generally tolerable considering the potential benefits for children.”
Proving the neuroprotective value of magnesium sulfate took many years, Dr. Burd explained in the Master Class, as none of the randomized controlled trials analyzed in eventual meta-analyses and systematic reviews had reached their primary endpoints. It wasn’t until researchers obtained unpublished data and conducted these analyses and reviews that a significant effect of magnesium sulfate on cerebral palsy could be seen. Dr. Burd and other researchers are now working to better understand its biologic plausibility and precise mechanisms of action.
Dr. Shepherd disclosed that she is a former editor for Cochrane Pregnancy and Childbirth and current sign-off editor for Cochrane Central Editorial Service but reported having no involvement in the editorial processing of the review. Other authors disclosed that they were investigators for included trials and/or have published opinions in medical journals related to magnesium sulfate to reduce cerebral palsy. Dr. Burd reported no disclosures.
An updated Cochrane Systematic Review of magnesium sulfate administered before preterm birth for neuroprotection has reaffirmed that the compound significantly reduces the risk of cerebral palsy and has added the finding that it also may reduce the risk of severe neonatal intraventricular hemorrhage.
Still unknown, however, is whether the effects of magnesium sulfate vary according to patient characteristics such as gestational age, or by treatment characteristics such as timing and dose. “We need further research to determine exactly who to treat, and when and how, to ideally standardize clinical practice recommendations across the world,” said Emily S. Shepherd, PhD, lead author of the review.
Magnesium sulfate is widely used for preterm cerebral palsy prevention but variance in national and local recommendations for its use may impede its optimal uptake in some places, she and her co-investigators wrote in the review.
In the United States, the American College of Obstetricians and Gynecologists advises institutions to develop their own guidelines regarding inclusion criteria and treatment regimens “in accordance with one of the larger trials.” (ACOG’s Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection was originally published in 2010 and was reaffirmed in 2023.)
In a Master Class column on magnesium sulfate for neuroprotection published earlier this year in Ob.Gyn. News, Irina Burd, MD, PhD, wrote that most hospitals in the United States have chosen a higher dose of magnesium sulfate administered up to 31 weeks’ gestation (6-g bolus, followed by 2 g/hour), in keeping with the protocols used in the BEAM trial published by the National Institute of Child Health and Human Development (NICHD). Dr. Burd is the Sylvan Frieman, MD, Endowed Professor and chair of the department of obstetrics, gynecology and reproductive sciences at the University of Maryland School of Medicine, Baltimore, Maryland.
The new Cochrane review included six randomized controlled trials (including the NICHD trial) covering 5917 pregnant participants and 6759 fetuses. Eligibility criteria varied, but all the RCTs included patients in preterm labor or with expected or planned imminent preterm birth at less than 34 weeks’ gestation.
Treatment regimens varied: three trials administered a 4-g loading dose only, and three included a maintenance dose (a 4-6-g loading dose and a 1-2 g/hour maintenance dose). “Although we attempted to explore variation through subgroup analyses, the ability to do this was limited,” the researchers wrote.
Up to 2 years of corrected age, magnesium sulfate reduced the risk of cerebral palsy compared with placebo (relative risk, 0.71; 95% confidence interval (CI), 0.57-0.89) and death or cerebral palsy (RR, 0.87; 95% CI, 0.77-0.98), with a high-certainty grade of evidence. The number needed to treat to prevent one case of cerebral palsy was 60 and the number needed to treat death or cerebral palsy was 56. The impact on severe intracranial hemorrhage (RR, 0.76; 95% CI, 0.60-0.98), a secondary outcome, was backed by moderate-certainty evidence.
Compared with the 2009 Cochrane review, the new study includes two new randomized controlled trials. One of which, the MAGENTA trial, administered magnesium sulfate at 30-34 weeks gestation and included new school-age follow-up data from two previously included trials. While the available data suggest little to no difference in outcomes at school age, more follow-up data are needed to assess this with greater certainty, the reviewers wrote.
While severe adverse outcomes (death, cardiac or respiratory arrest) for pregnant individuals appear not to have increased in pregnant patients who received magnesium sulfate (low-certainty evidence), the compound “probably increased maternal adverse effects severe enough to stop treatment,” the reviewers report (average RR, 3.21; 95% CI, 1.88-5.48; moderate-certainty evidence).
Side effects that were more frequent among women receiving magnesium sulfate include hypotension, tachycardia, warmth over body/flushing, nausea or vomiting, sweating, and dizziness.
“Treatment cessation due to such side effects was in the context of trials being conducted to establish benefit,” noted Dr. Shepherd, of the University of Adelaide in Australia. “With benefit now shown, these side effects may be viewed as comparatively minor/generally tolerable considering the potential benefits for children.”
Proving the neuroprotective value of magnesium sulfate took many years, Dr. Burd explained in the Master Class, as none of the randomized controlled trials analyzed in eventual meta-analyses and systematic reviews had reached their primary endpoints. It wasn’t until researchers obtained unpublished data and conducted these analyses and reviews that a significant effect of magnesium sulfate on cerebral palsy could be seen. Dr. Burd and other researchers are now working to better understand its biologic plausibility and precise mechanisms of action.
Dr. Shepherd disclosed that she is a former editor for Cochrane Pregnancy and Childbirth and current sign-off editor for Cochrane Central Editorial Service but reported having no involvement in the editorial processing of the review. Other authors disclosed that they were investigators for included trials and/or have published opinions in medical journals related to magnesium sulfate to reduce cerebral palsy. Dr. Burd reported no disclosures.
COCHRANE DATABASE SYSTEMATIC REVIEW
Study Highlights Atopic Dermatitis Features, Treatments Among Older Patients
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
.
The researchers reviewed charts of patients aged 60 years and older who were seen at either a private or county dermatology clinic in Houston between 2009 and 2020 and had been diagnosed with AD by a dermatologist. The findings of their cross-sectional study further supports that AD in this age group “presents as a unique phenotype compared to AD in younger ages, which may inform dermatologists’ diagnosis of AD in these patients” they wrote.
The 791 patients in the study had an average age of 69.3 years, were predominantly women (60.1%), and were racially diverse, with almost 40% being non-Hispanic White individuals. Others were non-Hispanic Black individuals (21.8%), Hispanics (20.4%), and non-Hispanic Asian/Pacific Islanders (11.7%).
Use of topicals, mainly topical corticosteroids (92.2%), was the most frequent treatment prescribed. Oral corticosteroids and antihistamines were “frequent systemic treatments” in this population, prescribed to 10.4% and 12.1%, respectively, “likely due to management prior to a diagnosis of AD by a dermatologist,” wrote first author Hannah Y. Wang, Baylor College of Medicine, Houston, and her coauthors, including Soo Jung Kim, MD, PhD, of the department of dermatology at Baylor.
Other treatments included dupilumab in 5.4%, systemic immunosuppressants (including methotrexate, cyclosporine, and mycophenolate) in 5.4%, and UVB-phototherapy in 2.7%.
Approximately 40% of the patients had a history of allergic rhinitis, while 20% had a history of asthma. Lichenification was noted in 14.5% of patients and nummular lesions in almost 13%. Other rash characteristics — ichthyosis and hyperpigmented patches — were less frequent, seen in 9.7% and 9.1%, respectively.
AD in this older population was most commonly documented on the extensors (49.9%) and the trunk (46%) and less commonly on the hands (19.8%) and feet (9%) — a distribution that is similar to past reports, the authors wrote.
Asked to comment on the findings, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization that the data relating to clinical morphology are consistent with past reports and with his own experiences. Lichenification is a “tell-tale sign of chronic disease” and may indicate undertreatment, and the frequency of nummular plaques is unsurprising because “nummular dermatitis as an independent eczema tends to occur more so in the elderly.”
More important, he said, was the finding regarding the use of oral corticosteroid and antihistamine, “both of which are advocated against in the management of AD.”
More research is “needed to elucidate the unique features of elderly AD in pathophysiology and optimal treatments,” the authors wrote, noting that age-related factors potentially affecting AD in this population include reduced skin barrier function, immune dysregulation, and environmental exposures.
The study, Dr. Friedman said, “shines a spotlight on this demographic — they exist, they suffer, and they are at times being managed with less-than-optimal options.” Clinical trials of “the welcome additions to our historically limited armament often lack a substantial elderly study population,” he said, and Medicare makes it “painful to get these game-changing drugs for this large patient population.”
The study authors and Dr. Friedman, who was not involved with the study, reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAAD INTERNATIONAL
Risk of Knee OA From Weight-Bearing Exercise Seen Only With Low Muscle Mass
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
Weight-bearing recreational activity was associated with a 22% increased odds of developing knee osteoarthritis (OA) in a large prospective cohort study in the Netherlands, but notably, the increased risk was seen only in those with low levels of lower-limb muscle mass.
The findings point toward the value of “tailored advice” for physical activity, and suggest that “caution is needed when engaging in weight-bearing activity, especially for individuals with low levels of lower-limb muscle mass,” Yahong Wu, MD, and coinvestigators, of the Erasmus Medical Center in Rotterdam, the Netherlands, wrote in JAMA Network Open.
Investigators used data from sequential cohorts of the longitudinal Rotterdam Study, which enrolled people aged 45 and older starting in 1990. The 5003 participants in this new analysis of physical activity and knee OA had complete records of baseline recreational physical activity, baseline knee pain, and knee radiographs from both baseline and at least one follow-up exam. Those with radiographically defined knee OA at baseline were excluded.
The incident rate of radiographically defined (x-ray) knee OA among all participants was 8.4%, with a mean follow-up time of 6.33 years. Among 3492 individuals without baseline knee pain, the researchers found no increased odds of incident radiographic OA with non–weight-bearing activity (odds ratio [OR], 1.04; 95% CI, 0.95-1.15; P = .37) but a significant association of weight-bearing activity with OA incidence (OR, 1.22; 95% CI, 1.10-1.35; P < .001).
A stratification analysis of a subset of participants whose lower-limb mass had been measured by dual-energy x-ray absorptiometry (DXA) showed, however, that the association of weight-bearing activity with incident OA was limited to patients in the lowest third of lower-limb muscle mass index (LMI), who had a 53% increased likelihood of developing knee OA (OR, 1.53; 95% CI, 1.15-2.04; P = .003).
For patients in the middle and upper tertiles, there was no significant association between weight-bearing activity and the odds of incident OA (OR, 0.93; P = .73, and OR, 1.15; P = .40, respectively).
The findings are reassuring overall, said Kelli D. Allen, PhD, research professor of medicine and exercise physiologist at the University of North Carolina at Chapel Hill, who was asked to comment on the study. “The study corroborates prior research showing that for most people, weight-bearing recreational activity does not increase the risk of knee osteoarthritis. This should be encouraging for people who want to increase their physical activity,” she said.
The study also suggests that “for people with low lower-limb muscle mass, there may be some considerations to make regarding the best type of physical activity to prevent future knee osteoarthritis,” she said in an e-mail. “The best approach may include non–weight-bearing activities, which could include biking, swimming, or other water exercises, along with strengthening exercises that help to increase muscle mass.”
Other studies, Dr. Allen said, have shown that low muscle mass itself is a risk factor for knee OA.
Physical Activity Types, Other Analyses
The researchers assessed total, weight-bearing, and non–weight-bearing physical activity using two validated questionnaires (an adapted version of the Zutphen Physical Activity Questionnaire and the Longitudinal Aging Study Amsterdam physical activity questionnaire) that asked participants about the frequency and duration of various types of physical activity. Activity was quantified as metabolic equivalent of task (MET) hours per week, and weight-bearing activities were defined as those in which the knee joint bears the body’s weight.
Walking, gardening, golf, dancing, and ball sports were among the activities qualifying as weight-bearing activities. Non–weight-bearing activities included cycling, rowing, and swimming.
Sex, body mass index, and follow-up time were among the covariates adjusted for in the primary analysis. Similar results were found when adjustments were also made for educational level, alcohol intake, lipid levels, and diabetes.
While incident radiographic knee OA (measured using the Kellgren & Lawrence grading system) was the primary outcome, the researchers also looked at symptomatic knee OA, as defined by x-ray and a knee pain questionnaire, and found no significant association of its incidence with any of the exercise categories (total, weight-bearing, or non-weight-bearing).
Coauthor Joyce B. J. van Meurs, PhD, of the departments of internal medicine and orthopedics & sports medicine at Erasmus Medical Center, told this news organization that “pain as a subjective, recurrent symptom is more difficult to study … [and] a larger sample size or more precise measurements [of pain] in future studies would help to better understand the true association” of symptomatic knee OA and physical activity.
Similarly, analyses of the 1511 patients (out of 5003) who had knee pain at baseline found no significant association of weight-bearing or non–weight-bearing physical activity with incident radiographic knee OA. The trends were similar to those found in the population without knee pain, however, which suggests the analysis was underpowered, the researchers wrote, noting too that patients with baseline pain had lower activity levels than those without pain. (Low case numbers precluded a stratification analysis on LMI for incident symptomatic OA.)
Thigh Circumference as an Indicator of Muscle Mass
The findings build upon an international meta-analysis published in 2021 that found no association between total physical activity and knee OA and align with other studies suggesting a link between greater mechanical stress/strain and greater OA risk, the researchers wrote. (The meta-analysis couldn’t investigate different types of activity.)
“Although we cannot establish a causal relationship … we hypothesize that the mechanical loading on joints and cartilage could explain the association of weight-bearing activity with osteoarthritis in the low LMI tertile group,” they said.
It is possible that thigh muscle-specific strength or mass may temper the risk of knee OA, they wrote, but the lack of thigh strength data in the Rotterdam Study precluded such evaluation. Still, in everyday practice, the researchers noted, lower limb muscle function could be assessed using thigh circumference.
Dr. Allen agreed. “ ‘Gold standard’ assessment of muscle mass is not common in routine practice, but clinicians can evaluate muscle mass in other ways, such as thigh circumference,” she told this news organization, noting that measurement should align with procedures described by the National Health and Nutrition Examination Survey in its anthropometry procedures manual.
“If low lower-limb muscle mass is suspected, a referral to a physical therapist can be helpful for more formally assessing muscle mass and muscle strength,” she added, “and for instructions for a safe and appropriate exercise program for building muscle and protecting joints.”
Among other limitations of the study, according to the researchers, are an ethnically nondiverse population, the unavailability of knee injury data, and the assessment of physical activity only at baseline.
Moving forward, Dr. van Meurs told this news organization, “the main question regarding physical activity and OA is still, if people already have pain or early OA complaints, what kinds of sports they can do without hurting their joints?” This “should be tested,” she said, “in a real-life, ideally trial-like intervention study.”
The study was funded by the Erasmus Medical Center and Erasmus University as well as through various government grants. Dr. Wu also had study support from the China Scholarship Council. Two of the authors reported relationships with arthritis-related organizations. Dr. Allen reported having no disclosures relevant to her comments.
FROM JAMA NETWORK OPEN
Dietary Factors Linked to Development of Spondyloarthritis, Preliminary Findings Suggest
Preliminary findings from a small case-control study at the Mayo Clinic in Rochester, Minnesota, suggest an association between diet and the development of spondyloarthritis (SpA), researchers reported in a poster at the Rheumatology Winter Clinical Symposium.
The small study involving 106 cases of incident spondyloarthritis matched 5:1 to individuals without SpA on the basis of age, sex, year, and geography found that risk was significantly higher with consumption of nondiet soda (adjusted odds ratio [aOR], 1.76), and with use of certain supplements: folate (aOR, 2.56), B vitamins (1.98), and fish oil (1.83). Moderate alcohol use ranging from two servings per month up to five per week was associated with a significantly lower risk of SpA (aOR, 0.63).
“We have seen an association between diet and RA. There is also strong literature showing an association between the microbiome and spondyloarthritis. Putting these two together, we wanted to see if the same was true for spondyloarthritis,” Vanessa Kronzer, MD, a rheumatologist at Mayo Clinic and a coauthor of the poster, said in an email. “Our results … do suggest an association between diet and developing spondyloarthritis as we suspected, for example, with soda.”
The researchers enrolled patients through the Mayo Clinic Biobank, which aims to engage a population-based sample of primary care patients, and administered questionnaires that assessed dietary and supplement exposures. They identified incident SpA using two diagnosis codes for ankylosing spondylitis or PsA ≥ 30 days apart along with use of disease-modifying antirheumatic drugs. To identify inflammatory bowel disease–associated SpAs, they used two diagnosis codes ≥ 30 days apart and age < 45 years. Follow-up questionnaires were administered 5 years later, Dr. Kronzer said.
Controls were matched on age, sex, year and geography. Logistic regression models adjusted for age, sex, race and ethnicity, education, and smoking, the researchers reported in their poster.
Dr. Kronzer and coauthors reported finding no significant associations with high-fat food, red meat, fish, poultry, diet soda, coffee and tea, and high alcohol use. They reported finding “trends of reduced risk with fruits and vegetables but higher risk with milk/dairy” and said these trends “should be replicated in larger studies.”
The 106 patients with incident spondyloarthritis had a mean age of 51. Three-fourths were female.
The research was funded by the Rheumatology Research Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kronzer and coauthors did not report any disclosures.
Preliminary findings from a small case-control study at the Mayo Clinic in Rochester, Minnesota, suggest an association between diet and the development of spondyloarthritis (SpA), researchers reported in a poster at the Rheumatology Winter Clinical Symposium.
The small study involving 106 cases of incident spondyloarthritis matched 5:1 to individuals without SpA on the basis of age, sex, year, and geography found that risk was significantly higher with consumption of nondiet soda (adjusted odds ratio [aOR], 1.76), and with use of certain supplements: folate (aOR, 2.56), B vitamins (1.98), and fish oil (1.83). Moderate alcohol use ranging from two servings per month up to five per week was associated with a significantly lower risk of SpA (aOR, 0.63).
“We have seen an association between diet and RA. There is also strong literature showing an association between the microbiome and spondyloarthritis. Putting these two together, we wanted to see if the same was true for spondyloarthritis,” Vanessa Kronzer, MD, a rheumatologist at Mayo Clinic and a coauthor of the poster, said in an email. “Our results … do suggest an association between diet and developing spondyloarthritis as we suspected, for example, with soda.”
The researchers enrolled patients through the Mayo Clinic Biobank, which aims to engage a population-based sample of primary care patients, and administered questionnaires that assessed dietary and supplement exposures. They identified incident SpA using two diagnosis codes for ankylosing spondylitis or PsA ≥ 30 days apart along with use of disease-modifying antirheumatic drugs. To identify inflammatory bowel disease–associated SpAs, they used two diagnosis codes ≥ 30 days apart and age < 45 years. Follow-up questionnaires were administered 5 years later, Dr. Kronzer said.
Controls were matched on age, sex, year and geography. Logistic regression models adjusted for age, sex, race and ethnicity, education, and smoking, the researchers reported in their poster.
Dr. Kronzer and coauthors reported finding no significant associations with high-fat food, red meat, fish, poultry, diet soda, coffee and tea, and high alcohol use. They reported finding “trends of reduced risk with fruits and vegetables but higher risk with milk/dairy” and said these trends “should be replicated in larger studies.”
The 106 patients with incident spondyloarthritis had a mean age of 51. Three-fourths were female.
The research was funded by the Rheumatology Research Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kronzer and coauthors did not report any disclosures.
Preliminary findings from a small case-control study at the Mayo Clinic in Rochester, Minnesota, suggest an association between diet and the development of spondyloarthritis (SpA), researchers reported in a poster at the Rheumatology Winter Clinical Symposium.
The small study involving 106 cases of incident spondyloarthritis matched 5:1 to individuals without SpA on the basis of age, sex, year, and geography found that risk was significantly higher with consumption of nondiet soda (adjusted odds ratio [aOR], 1.76), and with use of certain supplements: folate (aOR, 2.56), B vitamins (1.98), and fish oil (1.83). Moderate alcohol use ranging from two servings per month up to five per week was associated with a significantly lower risk of SpA (aOR, 0.63).
“We have seen an association between diet and RA. There is also strong literature showing an association between the microbiome and spondyloarthritis. Putting these two together, we wanted to see if the same was true for spondyloarthritis,” Vanessa Kronzer, MD, a rheumatologist at Mayo Clinic and a coauthor of the poster, said in an email. “Our results … do suggest an association between diet and developing spondyloarthritis as we suspected, for example, with soda.”
The researchers enrolled patients through the Mayo Clinic Biobank, which aims to engage a population-based sample of primary care patients, and administered questionnaires that assessed dietary and supplement exposures. They identified incident SpA using two diagnosis codes for ankylosing spondylitis or PsA ≥ 30 days apart along with use of disease-modifying antirheumatic drugs. To identify inflammatory bowel disease–associated SpAs, they used two diagnosis codes ≥ 30 days apart and age < 45 years. Follow-up questionnaires were administered 5 years later, Dr. Kronzer said.
Controls were matched on age, sex, year and geography. Logistic regression models adjusted for age, sex, race and ethnicity, education, and smoking, the researchers reported in their poster.
Dr. Kronzer and coauthors reported finding no significant associations with high-fat food, red meat, fish, poultry, diet soda, coffee and tea, and high alcohol use. They reported finding “trends of reduced risk with fruits and vegetables but higher risk with milk/dairy” and said these trends “should be replicated in larger studies.”
The 106 patients with incident spondyloarthritis had a mean age of 51. Three-fourths were female.
The research was funded by the Rheumatology Research Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kronzer and coauthors did not report any disclosures.
FROM RWCS 2024
New Research Dissects Transgenerational Obesity and Diabetes
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FROM DPSG-NA 2023
AI’s Future and Current Role in Rheumatology
The rheumatologist of the future will see patients who have been assessed and triaged with artificial intelligence utilizing data from remote kiosk-placed ultrasound scanners and physician-directed algorithms. Practices will be broadly fueled by AI, which will screen charts, produce notes, handle prior authorizations and insurance issues, aid in earlier diagnoses, find patients for clinical trials, and maybe even suggest the next best therapy for individual patients.
Such is the future envisioned by Alvin F. Wells, MD, PhD, and John J. Cush, MD, who discussed the current and forthcoming reach of AI — and their own uses of it — at the 2024 Rheumatology Winter Clinical Symposium.
“We’re not at the stage where ChatGPT and AI can tell us what the next best therapy is, but we’re getting there,” said Dr. Cush, a rheumatologist based in Dallas and executive director of RheumNow.com. For now, he said, “AI affords us a truly big-time increase in efficiency. It helps you deal with your time constraints in managing information overload and task overload.”
At a time when “PubMed doubles every 73 days ... and it’s getting harder and harder to stay abreast,” for example, new applications such as Scite, SciSpace, and Consensus can help curate, focus, and analyze the literature to match one’s own clinical interests. Such review tools are “just now getting into play and are evolving,” Dr. Cush said, noting that many but not all of them are based on ChatGPT, OpenAI’s chatbot that had a over 100 million users by January 2023 — just over a month after its version 3.5 was released.
For Dr. Wells, a rheumatologist and Midwest Region director in the department of rheumatology for the Advocate Health Medical Group in Franklin, Wisconsin, clinician-developed algorithms are helping his group assess patients — often remotely — and triage them to be seen fairly immediately by a rheumatologist versus in 4-6 weeks or in several months. “You can use AI to guide your access,” he said.
A patient “with a family history of RA, sed rate above 50, and osteopenia on x-rays” would be seen within a week, for example, while “another patient who’s had a [positive] ANA with no other symptoms, and maybe a family history, might be seen in 4-6 weeks,” said Dr. Wells, sharing his belief that “there is not a shortage of rheumatologists, [but a] shortage of using rheumatologists efficiently.”
AI for Improving Workflow
Current and future advances will enrich the intersection of AI and virtual medicine and improve outcomes and the rheumatologist-patient interaction, Dr. Wells said, pointing to research presented at the American College of Rheumatology (ACR) 2023 annual meeting on the use of computer vision technology for remotely assessing disease activity in rheumatoid arthritis (RA).
In the proof-of-concept “MeFisto” study, 28 patients with RA used an app that enabled computer vision inference of hand motion data. Upon recording, an algorithm tracked the mean degree change of joint angle on flexion and the mean time to maximal flexion for each joint.
The researchers found a strong correlation between flexion of the distal interphalangeal (DIP) joint and the Disease Activity Score in 28 joints, the Swollen Hand Joint Count, and the Tender Hand Joint Count. DIP flexion was found to be a significant predictor of low disease activity/remission and high disease activity, the researchers reported in their abstract.
“This blows you away — that a single camera on [one’s] smartphone can look at the manipulation of a hand … and that AI can tell me, there’s a chance this might be an inflammatory arthritis,” said Dr. Wells, noting that researchers are also developing ways to detect joint swelling in RA by AI.
AI can also be used for remote ultrasound scanning in RA, as evidenced by use of the ARTHUR system in Europe, he said. Developed by the Danish company ROPCA, the ARTHUR technology (Rheumatoid Arthritis Ultrasound Robot) interacts directly with the patient who has new joint pain or established RA to capture ultrasound images in grayscale and color flow of 11 joints per hand. AI analyzes the images and creates a report for the specialist.
“They’re trying to get a foothold in the US,” Dr. Wells said, sharing his prediction that similar technology will someday be seen not only in pharmacies but also — in support of equitable access — in locations such as grocery stores. “Again,” he said, “nothing will replace us. I’m taking all [such] information and saying, who needs to be seen in 7 days and who can wait.”
AI for Writing, for Improving Practice and Patient Care
To manage his “task overload,” Dr. Cush uses ChatGPT for jobs such as first drafts of articles and making PowerPoint slides. It must be used cautiously for medical writing, however, as inaccuracies and false data/fabricated information — some of which has been coined AI “hallucinations” — are not uncommon.
“It’s very good at manuscript drafts, at generating bibliographies … it can do systematic reviews, it can do network meta-analyses, and it can find trends and patterns that can very helpful when it comes to writing. But you have to know how it’s a tool, and how it can hurt you,” he said.
Researchers recently reported asking ChatGPT to write an editorial about “how AI may replace the rheumatologist in editorial writing,” Dr. Cush noted. ChatGPT was “very politically correct,” he quipped, because it wrote that AI is “a tool to help the rheumatologist, but not replace him.”
Publishers want to preserve human intelligence — critical thinking and the ability to interpret, for instance — and most of the top medical journals (those most often cited) have issued guidance on the use of generative AI. “One said AI can’t be attributed as an author because being an author carries with it accountability of the work, and AI can’t take responsibility,” Dr. Cush said. Journals also “are saying you can use AI but you have to be totally transparent about it … [how it’s used] has to be very well spelled out.”
In practice, chatbots can be used for summarizing medical records, drafting post-visit summaries, collecting patient feedback, reminding about vaccinations, and performing administrative functions. “It’s really limitless as to what chatbots can do,” Dr. Cush said. “The question is, [what is] really going to help you?”
Much of the research submitted for presentation at major rheumatology meetings over the years has had questionable real-world utility and value, he said. But in the future this will likely change. “Take the PsA [psoriatic arthritis] patient who hasn’t responded to methotrexate or apremilast [Otezla]. There are [so many] choices, and there really isn’t a clear one. Shouldn’t data guide us on whether an IL-23 is better than a JAK, or maybe a JAK preferred over a TNF for some reason?” Dr. Cush said. “That’s what we’re hoping will happen down the line.”
More realistic AI-guided clinical scenarios for now include the following: AI screens the chart of a 68-year-old with RA on methotrexate and etanercept who is following up, and retrieves pieces of history — an elevated C-reactive protein 3 months ago, for instance, and diverticulosis 5 years ago. “AI tells you, based on this, he may have active disease, and here are three medications covered by his insurance,” Dr. Wells said.
Or, in the case of a 58-year-old patient with RA who has scheduled a virtual follow-up visit after having been on methotrexate and hydroxychloroquine for 12 weeks, AI detects a low platelet count in her previsit labs and also sees that she received an MMR booster 5 weeks ago at a local CVS Minute Clinic. AI retrieves for the rheumatologist a review article about thrombocytopenic purpura after MMR vaccination.
AI for Drug Development, Clinical Trials
Dr. Cush is following with keen interest the integration of AI into the process of drug development, from drug discovery and biomarker evaluation to clinical trial efficiency and patient recruitment, as well as marketing. “A lot hasn’t been ‘rolled out’ or shown to us, but there’s a lot going on … everyone is investing,” he said. “The number one challenge is regulatory: How will the [Food and Drug Administration] handle AI-generated data sets or AI-generated or monitored trials?”
The FDA is working to ensure quality and utility of data and is rapidly “approving AI algorithms for use in medicine and healthcare,” he said.
AI’s ability to identify patients in populations can not only facilitate earlier diagnoses but can accelerate patient recruitment for clinical trials, Dr. Cush emphasized. He pointed to research presented at the ACR 2021 annual meeting in which a machine-learning algorithm was used with electronic health records in the United Kingdom to estimate the probability of a patient’s being diagnosed with axial spondyloarthritis (axSpA).
AI identified 89 best clinical predictors (out of 820 analyzed). When applying these predictors to the population, AI was able to differentiate patients with axSpA from healthy controls with a sensitivity of 75%, a specificity of 96%, and a positive predictive value of 81%. Such an application of AI “is ideal … It would make clinical trials more streamlined and productive,” he said.
The extent to which AI will lead to cost savings — in the pharmacology arena, for instance, or for Well’s medical group — is unknown, Dr. Cush and Dr. Wells said. And, of course, there are concerns about potential bias and abuse of AI. “The worry,” Dr. Cush said, “is, who’s watching?”
Dr. Wells disclosed that he has research support and has served as a member of advisory boards and/or speaker bureaus for 17 different pharmaceutical or medical technology companies. Dr. Cush disclosed relationships with AbbVie, Amgen, Bristol-Myers Squibb, Novartis, Sanofi, and UCB.
The rheumatologist of the future will see patients who have been assessed and triaged with artificial intelligence utilizing data from remote kiosk-placed ultrasound scanners and physician-directed algorithms. Practices will be broadly fueled by AI, which will screen charts, produce notes, handle prior authorizations and insurance issues, aid in earlier diagnoses, find patients for clinical trials, and maybe even suggest the next best therapy for individual patients.
Such is the future envisioned by Alvin F. Wells, MD, PhD, and John J. Cush, MD, who discussed the current and forthcoming reach of AI — and their own uses of it — at the 2024 Rheumatology Winter Clinical Symposium.
“We’re not at the stage where ChatGPT and AI can tell us what the next best therapy is, but we’re getting there,” said Dr. Cush, a rheumatologist based in Dallas and executive director of RheumNow.com. For now, he said, “AI affords us a truly big-time increase in efficiency. It helps you deal with your time constraints in managing information overload and task overload.”
At a time when “PubMed doubles every 73 days ... and it’s getting harder and harder to stay abreast,” for example, new applications such as Scite, SciSpace, and Consensus can help curate, focus, and analyze the literature to match one’s own clinical interests. Such review tools are “just now getting into play and are evolving,” Dr. Cush said, noting that many but not all of them are based on ChatGPT, OpenAI’s chatbot that had a over 100 million users by January 2023 — just over a month after its version 3.5 was released.
For Dr. Wells, a rheumatologist and Midwest Region director in the department of rheumatology for the Advocate Health Medical Group in Franklin, Wisconsin, clinician-developed algorithms are helping his group assess patients — often remotely — and triage them to be seen fairly immediately by a rheumatologist versus in 4-6 weeks or in several months. “You can use AI to guide your access,” he said.
A patient “with a family history of RA, sed rate above 50, and osteopenia on x-rays” would be seen within a week, for example, while “another patient who’s had a [positive] ANA with no other symptoms, and maybe a family history, might be seen in 4-6 weeks,” said Dr. Wells, sharing his belief that “there is not a shortage of rheumatologists, [but a] shortage of using rheumatologists efficiently.”
AI for Improving Workflow
Current and future advances will enrich the intersection of AI and virtual medicine and improve outcomes and the rheumatologist-patient interaction, Dr. Wells said, pointing to research presented at the American College of Rheumatology (ACR) 2023 annual meeting on the use of computer vision technology for remotely assessing disease activity in rheumatoid arthritis (RA).
In the proof-of-concept “MeFisto” study, 28 patients with RA used an app that enabled computer vision inference of hand motion data. Upon recording, an algorithm tracked the mean degree change of joint angle on flexion and the mean time to maximal flexion for each joint.
The researchers found a strong correlation between flexion of the distal interphalangeal (DIP) joint and the Disease Activity Score in 28 joints, the Swollen Hand Joint Count, and the Tender Hand Joint Count. DIP flexion was found to be a significant predictor of low disease activity/remission and high disease activity, the researchers reported in their abstract.
“This blows you away — that a single camera on [one’s] smartphone can look at the manipulation of a hand … and that AI can tell me, there’s a chance this might be an inflammatory arthritis,” said Dr. Wells, noting that researchers are also developing ways to detect joint swelling in RA by AI.
AI can also be used for remote ultrasound scanning in RA, as evidenced by use of the ARTHUR system in Europe, he said. Developed by the Danish company ROPCA, the ARTHUR technology (Rheumatoid Arthritis Ultrasound Robot) interacts directly with the patient who has new joint pain or established RA to capture ultrasound images in grayscale and color flow of 11 joints per hand. AI analyzes the images and creates a report for the specialist.
“They’re trying to get a foothold in the US,” Dr. Wells said, sharing his prediction that similar technology will someday be seen not only in pharmacies but also — in support of equitable access — in locations such as grocery stores. “Again,” he said, “nothing will replace us. I’m taking all [such] information and saying, who needs to be seen in 7 days and who can wait.”
AI for Writing, for Improving Practice and Patient Care
To manage his “task overload,” Dr. Cush uses ChatGPT for jobs such as first drafts of articles and making PowerPoint slides. It must be used cautiously for medical writing, however, as inaccuracies and false data/fabricated information — some of which has been coined AI “hallucinations” — are not uncommon.
“It’s very good at manuscript drafts, at generating bibliographies … it can do systematic reviews, it can do network meta-analyses, and it can find trends and patterns that can very helpful when it comes to writing. But you have to know how it’s a tool, and how it can hurt you,” he said.
Researchers recently reported asking ChatGPT to write an editorial about “how AI may replace the rheumatologist in editorial writing,” Dr. Cush noted. ChatGPT was “very politically correct,” he quipped, because it wrote that AI is “a tool to help the rheumatologist, but not replace him.”
Publishers want to preserve human intelligence — critical thinking and the ability to interpret, for instance — and most of the top medical journals (those most often cited) have issued guidance on the use of generative AI. “One said AI can’t be attributed as an author because being an author carries with it accountability of the work, and AI can’t take responsibility,” Dr. Cush said. Journals also “are saying you can use AI but you have to be totally transparent about it … [how it’s used] has to be very well spelled out.”
In practice, chatbots can be used for summarizing medical records, drafting post-visit summaries, collecting patient feedback, reminding about vaccinations, and performing administrative functions. “It’s really limitless as to what chatbots can do,” Dr. Cush said. “The question is, [what is] really going to help you?”
Much of the research submitted for presentation at major rheumatology meetings over the years has had questionable real-world utility and value, he said. But in the future this will likely change. “Take the PsA [psoriatic arthritis] patient who hasn’t responded to methotrexate or apremilast [Otezla]. There are [so many] choices, and there really isn’t a clear one. Shouldn’t data guide us on whether an IL-23 is better than a JAK, or maybe a JAK preferred over a TNF for some reason?” Dr. Cush said. “That’s what we’re hoping will happen down the line.”
More realistic AI-guided clinical scenarios for now include the following: AI screens the chart of a 68-year-old with RA on methotrexate and etanercept who is following up, and retrieves pieces of history — an elevated C-reactive protein 3 months ago, for instance, and diverticulosis 5 years ago. “AI tells you, based on this, he may have active disease, and here are three medications covered by his insurance,” Dr. Wells said.
Or, in the case of a 58-year-old patient with RA who has scheduled a virtual follow-up visit after having been on methotrexate and hydroxychloroquine for 12 weeks, AI detects a low platelet count in her previsit labs and also sees that she received an MMR booster 5 weeks ago at a local CVS Minute Clinic. AI retrieves for the rheumatologist a review article about thrombocytopenic purpura after MMR vaccination.
AI for Drug Development, Clinical Trials
Dr. Cush is following with keen interest the integration of AI into the process of drug development, from drug discovery and biomarker evaluation to clinical trial efficiency and patient recruitment, as well as marketing. “A lot hasn’t been ‘rolled out’ or shown to us, but there’s a lot going on … everyone is investing,” he said. “The number one challenge is regulatory: How will the [Food and Drug Administration] handle AI-generated data sets or AI-generated or monitored trials?”
The FDA is working to ensure quality and utility of data and is rapidly “approving AI algorithms for use in medicine and healthcare,” he said.
AI’s ability to identify patients in populations can not only facilitate earlier diagnoses but can accelerate patient recruitment for clinical trials, Dr. Cush emphasized. He pointed to research presented at the ACR 2021 annual meeting in which a machine-learning algorithm was used with electronic health records in the United Kingdom to estimate the probability of a patient’s being diagnosed with axial spondyloarthritis (axSpA).
AI identified 89 best clinical predictors (out of 820 analyzed). When applying these predictors to the population, AI was able to differentiate patients with axSpA from healthy controls with a sensitivity of 75%, a specificity of 96%, and a positive predictive value of 81%. Such an application of AI “is ideal … It would make clinical trials more streamlined and productive,” he said.
The extent to which AI will lead to cost savings — in the pharmacology arena, for instance, or for Well’s medical group — is unknown, Dr. Cush and Dr. Wells said. And, of course, there are concerns about potential bias and abuse of AI. “The worry,” Dr. Cush said, “is, who’s watching?”
Dr. Wells disclosed that he has research support and has served as a member of advisory boards and/or speaker bureaus for 17 different pharmaceutical or medical technology companies. Dr. Cush disclosed relationships with AbbVie, Amgen, Bristol-Myers Squibb, Novartis, Sanofi, and UCB.
The rheumatologist of the future will see patients who have been assessed and triaged with artificial intelligence utilizing data from remote kiosk-placed ultrasound scanners and physician-directed algorithms. Practices will be broadly fueled by AI, which will screen charts, produce notes, handle prior authorizations and insurance issues, aid in earlier diagnoses, find patients for clinical trials, and maybe even suggest the next best therapy for individual patients.
Such is the future envisioned by Alvin F. Wells, MD, PhD, and John J. Cush, MD, who discussed the current and forthcoming reach of AI — and their own uses of it — at the 2024 Rheumatology Winter Clinical Symposium.
“We’re not at the stage where ChatGPT and AI can tell us what the next best therapy is, but we’re getting there,” said Dr. Cush, a rheumatologist based in Dallas and executive director of RheumNow.com. For now, he said, “AI affords us a truly big-time increase in efficiency. It helps you deal with your time constraints in managing information overload and task overload.”
At a time when “PubMed doubles every 73 days ... and it’s getting harder and harder to stay abreast,” for example, new applications such as Scite, SciSpace, and Consensus can help curate, focus, and analyze the literature to match one’s own clinical interests. Such review tools are “just now getting into play and are evolving,” Dr. Cush said, noting that many but not all of them are based on ChatGPT, OpenAI’s chatbot that had a over 100 million users by January 2023 — just over a month after its version 3.5 was released.
For Dr. Wells, a rheumatologist and Midwest Region director in the department of rheumatology for the Advocate Health Medical Group in Franklin, Wisconsin, clinician-developed algorithms are helping his group assess patients — often remotely — and triage them to be seen fairly immediately by a rheumatologist versus in 4-6 weeks or in several months. “You can use AI to guide your access,” he said.
A patient “with a family history of RA, sed rate above 50, and osteopenia on x-rays” would be seen within a week, for example, while “another patient who’s had a [positive] ANA with no other symptoms, and maybe a family history, might be seen in 4-6 weeks,” said Dr. Wells, sharing his belief that “there is not a shortage of rheumatologists, [but a] shortage of using rheumatologists efficiently.”
AI for Improving Workflow
Current and future advances will enrich the intersection of AI and virtual medicine and improve outcomes and the rheumatologist-patient interaction, Dr. Wells said, pointing to research presented at the American College of Rheumatology (ACR) 2023 annual meeting on the use of computer vision technology for remotely assessing disease activity in rheumatoid arthritis (RA).
In the proof-of-concept “MeFisto” study, 28 patients with RA used an app that enabled computer vision inference of hand motion data. Upon recording, an algorithm tracked the mean degree change of joint angle on flexion and the mean time to maximal flexion for each joint.
The researchers found a strong correlation between flexion of the distal interphalangeal (DIP) joint and the Disease Activity Score in 28 joints, the Swollen Hand Joint Count, and the Tender Hand Joint Count. DIP flexion was found to be a significant predictor of low disease activity/remission and high disease activity, the researchers reported in their abstract.
“This blows you away — that a single camera on [one’s] smartphone can look at the manipulation of a hand … and that AI can tell me, there’s a chance this might be an inflammatory arthritis,” said Dr. Wells, noting that researchers are also developing ways to detect joint swelling in RA by AI.
AI can also be used for remote ultrasound scanning in RA, as evidenced by use of the ARTHUR system in Europe, he said. Developed by the Danish company ROPCA, the ARTHUR technology (Rheumatoid Arthritis Ultrasound Robot) interacts directly with the patient who has new joint pain or established RA to capture ultrasound images in grayscale and color flow of 11 joints per hand. AI analyzes the images and creates a report for the specialist.
“They’re trying to get a foothold in the US,” Dr. Wells said, sharing his prediction that similar technology will someday be seen not only in pharmacies but also — in support of equitable access — in locations such as grocery stores. “Again,” he said, “nothing will replace us. I’m taking all [such] information and saying, who needs to be seen in 7 days and who can wait.”
AI for Writing, for Improving Practice and Patient Care
To manage his “task overload,” Dr. Cush uses ChatGPT for jobs such as first drafts of articles and making PowerPoint slides. It must be used cautiously for medical writing, however, as inaccuracies and false data/fabricated information — some of which has been coined AI “hallucinations” — are not uncommon.
“It’s very good at manuscript drafts, at generating bibliographies … it can do systematic reviews, it can do network meta-analyses, and it can find trends and patterns that can very helpful when it comes to writing. But you have to know how it’s a tool, and how it can hurt you,” he said.
Researchers recently reported asking ChatGPT to write an editorial about “how AI may replace the rheumatologist in editorial writing,” Dr. Cush noted. ChatGPT was “very politically correct,” he quipped, because it wrote that AI is “a tool to help the rheumatologist, but not replace him.”
Publishers want to preserve human intelligence — critical thinking and the ability to interpret, for instance — and most of the top medical journals (those most often cited) have issued guidance on the use of generative AI. “One said AI can’t be attributed as an author because being an author carries with it accountability of the work, and AI can’t take responsibility,” Dr. Cush said. Journals also “are saying you can use AI but you have to be totally transparent about it … [how it’s used] has to be very well spelled out.”
In practice, chatbots can be used for summarizing medical records, drafting post-visit summaries, collecting patient feedback, reminding about vaccinations, and performing administrative functions. “It’s really limitless as to what chatbots can do,” Dr. Cush said. “The question is, [what is] really going to help you?”
Much of the research submitted for presentation at major rheumatology meetings over the years has had questionable real-world utility and value, he said. But in the future this will likely change. “Take the PsA [psoriatic arthritis] patient who hasn’t responded to methotrexate or apremilast [Otezla]. There are [so many] choices, and there really isn’t a clear one. Shouldn’t data guide us on whether an IL-23 is better than a JAK, or maybe a JAK preferred over a TNF for some reason?” Dr. Cush said. “That’s what we’re hoping will happen down the line.”
More realistic AI-guided clinical scenarios for now include the following: AI screens the chart of a 68-year-old with RA on methotrexate and etanercept who is following up, and retrieves pieces of history — an elevated C-reactive protein 3 months ago, for instance, and diverticulosis 5 years ago. “AI tells you, based on this, he may have active disease, and here are three medications covered by his insurance,” Dr. Wells said.
Or, in the case of a 58-year-old patient with RA who has scheduled a virtual follow-up visit after having been on methotrexate and hydroxychloroquine for 12 weeks, AI detects a low platelet count in her previsit labs and also sees that she received an MMR booster 5 weeks ago at a local CVS Minute Clinic. AI retrieves for the rheumatologist a review article about thrombocytopenic purpura after MMR vaccination.
AI for Drug Development, Clinical Trials
Dr. Cush is following with keen interest the integration of AI into the process of drug development, from drug discovery and biomarker evaluation to clinical trial efficiency and patient recruitment, as well as marketing. “A lot hasn’t been ‘rolled out’ or shown to us, but there’s a lot going on … everyone is investing,” he said. “The number one challenge is regulatory: How will the [Food and Drug Administration] handle AI-generated data sets or AI-generated or monitored trials?”
The FDA is working to ensure quality and utility of data and is rapidly “approving AI algorithms for use in medicine and healthcare,” he said.
AI’s ability to identify patients in populations can not only facilitate earlier diagnoses but can accelerate patient recruitment for clinical trials, Dr. Cush emphasized. He pointed to research presented at the ACR 2021 annual meeting in which a machine-learning algorithm was used with electronic health records in the United Kingdom to estimate the probability of a patient’s being diagnosed with axial spondyloarthritis (axSpA).
AI identified 89 best clinical predictors (out of 820 analyzed). When applying these predictors to the population, AI was able to differentiate patients with axSpA from healthy controls with a sensitivity of 75%, a specificity of 96%, and a positive predictive value of 81%. Such an application of AI “is ideal … It would make clinical trials more streamlined and productive,” he said.
The extent to which AI will lead to cost savings — in the pharmacology arena, for instance, or for Well’s medical group — is unknown, Dr. Cush and Dr. Wells said. And, of course, there are concerns about potential bias and abuse of AI. “The worry,” Dr. Cush said, “is, who’s watching?”
Dr. Wells disclosed that he has research support and has served as a member of advisory boards and/or speaker bureaus for 17 different pharmaceutical or medical technology companies. Dr. Cush disclosed relationships with AbbVie, Amgen, Bristol-Myers Squibb, Novartis, Sanofi, and UCB.
FROM RWCS 2024
Leflunomide: A Fresh Look at an Old Drug
The Food and Drug Administration’s approval of leflunomide in September 1998 as a treatment for rheumatoid arthritis was sandwiched between the debuts of infliximab (Remicade and biosimilars) and etanercept (Enbrel) in August and November of that year, the latter of which was so exciting that “within 2 months you couldn’t get [it],” recalled Eric M. Ruderman, MD. And “like every middle child, [leflunomide] was underloved, underappreciated, and largely dismissed.”
Yet should it have been? Is it worth another look today?
At the 2024 Rheumatology Winter Clinical Symposium, Dr. Ruderman reflected on some of the clinical trial data published after leflunomide’s approval that “got lost in the shuffle” of the rightful embrace of biologics in United States practice, and urged reconsideration of the loading strategy still advised in the drug’s labeling.
“I’m not telling you that you should be using [leflunomide] in place of biologics, instead of biologics, or before biologics … but it should be in your toolkit,” said Dr. Ruderman, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago. The drug “still has a role in RA, including in combination with methotrexate, and a potential role in other rheumatic diseases.”
“In our PsA clinic,” he noted, “we’ve actually not infrequently added leflunomide to some of the other agents we’ve been using.”
Key Findings Over the Years in RA
Leflunomide showed efficacy similar to that of sulfasalazine in a randomized trial published in 1999 that used primary endpoints of tender/swollen joints and physician and patient global scores. Then, against methotrexate, it proved just as efficacious in achieving at least 20% improvement in American College of Rheumatology composite response criteria (ACR20) over 52 weeks, and in meeting endpoints similar to those of the sulfasalazine trial, in two trials, one published in 1999 and another in 2000.
“So here were two big trials [comparing it with methotrexate] that suggested the drug was just as good as what had become our standard of care by that point,” Dr. Ruderman said.
Each of these three trials used a loading dose of 100 mg leflunomide for 3 days, followed by 20 mg daily. Sulfasalazine was initiated at 2 g and escalated over 4 weeks. Methotrexate was initiated in one of the trials at a dose of 7.5 mg, then increased to 15 mg in almost two-thirds of patients; in the other methotrexate trial the initial dose was 15 mg escalated over 3 months.
Side effects of leflunomide — GI issues, rash, alopecia (reversible), and elevated liver function tests — were similar across the trials, and represented “about the same toxicities as methotrexate,” he said.
Researchers then tested leflunomide as an add-on to methotrexate in patients who had inadequate response, which “was a little bit daunting since we were still concerned about the toxicity of methotrexate at this point,” Dr. Ruderman said. “The idea that we’d take another drug with similar toxicities and add it on to the methotrexate was a little scary.”
But it worked. Patients on a mean background dose of 16.5 mg methotrexate were randomized to placebo or to a 2-day leflunomide loading dose followed by 10 mg/day that could be escalated at 8 weeks to 20 mg if needed. At 6 months, 19.5% and 46.2%, respectively, met ACR20 (P < .001), and “interestingly,” he said, “adverse events were pretty similar” between combination therapy and methotrexate monotherapy.
“This was very much like all the studies we’ve seen over the years with new biologics — they were all added to background methotrexate,” he said. “And the truth is, the [46%] response seen when adding leflunomide to background methotrexate wasn’t very different from the 50% [ACR20] response you tend to see when you add a biologic.”
However, despite the study’s conclusion that combination therapy provided significant benefit to patients with inadequate response to methotrexate alone, “the drug got lost, because everyone was prescribing the biologics,” Dr. Ruderman said.
He said he found only one study comparing leflunomide with a biologic. In a notably small but well-designed study from Sri Lanka published in 2017, 40 patients with an inadequate response to methotrexate were randomized to low-dose rituximab (500 mg x 2) or 20 mg/day leflunomide (no loading dose). At week 24, ACR20 was nearly identical (85% vs 84%), with a similar rate of adverse events.
The researchers pointed out “that there’s a potential cost benefit in developing countries where biologics aren’t as accessible,” he said, agreeing that “the big opportunity for a drug like leflunomide is outside the US, where you don’t have access to the drugs we take advantage of all the time.”
A meeting participant from Canada pointed out that rheumatologists there are “mandated to use it for PsA in combination with methotrexate before we can get a biologic, and for RA we can use it with Plaquenil [hydroxychloroquine] and methotrexate before we get a biologic, so we’re using it all the time.”
Asked about efficacy, the physician said the combination with methotrexate is “absolutely” efficacious. “It works really well” he said. “The problem is, you really have to watch the white cell count and liver function … and the half-life is long.”
Indeed, Dr. Ruderman said during his talk, the plasma half-life of teriflunomide, its active metabolite, is 15.5 days, which is challenging when adverse events occur. “And it’s a terrible drug in young women thinking about pregnancy because it’s teratogenic and stays around,” he said.
Leflunomide, which, notably, was “developed specifically for RA from the get-go” and not borrowed from another specialty, works by blocking de novo pyrimidine synthesis, Dr. Ruderman said. T-cell activation requires the upregulation of pyrimidine production (salvage pathways are insufficient); the “drug prevents that” by inhibiting an enzyme that catalyzes conversion of dihydroorotate to orotate, which, in turn, is converted to pyrimidine ribonucleotides, he explained.
Other potential mechanisms of action have been proposed — mainly, inhibition of tumor necrosis factor signaling and inhibition of kinase activity, including the JAK/STAT pathway — but “there’s not great data for any of them,” he said.
Loading vs Not Loading, and Its Role in PsA and Other Diseases
“We stopped loading years ago because at 100 mg for 3 days in a row, everyone has GI issues,” Dr. Ruderman said. “It may have made sense from a pharmacokinetic standpoint because [based on the long half-life] you could get to a higher drug level quicker, but not a practical standpoint, because patients would stop the drug — they couldn’t take it.” The first study to examine the necessity of loading leflunomide in a “prospective, careful way” was published in 2013. It randomized 120 patients to 100 mg or 20 mg for 3 days, followed by a 3-month open-label period of 20 mg, and found no clinical benefit with loading but more diarrhea and elevated liver enzymes.
“It tells us something about how we need to think about half-lives,” he said. “Maybe [loading is] not necessary because the biological effects are different than the drug levels.”
In the PsA space, in 2004, researchers reported a double-blind randomized trial in which 190 patients with active PsA and cutaneous psoriasis with at least 3% body surface area involvement were randomized to receive leflunomide (a loading dose followed by 20 mg/day) or placebo for 24 weeks. Almost 60% of leflunomide-treated patients, compared with 30% of placebo-treated patients, were classified as responders by the Psoriatic Arthritis Response criteria (P < .0001), “which is a soft endpoint” but was utilized at the time, Dr. Ruderman said. The researchers noted improvements in ACR20 and skin responses as well, and toxicity was similar to that reported in the RA studies.
However, approval was never sought, and the drug was infrequently prescribed, “because etanercept came out for this disease, and then adalimumab … and then the world changed,” he said.
More recently, a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA tested leflunomide plus methotrexate vs methotrexate monotherapy and was published in The Lancet Rheumatology. After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1 [standard deviation (SD), 1.4] vs 3.7 [SD, 1.3]; treatment difference, -0.6; 90% CI, -1.0 to -0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than that of the monotherapy group (34%; P = .019). Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group.
In an interview after the meeting, Dr. Ruderman explained that in his practice, about 15 years ago, leflunomide was sometimes prescribed as an alternative to a biologic change for patients whose skin disease improved significantly with ustekinumab (Stelara) but who “suddenly had more joint symptoms that they didn’t have before.”
And “we’ve found ourselves a bit recently with the same sort of story, where patients are prescribed IL-23 inhibitors like Skyrizi [risankizumab] and Tremfya [guselkumab] and their skin does really well but now they’re having more joint symptoms than previously,” he said. “Our choices are to switch to a whole different biologic, or to think about adding something as an adjunct — and maybe leflunomide is a reasonable option.”
In the last 5 years, Dr. Ruderman noted, randomized trial data has been published on leflunomide in lupus nephritis induction, and in lupus nephritis maintenance, as well as in IgG4-related disease.
Dr. Ruderman disclosed consulting and/or drug safety monitoring board work for AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Lilly, Merck, Novartis, NS Pharma, and UCB.
The Food and Drug Administration’s approval of leflunomide in September 1998 as a treatment for rheumatoid arthritis was sandwiched between the debuts of infliximab (Remicade and biosimilars) and etanercept (Enbrel) in August and November of that year, the latter of which was so exciting that “within 2 months you couldn’t get [it],” recalled Eric M. Ruderman, MD. And “like every middle child, [leflunomide] was underloved, underappreciated, and largely dismissed.”
Yet should it have been? Is it worth another look today?
At the 2024 Rheumatology Winter Clinical Symposium, Dr. Ruderman reflected on some of the clinical trial data published after leflunomide’s approval that “got lost in the shuffle” of the rightful embrace of biologics in United States practice, and urged reconsideration of the loading strategy still advised in the drug’s labeling.
“I’m not telling you that you should be using [leflunomide] in place of biologics, instead of biologics, or before biologics … but it should be in your toolkit,” said Dr. Ruderman, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago. The drug “still has a role in RA, including in combination with methotrexate, and a potential role in other rheumatic diseases.”
“In our PsA clinic,” he noted, “we’ve actually not infrequently added leflunomide to some of the other agents we’ve been using.”
Key Findings Over the Years in RA
Leflunomide showed efficacy similar to that of sulfasalazine in a randomized trial published in 1999 that used primary endpoints of tender/swollen joints and physician and patient global scores. Then, against methotrexate, it proved just as efficacious in achieving at least 20% improvement in American College of Rheumatology composite response criteria (ACR20) over 52 weeks, and in meeting endpoints similar to those of the sulfasalazine trial, in two trials, one published in 1999 and another in 2000.
“So here were two big trials [comparing it with methotrexate] that suggested the drug was just as good as what had become our standard of care by that point,” Dr. Ruderman said.
Each of these three trials used a loading dose of 100 mg leflunomide for 3 days, followed by 20 mg daily. Sulfasalazine was initiated at 2 g and escalated over 4 weeks. Methotrexate was initiated in one of the trials at a dose of 7.5 mg, then increased to 15 mg in almost two-thirds of patients; in the other methotrexate trial the initial dose was 15 mg escalated over 3 months.
Side effects of leflunomide — GI issues, rash, alopecia (reversible), and elevated liver function tests — were similar across the trials, and represented “about the same toxicities as methotrexate,” he said.
Researchers then tested leflunomide as an add-on to methotrexate in patients who had inadequate response, which “was a little bit daunting since we were still concerned about the toxicity of methotrexate at this point,” Dr. Ruderman said. “The idea that we’d take another drug with similar toxicities and add it on to the methotrexate was a little scary.”
But it worked. Patients on a mean background dose of 16.5 mg methotrexate were randomized to placebo or to a 2-day leflunomide loading dose followed by 10 mg/day that could be escalated at 8 weeks to 20 mg if needed. At 6 months, 19.5% and 46.2%, respectively, met ACR20 (P < .001), and “interestingly,” he said, “adverse events were pretty similar” between combination therapy and methotrexate monotherapy.
“This was very much like all the studies we’ve seen over the years with new biologics — they were all added to background methotrexate,” he said. “And the truth is, the [46%] response seen when adding leflunomide to background methotrexate wasn’t very different from the 50% [ACR20] response you tend to see when you add a biologic.”
However, despite the study’s conclusion that combination therapy provided significant benefit to patients with inadequate response to methotrexate alone, “the drug got lost, because everyone was prescribing the biologics,” Dr. Ruderman said.
He said he found only one study comparing leflunomide with a biologic. In a notably small but well-designed study from Sri Lanka published in 2017, 40 patients with an inadequate response to methotrexate were randomized to low-dose rituximab (500 mg x 2) or 20 mg/day leflunomide (no loading dose). At week 24, ACR20 was nearly identical (85% vs 84%), with a similar rate of adverse events.
The researchers pointed out “that there’s a potential cost benefit in developing countries where biologics aren’t as accessible,” he said, agreeing that “the big opportunity for a drug like leflunomide is outside the US, where you don’t have access to the drugs we take advantage of all the time.”
A meeting participant from Canada pointed out that rheumatologists there are “mandated to use it for PsA in combination with methotrexate before we can get a biologic, and for RA we can use it with Plaquenil [hydroxychloroquine] and methotrexate before we get a biologic, so we’re using it all the time.”
Asked about efficacy, the physician said the combination with methotrexate is “absolutely” efficacious. “It works really well” he said. “The problem is, you really have to watch the white cell count and liver function … and the half-life is long.”
Indeed, Dr. Ruderman said during his talk, the plasma half-life of teriflunomide, its active metabolite, is 15.5 days, which is challenging when adverse events occur. “And it’s a terrible drug in young women thinking about pregnancy because it’s teratogenic and stays around,” he said.
Leflunomide, which, notably, was “developed specifically for RA from the get-go” and not borrowed from another specialty, works by blocking de novo pyrimidine synthesis, Dr. Ruderman said. T-cell activation requires the upregulation of pyrimidine production (salvage pathways are insufficient); the “drug prevents that” by inhibiting an enzyme that catalyzes conversion of dihydroorotate to orotate, which, in turn, is converted to pyrimidine ribonucleotides, he explained.
Other potential mechanisms of action have been proposed — mainly, inhibition of tumor necrosis factor signaling and inhibition of kinase activity, including the JAK/STAT pathway — but “there’s not great data for any of them,” he said.
Loading vs Not Loading, and Its Role in PsA and Other Diseases
“We stopped loading years ago because at 100 mg for 3 days in a row, everyone has GI issues,” Dr. Ruderman said. “It may have made sense from a pharmacokinetic standpoint because [based on the long half-life] you could get to a higher drug level quicker, but not a practical standpoint, because patients would stop the drug — they couldn’t take it.” The first study to examine the necessity of loading leflunomide in a “prospective, careful way” was published in 2013. It randomized 120 patients to 100 mg or 20 mg for 3 days, followed by a 3-month open-label period of 20 mg, and found no clinical benefit with loading but more diarrhea and elevated liver enzymes.
“It tells us something about how we need to think about half-lives,” he said. “Maybe [loading is] not necessary because the biological effects are different than the drug levels.”
In the PsA space, in 2004, researchers reported a double-blind randomized trial in which 190 patients with active PsA and cutaneous psoriasis with at least 3% body surface area involvement were randomized to receive leflunomide (a loading dose followed by 20 mg/day) or placebo for 24 weeks. Almost 60% of leflunomide-treated patients, compared with 30% of placebo-treated patients, were classified as responders by the Psoriatic Arthritis Response criteria (P < .0001), “which is a soft endpoint” but was utilized at the time, Dr. Ruderman said. The researchers noted improvements in ACR20 and skin responses as well, and toxicity was similar to that reported in the RA studies.
However, approval was never sought, and the drug was infrequently prescribed, “because etanercept came out for this disease, and then adalimumab … and then the world changed,” he said.
More recently, a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA tested leflunomide plus methotrexate vs methotrexate monotherapy and was published in The Lancet Rheumatology. After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1 [standard deviation (SD), 1.4] vs 3.7 [SD, 1.3]; treatment difference, -0.6; 90% CI, -1.0 to -0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than that of the monotherapy group (34%; P = .019). Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group.
In an interview after the meeting, Dr. Ruderman explained that in his practice, about 15 years ago, leflunomide was sometimes prescribed as an alternative to a biologic change for patients whose skin disease improved significantly with ustekinumab (Stelara) but who “suddenly had more joint symptoms that they didn’t have before.”
And “we’ve found ourselves a bit recently with the same sort of story, where patients are prescribed IL-23 inhibitors like Skyrizi [risankizumab] and Tremfya [guselkumab] and their skin does really well but now they’re having more joint symptoms than previously,” he said. “Our choices are to switch to a whole different biologic, or to think about adding something as an adjunct — and maybe leflunomide is a reasonable option.”
In the last 5 years, Dr. Ruderman noted, randomized trial data has been published on leflunomide in lupus nephritis induction, and in lupus nephritis maintenance, as well as in IgG4-related disease.
Dr. Ruderman disclosed consulting and/or drug safety monitoring board work for AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Lilly, Merck, Novartis, NS Pharma, and UCB.
The Food and Drug Administration’s approval of leflunomide in September 1998 as a treatment for rheumatoid arthritis was sandwiched between the debuts of infliximab (Remicade and biosimilars) and etanercept (Enbrel) in August and November of that year, the latter of which was so exciting that “within 2 months you couldn’t get [it],” recalled Eric M. Ruderman, MD. And “like every middle child, [leflunomide] was underloved, underappreciated, and largely dismissed.”
Yet should it have been? Is it worth another look today?
At the 2024 Rheumatology Winter Clinical Symposium, Dr. Ruderman reflected on some of the clinical trial data published after leflunomide’s approval that “got lost in the shuffle” of the rightful embrace of biologics in United States practice, and urged reconsideration of the loading strategy still advised in the drug’s labeling.
“I’m not telling you that you should be using [leflunomide] in place of biologics, instead of biologics, or before biologics … but it should be in your toolkit,” said Dr. Ruderman, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago. The drug “still has a role in RA, including in combination with methotrexate, and a potential role in other rheumatic diseases.”
“In our PsA clinic,” he noted, “we’ve actually not infrequently added leflunomide to some of the other agents we’ve been using.”
Key Findings Over the Years in RA
Leflunomide showed efficacy similar to that of sulfasalazine in a randomized trial published in 1999 that used primary endpoints of tender/swollen joints and physician and patient global scores. Then, against methotrexate, it proved just as efficacious in achieving at least 20% improvement in American College of Rheumatology composite response criteria (ACR20) over 52 weeks, and in meeting endpoints similar to those of the sulfasalazine trial, in two trials, one published in 1999 and another in 2000.
“So here were two big trials [comparing it with methotrexate] that suggested the drug was just as good as what had become our standard of care by that point,” Dr. Ruderman said.
Each of these three trials used a loading dose of 100 mg leflunomide for 3 days, followed by 20 mg daily. Sulfasalazine was initiated at 2 g and escalated over 4 weeks. Methotrexate was initiated in one of the trials at a dose of 7.5 mg, then increased to 15 mg in almost two-thirds of patients; in the other methotrexate trial the initial dose was 15 mg escalated over 3 months.
Side effects of leflunomide — GI issues, rash, alopecia (reversible), and elevated liver function tests — were similar across the trials, and represented “about the same toxicities as methotrexate,” he said.
Researchers then tested leflunomide as an add-on to methotrexate in patients who had inadequate response, which “was a little bit daunting since we were still concerned about the toxicity of methotrexate at this point,” Dr. Ruderman said. “The idea that we’d take another drug with similar toxicities and add it on to the methotrexate was a little scary.”
But it worked. Patients on a mean background dose of 16.5 mg methotrexate were randomized to placebo or to a 2-day leflunomide loading dose followed by 10 mg/day that could be escalated at 8 weeks to 20 mg if needed. At 6 months, 19.5% and 46.2%, respectively, met ACR20 (P < .001), and “interestingly,” he said, “adverse events were pretty similar” between combination therapy and methotrexate monotherapy.
“This was very much like all the studies we’ve seen over the years with new biologics — they were all added to background methotrexate,” he said. “And the truth is, the [46%] response seen when adding leflunomide to background methotrexate wasn’t very different from the 50% [ACR20] response you tend to see when you add a biologic.”
However, despite the study’s conclusion that combination therapy provided significant benefit to patients with inadequate response to methotrexate alone, “the drug got lost, because everyone was prescribing the biologics,” Dr. Ruderman said.
He said he found only one study comparing leflunomide with a biologic. In a notably small but well-designed study from Sri Lanka published in 2017, 40 patients with an inadequate response to methotrexate were randomized to low-dose rituximab (500 mg x 2) or 20 mg/day leflunomide (no loading dose). At week 24, ACR20 was nearly identical (85% vs 84%), with a similar rate of adverse events.
The researchers pointed out “that there’s a potential cost benefit in developing countries where biologics aren’t as accessible,” he said, agreeing that “the big opportunity for a drug like leflunomide is outside the US, where you don’t have access to the drugs we take advantage of all the time.”
A meeting participant from Canada pointed out that rheumatologists there are “mandated to use it for PsA in combination with methotrexate before we can get a biologic, and for RA we can use it with Plaquenil [hydroxychloroquine] and methotrexate before we get a biologic, so we’re using it all the time.”
Asked about efficacy, the physician said the combination with methotrexate is “absolutely” efficacious. “It works really well” he said. “The problem is, you really have to watch the white cell count and liver function … and the half-life is long.”
Indeed, Dr. Ruderman said during his talk, the plasma half-life of teriflunomide, its active metabolite, is 15.5 days, which is challenging when adverse events occur. “And it’s a terrible drug in young women thinking about pregnancy because it’s teratogenic and stays around,” he said.
Leflunomide, which, notably, was “developed specifically for RA from the get-go” and not borrowed from another specialty, works by blocking de novo pyrimidine synthesis, Dr. Ruderman said. T-cell activation requires the upregulation of pyrimidine production (salvage pathways are insufficient); the “drug prevents that” by inhibiting an enzyme that catalyzes conversion of dihydroorotate to orotate, which, in turn, is converted to pyrimidine ribonucleotides, he explained.
Other potential mechanisms of action have been proposed — mainly, inhibition of tumor necrosis factor signaling and inhibition of kinase activity, including the JAK/STAT pathway — but “there’s not great data for any of them,” he said.
Loading vs Not Loading, and Its Role in PsA and Other Diseases
“We stopped loading years ago because at 100 mg for 3 days in a row, everyone has GI issues,” Dr. Ruderman said. “It may have made sense from a pharmacokinetic standpoint because [based on the long half-life] you could get to a higher drug level quicker, but not a practical standpoint, because patients would stop the drug — they couldn’t take it.” The first study to examine the necessity of loading leflunomide in a “prospective, careful way” was published in 2013. It randomized 120 patients to 100 mg or 20 mg for 3 days, followed by a 3-month open-label period of 20 mg, and found no clinical benefit with loading but more diarrhea and elevated liver enzymes.
“It tells us something about how we need to think about half-lives,” he said. “Maybe [loading is] not necessary because the biological effects are different than the drug levels.”
In the PsA space, in 2004, researchers reported a double-blind randomized trial in which 190 patients with active PsA and cutaneous psoriasis with at least 3% body surface area involvement were randomized to receive leflunomide (a loading dose followed by 20 mg/day) or placebo for 24 weeks. Almost 60% of leflunomide-treated patients, compared with 30% of placebo-treated patients, were classified as responders by the Psoriatic Arthritis Response criteria (P < .0001), “which is a soft endpoint” but was utilized at the time, Dr. Ruderman said. The researchers noted improvements in ACR20 and skin responses as well, and toxicity was similar to that reported in the RA studies.
However, approval was never sought, and the drug was infrequently prescribed, “because etanercept came out for this disease, and then adalimumab … and then the world changed,” he said.
More recently, a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA tested leflunomide plus methotrexate vs methotrexate monotherapy and was published in The Lancet Rheumatology. After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1 [standard deviation (SD), 1.4] vs 3.7 [SD, 1.3]; treatment difference, -0.6; 90% CI, -1.0 to -0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than that of the monotherapy group (34%; P = .019). Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group.
In an interview after the meeting, Dr. Ruderman explained that in his practice, about 15 years ago, leflunomide was sometimes prescribed as an alternative to a biologic change for patients whose skin disease improved significantly with ustekinumab (Stelara) but who “suddenly had more joint symptoms that they didn’t have before.”
And “we’ve found ourselves a bit recently with the same sort of story, where patients are prescribed IL-23 inhibitors like Skyrizi [risankizumab] and Tremfya [guselkumab] and their skin does really well but now they’re having more joint symptoms than previously,” he said. “Our choices are to switch to a whole different biologic, or to think about adding something as an adjunct — and maybe leflunomide is a reasonable option.”
In the last 5 years, Dr. Ruderman noted, randomized trial data has been published on leflunomide in lupus nephritis induction, and in lupus nephritis maintenance, as well as in IgG4-related disease.
Dr. Ruderman disclosed consulting and/or drug safety monitoring board work for AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Lilly, Merck, Novartis, NS Pharma, and UCB.
FROM RWCS 2024