User login
Top Spondyloarthritis Studies of 2023 Include Underdiagnosis and Treatment in IBD
A Danish study showing that about half of patients with newly diagnosed inflammatory bowel disease (IBD) had findings consistent with spondyloarthritis (SpA) was highlighted as one of last year’s more actionable studies on SpA and axial SpA (axSpa) at the 2024 Rheumatology Winter Clinical Symposium (RWCS).
“There’s a lesson here,” said Eric M. Ruderman, MD, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago, Illinois. “We’ve spent a lot of time working with the dermatologists in the last 10 years to try to coordinate what we’re doing [for psoriatic disease]. It’s time to start working with the gastroenterologists more.”
The findings offer “more evidence” for an increasingly documented overlap of IBD with SpA — whether axial or peripheral — and suggest there is underdiagnosis of SpA among patients with IBD. “It’s important,” he said at the meeting, “because if there are meaningful joint symptoms, this should be considered when making treatment choices [for IBD],” just as rheumatologists must be aware of the potential for IBD in choosing therapies.
Dr. Ruderman also urged rheumatologists making treatment decisions for axSpA to more carefully consider the role of central pain in driving residual symptoms in patients on biologic disease-modifying antirheumatic drugs (bDMARDs). He pointed to a 2023 study of patients with radiographic axSpA (r-axSpA) receiving bDMARDs that showed significant associations between high central pain and a greater odds of having higher disease activity, independent of elevated C-reactive protein (CRP) levels.
“I’ve come to the conclusion that there’s a huge amount of central pain in our patients — that it [affects] 20%-30% of our patients, no matter what rheumatologic disease they have,” he said, “and if you don’t acknowledge and consider that, you’ll keep churning through medications that aren’t going to work because you’re not addressing a fundamental issue.”
Among other key studies of 2023 highlighted by Dr. Ruderman was a large retrospective cohort study showing a similar incidence of ankylosing spondylitis (AS) in US military men and women screened for chronic back pain and the GO-BACK withdrawal and retreatment trial of golimumab suggesting that dosing can be extended.
Meanwhile, last year brought more bad news for interleukin (IL)-23 inhibition in axSpA, with the termination of a phase 2 study of tildrakizumab (Ilumya). Good news came with the US Food and Drug Administration approval in 2023 of an intravenous formulation of the IL-17 inhibitor secukinumab (Cosentyx), which will be helpful for some Medicare patients. And moving forward, the biologic pipeline is SpA is “almost all about new pathways in the IL-17 arena,” Dr. Ruderman said.
Making Good Drug Choices for the Gut and the Joints
In the study of SpA among patients with IBD, reported at the EULAR 2023 meeting in Milan, Italy, rheumatologists assessed 110 consecutive patients — 34% of whom were diagnosed with Crohn’s disease and 59% of whom had ulcerative colitis — from a Danish IBD inception cohort. The patients, about 40% of whom were male, had a mean age of 42.
At the time of IBD diagnosis, 49% had arthralgias/musculoskeletal symptoms, 52% fulfilled Assessment of SpondyloArthritis International Society (ASAS) classification criteria for peripheral SpA, and 49% had synovitis and/or enthesitis verified by ultrasound, Dr. Ruderman said.
Gastroenterologists like the integrin antagonist vedolizumab (Entyvio) for some patients with IBD because “it’s a very gut-specific drug and doesn’t have as much impact on the systemic immune system as other drugs, but because it’s gut specific, it does nothing for peripheral or axial joint symptoms,” Dr. Ruderman said in an interview after the meeting. “We’ve seen patients switched to this drug from Humira [or other biologics] and suddenly they have joint pains they never had before.”
The IL-12/23 inhibitor ustekinumab (Stelara) and the IL-23 inhibitor risankizumab (Skyrizi) are also sometimes selected for IBD, but “neither work well for patients with confirmed axSpA or inflammatory axial spine pain and arthritis,” he said. “Maybe these patients belong on a TNF [tumor necrosis factor] inhibitor or a JAK [Janus kinase] inhibitor, which will manage both the joints and the gut.”
“It’s not that we don’t talk to one another, but as we get more and more drugs in this space — both us and the gastroenterologists — it behooves us to communicate better to make sure we’re making the right choices for patients,” Dr. Ruderman said in the interview.
On the flip side, there’s a clear link between patients with axSpA who have or later develop IBD, as was further documented in 2023 by a multicenter Spanish study that evaluated patients with SpA (including both radiographic and nonradiographic axSpA) for the prevalence of undiagnosed IBD, Dr. Ruderman said at the RWCS.
The study, reported at the American College of Rheumatology (ACR) 2023 annual meeting, included only patients who were bDAMRD-naive and off of steroids for at least 30 days. The researchers used elevated fecal calprotectin levels (≥ 80 mcg/g) followed by colonoscopy — and an endoscopic capsule study or MRI if colonoscopy was normal — to confirm a diagnosis of IBD. Of 559 patients, 4.4% had such a confirmed diagnosis (95% with Crohn’s disease), and interestingly, only 30% of these patients had clinical IBD symptoms.
“These are people who had no suspicion,” Dr. Ruderman said at the meeting. “You could say that maybe not having symptoms is not a big deal, but over time, maybe there will be consequences.”
The IL-17 inhibitors ixekizumab (Taltz), secukinumab, and bimekizumab (Bimzelx) are generally felt to be contraindicated in patients who have confirmed IBD, Dr. Ruderman noted in the interview. “While we don’t want to necessarily avoid those drugs, we need to be aware of the potential [for IBD],” he said, “and we need to have a low threshold of suspicion if our patients develop any GI symptoms.”
Considering Noninflammatory Residual Pain
The 2023 central pain study that caught Dr. Ruderman’s attention — research reported at the EULAR 2023 meeting — looked at 70 patients with r-axSpA receiving bDMARD treatment (mostly TNF inhibitors) who were being followed in an extension of the German Spondyloarthritis Inception Cohort. Investigators used the Widespread Pain Index (WPI) to help quantify central pain/central sensitization and the Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) to measure disease activity.
“Central pain was actually associated with having residual symptoms,” Dr. Ruderman said at the RWCS. Higher WPI scores were significantly associated with higher ASDAS-CRP scores, and a high WPI was also associated with higher odds of having high or very high disease activity (ASDAS > 2.1), independent of other factors including elevated CRP, the investigators reported in their abstract.
Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, commented that “we don’t have great [non-opioid] treatments for pain,” prompting Dr. Ruderman to emphasize the importance of “resisting the urge to [automatically] switch to another biologic” without trying to discern whether residual pain is inflammatory or noninflammatory in nature.
“I’m really comfortable with this,” Dr. Ruderman said, noting that he prescribes drugs like duloxetine or pregabalin for suspected central pain. “For the statin (for cardiovascular disease prevention), I’m more likely to turn back to the primary care physician and work with them, but here it’s part of what we’re treating — it becomes part of our tool kits.”
The central pain issue, Dr. Ruderman said after the meeting, is one of recognition and nomenclature. In the last few years, “there’s been a tendency to get away from secondary fibromyalgia as a label. There’s a lot of baggage with the diagnosis, unfortunately,” he said in the interview. “And it’s all connected. … It’s very likely that the [central] pain signaling is triggered by the inflammatory pain in the first place.”
A New Look at Sex-Specific Incidence of AS
The study on AS in a retrospective cohort of 729,000 working-age US military service members “flew under the radar,” but its finding of a similar incidence in men and women who underwent screening for chronic back pain is “fascinating,” Dr. Ruderman said. Compared with females, men were not significantly more likely to have a diagnosis of AS (adjusted odds ratio [OR], 0.79; 95% CI, 0.61-1.02; P = .072), the researchers reported.
“We’ve always assumed that AS is a male disease, and that, as we got into nonradiographic axSpA, we would see more women. This study calls that into question,” he said.
More Light on bDMARD Dosage Extension and Withdrawal
The GO-BACK study of the TNF inhibitor golimumab (Simponi) randomized 188 patients with inactive nonradiographic axSpA after 6 months of 50 mg golimumab monthly to treatment withdrawal/monthly placebo, continued monthly treatment, or treatment every 2 months. The take-home message, Dr. Ruderman said, is that “withdrawal, but not reduction in dose, led to a higher risk of flare.”
Also notable in this study published in 2023 is that “almost 100% of those who flared were recaptured with the reinitiation of monthly dosing,” he said. “So you don’t lose if you try to stop … [although] I don’t think that will ever be a successful strategy.” (The proportion of patients without a disease flare over 12 months was 34% in the withdrawal group, 68% in the extended dosing group, and 84% in the continued monthly treatment group.)
Dosing extensions have been shown to be potentially viable with other biologics, “but with this one, it looks like you can spread it out almost with impunity because it doesn’t look like there’s much difference” between continuing monthly and extending, Dr. Kavanaugh commented.
Another study from 2023 of the IL-17A inhibitor ixekizumab in axSpA similarly showed a high recapture rate for patients who withdrew from therapy and then flared. In this phase 3 extension study in which 155 patients with inactive or low-level disease were randomized at week 24 to continued ixekizumab or placebo, 53% of placebo patients flared by 2 years, compared with 13% in the ixekizumab arm. Of those who flared, 96% recaptured low disease activity with re-initiation of therapy.
“It’s the same story. You might get away with [stopping the therapy] because it’s not 100% who flared. But is it worth it?” Dr. Ruderman said.
IL-23 Inhibition in Axial Disease and the Pipeline
Is the chapter on IL-23 inhibitors closed for axSpA? Aside from a possible role for axial disease in psoriatic arthritis (PsA), it likely is, Dr. Ruderman said, pointing to the phase 2 randomized, double-blind, placebo-controlled study of tildrakizumab in patients with AS that was terminated at week 24 after the drug showed no difference in efficacy from placebo.
Dr. Kavanaugh agreed. “This adds to the data on risankizumab and ustekinumab in studies done properly in AS,” he said. “There’s no benefit.”
The “real issue” still to be determined, said Dr. Ruderman, “is what is the role of IL-23 inhibitors in patients with axial PsA?”
A post-hoc analysis of data from the SELECT PsA 1 and 2 trials, published in 2023, showed greater improvement in the overall Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score in patients with axial disease who received 15 mg upadacitinib (Rinvoq), compared with placebo.
“It suggests there’s improvement in the patients with axial PsA as defined [by a high BASDAI score], but they didn’t compare this with patients without axial disease … it’s muddy,” Dr. Ruderman said. Other research that’s underway should provide clarity, Dr. Kavanaugh said.
The pipeline for new treatments for SpA, including axSpA, is focused on new biologics targeting the IL-17 pathways, as well as a fair number of targeted synthetics, Dr. Ruderman said. “What will be interesting to me is what happens with the TYK2 inhibitors … because one of the postulated mechanisms is that the IL-23 signals through TYK-2,” he said. “So if that’s the mechanism, will they really help our patients with axial disease? We need the trials to find out.”
The intravenous formulation of secukinumab, approved in 2023 for AS, nr-axSpA, and PsA, is a “nice addition to our armamentarium, Dr. Ruderman noted in his 2023 review. “For years, a patient doing well on an IL-17 inhibitor for their axial disease or their psoriatic disease would hit Medicare age and suddenly couldn’t afford subcutaneous administration, and we had to switch them over to an IV-TNF inhibitor,” he said. “Now we have an IV IL-17 inhibitor.”
A Danish study showing that about half of patients with newly diagnosed inflammatory bowel disease (IBD) had findings consistent with spondyloarthritis (SpA) was highlighted as one of last year’s more actionable studies on SpA and axial SpA (axSpa) at the 2024 Rheumatology Winter Clinical Symposium (RWCS).
“There’s a lesson here,” said Eric M. Ruderman, MD, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago, Illinois. “We’ve spent a lot of time working with the dermatologists in the last 10 years to try to coordinate what we’re doing [for psoriatic disease]. It’s time to start working with the gastroenterologists more.”
The findings offer “more evidence” for an increasingly documented overlap of IBD with SpA — whether axial or peripheral — and suggest there is underdiagnosis of SpA among patients with IBD. “It’s important,” he said at the meeting, “because if there are meaningful joint symptoms, this should be considered when making treatment choices [for IBD],” just as rheumatologists must be aware of the potential for IBD in choosing therapies.
Dr. Ruderman also urged rheumatologists making treatment decisions for axSpA to more carefully consider the role of central pain in driving residual symptoms in patients on biologic disease-modifying antirheumatic drugs (bDMARDs). He pointed to a 2023 study of patients with radiographic axSpA (r-axSpA) receiving bDMARDs that showed significant associations between high central pain and a greater odds of having higher disease activity, independent of elevated C-reactive protein (CRP) levels.
“I’ve come to the conclusion that there’s a huge amount of central pain in our patients — that it [affects] 20%-30% of our patients, no matter what rheumatologic disease they have,” he said, “and if you don’t acknowledge and consider that, you’ll keep churning through medications that aren’t going to work because you’re not addressing a fundamental issue.”
Among other key studies of 2023 highlighted by Dr. Ruderman was a large retrospective cohort study showing a similar incidence of ankylosing spondylitis (AS) in US military men and women screened for chronic back pain and the GO-BACK withdrawal and retreatment trial of golimumab suggesting that dosing can be extended.
Meanwhile, last year brought more bad news for interleukin (IL)-23 inhibition in axSpA, with the termination of a phase 2 study of tildrakizumab (Ilumya). Good news came with the US Food and Drug Administration approval in 2023 of an intravenous formulation of the IL-17 inhibitor secukinumab (Cosentyx), which will be helpful for some Medicare patients. And moving forward, the biologic pipeline is SpA is “almost all about new pathways in the IL-17 arena,” Dr. Ruderman said.
Making Good Drug Choices for the Gut and the Joints
In the study of SpA among patients with IBD, reported at the EULAR 2023 meeting in Milan, Italy, rheumatologists assessed 110 consecutive patients — 34% of whom were diagnosed with Crohn’s disease and 59% of whom had ulcerative colitis — from a Danish IBD inception cohort. The patients, about 40% of whom were male, had a mean age of 42.
At the time of IBD diagnosis, 49% had arthralgias/musculoskeletal symptoms, 52% fulfilled Assessment of SpondyloArthritis International Society (ASAS) classification criteria for peripheral SpA, and 49% had synovitis and/or enthesitis verified by ultrasound, Dr. Ruderman said.
Gastroenterologists like the integrin antagonist vedolizumab (Entyvio) for some patients with IBD because “it’s a very gut-specific drug and doesn’t have as much impact on the systemic immune system as other drugs, but because it’s gut specific, it does nothing for peripheral or axial joint symptoms,” Dr. Ruderman said in an interview after the meeting. “We’ve seen patients switched to this drug from Humira [or other biologics] and suddenly they have joint pains they never had before.”
The IL-12/23 inhibitor ustekinumab (Stelara) and the IL-23 inhibitor risankizumab (Skyrizi) are also sometimes selected for IBD, but “neither work well for patients with confirmed axSpA or inflammatory axial spine pain and arthritis,” he said. “Maybe these patients belong on a TNF [tumor necrosis factor] inhibitor or a JAK [Janus kinase] inhibitor, which will manage both the joints and the gut.”
“It’s not that we don’t talk to one another, but as we get more and more drugs in this space — both us and the gastroenterologists — it behooves us to communicate better to make sure we’re making the right choices for patients,” Dr. Ruderman said in the interview.
On the flip side, there’s a clear link between patients with axSpA who have or later develop IBD, as was further documented in 2023 by a multicenter Spanish study that evaluated patients with SpA (including both radiographic and nonradiographic axSpA) for the prevalence of undiagnosed IBD, Dr. Ruderman said at the RWCS.
The study, reported at the American College of Rheumatology (ACR) 2023 annual meeting, included only patients who were bDAMRD-naive and off of steroids for at least 30 days. The researchers used elevated fecal calprotectin levels (≥ 80 mcg/g) followed by colonoscopy — and an endoscopic capsule study or MRI if colonoscopy was normal — to confirm a diagnosis of IBD. Of 559 patients, 4.4% had such a confirmed diagnosis (95% with Crohn’s disease), and interestingly, only 30% of these patients had clinical IBD symptoms.
“These are people who had no suspicion,” Dr. Ruderman said at the meeting. “You could say that maybe not having symptoms is not a big deal, but over time, maybe there will be consequences.”
The IL-17 inhibitors ixekizumab (Taltz), secukinumab, and bimekizumab (Bimzelx) are generally felt to be contraindicated in patients who have confirmed IBD, Dr. Ruderman noted in the interview. “While we don’t want to necessarily avoid those drugs, we need to be aware of the potential [for IBD],” he said, “and we need to have a low threshold of suspicion if our patients develop any GI symptoms.”
Considering Noninflammatory Residual Pain
The 2023 central pain study that caught Dr. Ruderman’s attention — research reported at the EULAR 2023 meeting — looked at 70 patients with r-axSpA receiving bDMARD treatment (mostly TNF inhibitors) who were being followed in an extension of the German Spondyloarthritis Inception Cohort. Investigators used the Widespread Pain Index (WPI) to help quantify central pain/central sensitization and the Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) to measure disease activity.
“Central pain was actually associated with having residual symptoms,” Dr. Ruderman said at the RWCS. Higher WPI scores were significantly associated with higher ASDAS-CRP scores, and a high WPI was also associated with higher odds of having high or very high disease activity (ASDAS > 2.1), independent of other factors including elevated CRP, the investigators reported in their abstract.
Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, commented that “we don’t have great [non-opioid] treatments for pain,” prompting Dr. Ruderman to emphasize the importance of “resisting the urge to [automatically] switch to another biologic” without trying to discern whether residual pain is inflammatory or noninflammatory in nature.
“I’m really comfortable with this,” Dr. Ruderman said, noting that he prescribes drugs like duloxetine or pregabalin for suspected central pain. “For the statin (for cardiovascular disease prevention), I’m more likely to turn back to the primary care physician and work with them, but here it’s part of what we’re treating — it becomes part of our tool kits.”
The central pain issue, Dr. Ruderman said after the meeting, is one of recognition and nomenclature. In the last few years, “there’s been a tendency to get away from secondary fibromyalgia as a label. There’s a lot of baggage with the diagnosis, unfortunately,” he said in the interview. “And it’s all connected. … It’s very likely that the [central] pain signaling is triggered by the inflammatory pain in the first place.”
A New Look at Sex-Specific Incidence of AS
The study on AS in a retrospective cohort of 729,000 working-age US military service members “flew under the radar,” but its finding of a similar incidence in men and women who underwent screening for chronic back pain is “fascinating,” Dr. Ruderman said. Compared with females, men were not significantly more likely to have a diagnosis of AS (adjusted odds ratio [OR], 0.79; 95% CI, 0.61-1.02; P = .072), the researchers reported.
“We’ve always assumed that AS is a male disease, and that, as we got into nonradiographic axSpA, we would see more women. This study calls that into question,” he said.
More Light on bDMARD Dosage Extension and Withdrawal
The GO-BACK study of the TNF inhibitor golimumab (Simponi) randomized 188 patients with inactive nonradiographic axSpA after 6 months of 50 mg golimumab monthly to treatment withdrawal/monthly placebo, continued monthly treatment, or treatment every 2 months. The take-home message, Dr. Ruderman said, is that “withdrawal, but not reduction in dose, led to a higher risk of flare.”
Also notable in this study published in 2023 is that “almost 100% of those who flared were recaptured with the reinitiation of monthly dosing,” he said. “So you don’t lose if you try to stop … [although] I don’t think that will ever be a successful strategy.” (The proportion of patients without a disease flare over 12 months was 34% in the withdrawal group, 68% in the extended dosing group, and 84% in the continued monthly treatment group.)
Dosing extensions have been shown to be potentially viable with other biologics, “but with this one, it looks like you can spread it out almost with impunity because it doesn’t look like there’s much difference” between continuing monthly and extending, Dr. Kavanaugh commented.
Another study from 2023 of the IL-17A inhibitor ixekizumab in axSpA similarly showed a high recapture rate for patients who withdrew from therapy and then flared. In this phase 3 extension study in which 155 patients with inactive or low-level disease were randomized at week 24 to continued ixekizumab or placebo, 53% of placebo patients flared by 2 years, compared with 13% in the ixekizumab arm. Of those who flared, 96% recaptured low disease activity with re-initiation of therapy.
“It’s the same story. You might get away with [stopping the therapy] because it’s not 100% who flared. But is it worth it?” Dr. Ruderman said.
IL-23 Inhibition in Axial Disease and the Pipeline
Is the chapter on IL-23 inhibitors closed for axSpA? Aside from a possible role for axial disease in psoriatic arthritis (PsA), it likely is, Dr. Ruderman said, pointing to the phase 2 randomized, double-blind, placebo-controlled study of tildrakizumab in patients with AS that was terminated at week 24 after the drug showed no difference in efficacy from placebo.
Dr. Kavanaugh agreed. “This adds to the data on risankizumab and ustekinumab in studies done properly in AS,” he said. “There’s no benefit.”
The “real issue” still to be determined, said Dr. Ruderman, “is what is the role of IL-23 inhibitors in patients with axial PsA?”
A post-hoc analysis of data from the SELECT PsA 1 and 2 trials, published in 2023, showed greater improvement in the overall Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score in patients with axial disease who received 15 mg upadacitinib (Rinvoq), compared with placebo.
“It suggests there’s improvement in the patients with axial PsA as defined [by a high BASDAI score], but they didn’t compare this with patients without axial disease … it’s muddy,” Dr. Ruderman said. Other research that’s underway should provide clarity, Dr. Kavanaugh said.
The pipeline for new treatments for SpA, including axSpA, is focused on new biologics targeting the IL-17 pathways, as well as a fair number of targeted synthetics, Dr. Ruderman said. “What will be interesting to me is what happens with the TYK2 inhibitors … because one of the postulated mechanisms is that the IL-23 signals through TYK-2,” he said. “So if that’s the mechanism, will they really help our patients with axial disease? We need the trials to find out.”
The intravenous formulation of secukinumab, approved in 2023 for AS, nr-axSpA, and PsA, is a “nice addition to our armamentarium, Dr. Ruderman noted in his 2023 review. “For years, a patient doing well on an IL-17 inhibitor for their axial disease or their psoriatic disease would hit Medicare age and suddenly couldn’t afford subcutaneous administration, and we had to switch them over to an IV-TNF inhibitor,” he said. “Now we have an IV IL-17 inhibitor.”
A Danish study showing that about half of patients with newly diagnosed inflammatory bowel disease (IBD) had findings consistent with spondyloarthritis (SpA) was highlighted as one of last year’s more actionable studies on SpA and axial SpA (axSpa) at the 2024 Rheumatology Winter Clinical Symposium (RWCS).
“There’s a lesson here,” said Eric M. Ruderman, MD, professor of medicine and associate chief of clinical affairs in the division of rheumatology at Northwestern University Feinberg School of Medicine, Chicago, Illinois. “We’ve spent a lot of time working with the dermatologists in the last 10 years to try to coordinate what we’re doing [for psoriatic disease]. It’s time to start working with the gastroenterologists more.”
The findings offer “more evidence” for an increasingly documented overlap of IBD with SpA — whether axial or peripheral — and suggest there is underdiagnosis of SpA among patients with IBD. “It’s important,” he said at the meeting, “because if there are meaningful joint symptoms, this should be considered when making treatment choices [for IBD],” just as rheumatologists must be aware of the potential for IBD in choosing therapies.
Dr. Ruderman also urged rheumatologists making treatment decisions for axSpA to more carefully consider the role of central pain in driving residual symptoms in patients on biologic disease-modifying antirheumatic drugs (bDMARDs). He pointed to a 2023 study of patients with radiographic axSpA (r-axSpA) receiving bDMARDs that showed significant associations between high central pain and a greater odds of having higher disease activity, independent of elevated C-reactive protein (CRP) levels.
“I’ve come to the conclusion that there’s a huge amount of central pain in our patients — that it [affects] 20%-30% of our patients, no matter what rheumatologic disease they have,” he said, “and if you don’t acknowledge and consider that, you’ll keep churning through medications that aren’t going to work because you’re not addressing a fundamental issue.”
Among other key studies of 2023 highlighted by Dr. Ruderman was a large retrospective cohort study showing a similar incidence of ankylosing spondylitis (AS) in US military men and women screened for chronic back pain and the GO-BACK withdrawal and retreatment trial of golimumab suggesting that dosing can be extended.
Meanwhile, last year brought more bad news for interleukin (IL)-23 inhibition in axSpA, with the termination of a phase 2 study of tildrakizumab (Ilumya). Good news came with the US Food and Drug Administration approval in 2023 of an intravenous formulation of the IL-17 inhibitor secukinumab (Cosentyx), which will be helpful for some Medicare patients. And moving forward, the biologic pipeline is SpA is “almost all about new pathways in the IL-17 arena,” Dr. Ruderman said.
Making Good Drug Choices for the Gut and the Joints
In the study of SpA among patients with IBD, reported at the EULAR 2023 meeting in Milan, Italy, rheumatologists assessed 110 consecutive patients — 34% of whom were diagnosed with Crohn’s disease and 59% of whom had ulcerative colitis — from a Danish IBD inception cohort. The patients, about 40% of whom were male, had a mean age of 42.
At the time of IBD diagnosis, 49% had arthralgias/musculoskeletal symptoms, 52% fulfilled Assessment of SpondyloArthritis International Society (ASAS) classification criteria for peripheral SpA, and 49% had synovitis and/or enthesitis verified by ultrasound, Dr. Ruderman said.
Gastroenterologists like the integrin antagonist vedolizumab (Entyvio) for some patients with IBD because “it’s a very gut-specific drug and doesn’t have as much impact on the systemic immune system as other drugs, but because it’s gut specific, it does nothing for peripheral or axial joint symptoms,” Dr. Ruderman said in an interview after the meeting. “We’ve seen patients switched to this drug from Humira [or other biologics] and suddenly they have joint pains they never had before.”
The IL-12/23 inhibitor ustekinumab (Stelara) and the IL-23 inhibitor risankizumab (Skyrizi) are also sometimes selected for IBD, but “neither work well for patients with confirmed axSpA or inflammatory axial spine pain and arthritis,” he said. “Maybe these patients belong on a TNF [tumor necrosis factor] inhibitor or a JAK [Janus kinase] inhibitor, which will manage both the joints and the gut.”
“It’s not that we don’t talk to one another, but as we get more and more drugs in this space — both us and the gastroenterologists — it behooves us to communicate better to make sure we’re making the right choices for patients,” Dr. Ruderman said in the interview.
On the flip side, there’s a clear link between patients with axSpA who have or later develop IBD, as was further documented in 2023 by a multicenter Spanish study that evaluated patients with SpA (including both radiographic and nonradiographic axSpA) for the prevalence of undiagnosed IBD, Dr. Ruderman said at the RWCS.
The study, reported at the American College of Rheumatology (ACR) 2023 annual meeting, included only patients who were bDAMRD-naive and off of steroids for at least 30 days. The researchers used elevated fecal calprotectin levels (≥ 80 mcg/g) followed by colonoscopy — and an endoscopic capsule study or MRI if colonoscopy was normal — to confirm a diagnosis of IBD. Of 559 patients, 4.4% had such a confirmed diagnosis (95% with Crohn’s disease), and interestingly, only 30% of these patients had clinical IBD symptoms.
“These are people who had no suspicion,” Dr. Ruderman said at the meeting. “You could say that maybe not having symptoms is not a big deal, but over time, maybe there will be consequences.”
The IL-17 inhibitors ixekizumab (Taltz), secukinumab, and bimekizumab (Bimzelx) are generally felt to be contraindicated in patients who have confirmed IBD, Dr. Ruderman noted in the interview. “While we don’t want to necessarily avoid those drugs, we need to be aware of the potential [for IBD],” he said, “and we need to have a low threshold of suspicion if our patients develop any GI symptoms.”
Considering Noninflammatory Residual Pain
The 2023 central pain study that caught Dr. Ruderman’s attention — research reported at the EULAR 2023 meeting — looked at 70 patients with r-axSpA receiving bDMARD treatment (mostly TNF inhibitors) who were being followed in an extension of the German Spondyloarthritis Inception Cohort. Investigators used the Widespread Pain Index (WPI) to help quantify central pain/central sensitization and the Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) to measure disease activity.
“Central pain was actually associated with having residual symptoms,” Dr. Ruderman said at the RWCS. Higher WPI scores were significantly associated with higher ASDAS-CRP scores, and a high WPI was also associated with higher odds of having high or very high disease activity (ASDAS > 2.1), independent of other factors including elevated CRP, the investigators reported in their abstract.
Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, commented that “we don’t have great [non-opioid] treatments for pain,” prompting Dr. Ruderman to emphasize the importance of “resisting the urge to [automatically] switch to another biologic” without trying to discern whether residual pain is inflammatory or noninflammatory in nature.
“I’m really comfortable with this,” Dr. Ruderman said, noting that he prescribes drugs like duloxetine or pregabalin for suspected central pain. “For the statin (for cardiovascular disease prevention), I’m more likely to turn back to the primary care physician and work with them, but here it’s part of what we’re treating — it becomes part of our tool kits.”
The central pain issue, Dr. Ruderman said after the meeting, is one of recognition and nomenclature. In the last few years, “there’s been a tendency to get away from secondary fibromyalgia as a label. There’s a lot of baggage with the diagnosis, unfortunately,” he said in the interview. “And it’s all connected. … It’s very likely that the [central] pain signaling is triggered by the inflammatory pain in the first place.”
A New Look at Sex-Specific Incidence of AS
The study on AS in a retrospective cohort of 729,000 working-age US military service members “flew under the radar,” but its finding of a similar incidence in men and women who underwent screening for chronic back pain is “fascinating,” Dr. Ruderman said. Compared with females, men were not significantly more likely to have a diagnosis of AS (adjusted odds ratio [OR], 0.79; 95% CI, 0.61-1.02; P = .072), the researchers reported.
“We’ve always assumed that AS is a male disease, and that, as we got into nonradiographic axSpA, we would see more women. This study calls that into question,” he said.
More Light on bDMARD Dosage Extension and Withdrawal
The GO-BACK study of the TNF inhibitor golimumab (Simponi) randomized 188 patients with inactive nonradiographic axSpA after 6 months of 50 mg golimumab monthly to treatment withdrawal/monthly placebo, continued monthly treatment, or treatment every 2 months. The take-home message, Dr. Ruderman said, is that “withdrawal, but not reduction in dose, led to a higher risk of flare.”
Also notable in this study published in 2023 is that “almost 100% of those who flared were recaptured with the reinitiation of monthly dosing,” he said. “So you don’t lose if you try to stop … [although] I don’t think that will ever be a successful strategy.” (The proportion of patients without a disease flare over 12 months was 34% in the withdrawal group, 68% in the extended dosing group, and 84% in the continued monthly treatment group.)
Dosing extensions have been shown to be potentially viable with other biologics, “but with this one, it looks like you can spread it out almost with impunity because it doesn’t look like there’s much difference” between continuing monthly and extending, Dr. Kavanaugh commented.
Another study from 2023 of the IL-17A inhibitor ixekizumab in axSpA similarly showed a high recapture rate for patients who withdrew from therapy and then flared. In this phase 3 extension study in which 155 patients with inactive or low-level disease were randomized at week 24 to continued ixekizumab or placebo, 53% of placebo patients flared by 2 years, compared with 13% in the ixekizumab arm. Of those who flared, 96% recaptured low disease activity with re-initiation of therapy.
“It’s the same story. You might get away with [stopping the therapy] because it’s not 100% who flared. But is it worth it?” Dr. Ruderman said.
IL-23 Inhibition in Axial Disease and the Pipeline
Is the chapter on IL-23 inhibitors closed for axSpA? Aside from a possible role for axial disease in psoriatic arthritis (PsA), it likely is, Dr. Ruderman said, pointing to the phase 2 randomized, double-blind, placebo-controlled study of tildrakizumab in patients with AS that was terminated at week 24 after the drug showed no difference in efficacy from placebo.
Dr. Kavanaugh agreed. “This adds to the data on risankizumab and ustekinumab in studies done properly in AS,” he said. “There’s no benefit.”
The “real issue” still to be determined, said Dr. Ruderman, “is what is the role of IL-23 inhibitors in patients with axial PsA?”
A post-hoc analysis of data from the SELECT PsA 1 and 2 trials, published in 2023, showed greater improvement in the overall Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score in patients with axial disease who received 15 mg upadacitinib (Rinvoq), compared with placebo.
“It suggests there’s improvement in the patients with axial PsA as defined [by a high BASDAI score], but they didn’t compare this with patients without axial disease … it’s muddy,” Dr. Ruderman said. Other research that’s underway should provide clarity, Dr. Kavanaugh said.
The pipeline for new treatments for SpA, including axSpA, is focused on new biologics targeting the IL-17 pathways, as well as a fair number of targeted synthetics, Dr. Ruderman said. “What will be interesting to me is what happens with the TYK2 inhibitors … because one of the postulated mechanisms is that the IL-23 signals through TYK-2,” he said. “So if that’s the mechanism, will they really help our patients with axial disease? We need the trials to find out.”
The intravenous formulation of secukinumab, approved in 2023 for AS, nr-axSpA, and PsA, is a “nice addition to our armamentarium, Dr. Ruderman noted in his 2023 review. “For years, a patient doing well on an IL-17 inhibitor for their axial disease or their psoriatic disease would hit Medicare age and suddenly couldn’t afford subcutaneous administration, and we had to switch them over to an IV-TNF inhibitor,” he said. “Now we have an IV IL-17 inhibitor.”
FROM RWCS 2024
Climate Change and AD: New Review Shows Negative Impacts and Unknowns
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
A new . But it also showed the extent to which research is lacking.
“There’s not as much out there as one might expect, given that this is the most common dermatologic disease and one of the most burdensome diseases worldwide,” said Katrina Abuabara, MD, of the department of dermatology at the University of California, San Francisco, one of the senior authors of the review.
“There’s a genetic predisposition to AD, but it’s certainly very environmentally patterned,” she said in an interview. “Given that we know there are strong environmental influences, it’s an obvious example of how climate change affects our health ... It is one that may be underappreciated and that could give us near-term information.”
Indeed, she and her coauthors emphasized in their paper, “AD could serve as a case study for climatic impacts on health.” The review, which looked beyond the realm of air pollution, was published in Allergy, the journal of the European Academy of Allergy and Clinical Immunology.
Dr. Abuabara, UCSF dermatologist Sheng-Pei Wang, MD, MPH, and their coauthors — dermatologists and others from the United States, Europe, Brazil, and India — were convened by the International Eczema Council and teamed up with a biologist and climate science expert, Camilo Mora, PhD, of the University of Hawaii at Mānoa, Honolulu. Because research to date has focused on air pollution, with the impact of other hazards that Dr. Abuabara said were “a lot less developed and organized,” they used a framework and search strategy developed by Dr. Mora that looks at 10 climatic hazards related to greenhouse gas emissions, including heat waves, drought, precipitation, wildfires, and sea level rise.
“Given that this [framework] was already out there in the literature, we thought it would give us a structure and a nice way to organize the literature,” Dr. Abuabara said. While the literature is too heterogeneous for a systematic review and meta-analysis, the researchers used a systematic approach, she explained.
Lawrence Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and a coauthor of the paper, said in an e-mail that the review raises “our consciousness about how these [climate] changes may be impacting atopic dermatitis.”
Researchers have “much work to do to understand the evolving impact on AD development and course, and even more to figure out how to avoid extreme weather’s impact to minimize its effects on inflammatory skin diseases,” he said. “In the meantime, this paper is a call for the health care community to recognize a set of factors that can influence our patients’ dermatitis and lives.”
Mixed Results, But Negative Impacts Overall
The researchers identified 18 studies across most of the 10 climatic hazards with evidence for an impact on AD, the majority of which demonstrated harmful effects on various aspects of AD — most commonly on AD-related health care utilization and severity/flares. Only three of the studies examined AD prevalence and notably, none looked at incidence.
The impact of climatic hazards on AD appears to vary depending on the geographic region and its baseline climate, the authors said. A study in South Korea, for instance, found that in areas declared as disaster zones after storms and heavy rains, the number of AD-related outpatient visits increased for all ages. And a study in the United States showed an increased prevalence of childhood eczema in states with higher mean annual precipitation. However, some other studies on precipitation found no associations.
Just as published studies on precipitation yielded mixed results, so have studies on warming temperatures, Dr. Abuabara and her colleagues reported in their paper, with higher temperatures found to be positively associated with severity of AD symptoms in a study among patients with AD living in a region of Southern Italy, but decreased AD-related health care utilization in a study in Denmark.
In another study of over 5,500 children enrolled in an eczema registry in the United States between 2004 and 2012, higher temperature (odds ratio [OR] = 0.90, P < .001) and increased sun exposure (OR = 0.93, P = .009) were associated with poorly controlled eczema, after the researchers controlled for gender, race, income, and topical medication use.
Studies From 10 Countries Reviewed
Across the 18 studies identified in the review, data were collected in 10 countries. Five studies were conducted in the United States, one used global data, six were from Asia, and the others were from Europe and Africa. Data are lacking, the researchers wrote, in many parts of the world, including coastal regions of the tropics that are projected to experience the largest cumulative climatic hazards.
Future research should not only cover more geographic areas — especially those most impacted by climate change — but should examine impacts on AD incidence, prevalence, and “long-term monitoring of disease activity over time at the individual level,” the researchers recommended. Research should also aim to integrate multiple climatic factors and types of climate data, they said.
“As researchers, we always like to distill things down, but with climatic hazards like warming, you have to integrate other factors such as what the baseline temperature is and how precipitation is involved,” Dr. Abuabara said in the interview. With precipitation, similarly, associated factors such as outdoor humidity, pollen, and pollution exposure may also be at play for AD. Overall, she said, “you have to integrate many types of data.”
In addition to their literature review, the researchers created maps comparing the past, present, and future burden of climatic hazards to AD prevalence data. One pair of maps illustrates global cumulative exposure to climatic hazards in 2005 in parallel with the estimated annual change in AD prevalence in the subsequent decade. “It’s meant to be descriptive,” Dr. Abuabara said in the interview. The maps show alignment “between the areas experiencing the most climatic hazards and those where we subsequently saw the most rapid changes in AD.”
The paper also describes how climatic factors impact skin physiology and AD — exacerbating barrier impairment, immune dysregulation, dysbiosis, and pruritus — and how there are differential impacts on vulnerable and displaced populations with AD. It also briefly addresses air pollution, which was not included in the review framework but is impacted by wildfire and other included climatic factors.
The Need to Better Track AD, Anticipate Clinical Impact
“Outside of epidemiology, [clinicians and others] may not realize we actually have fairly poor measures of prevalence and severity of AD and disease flare over time,” Dr. Abuabara said. So “improving the ways we can measure this disease and getting more detailed data is important” for assessing the impact of climate changes.
More skin measures should be incorporated into large national health surveys, for one. “Skin doesn’t come to mind as much as diseases like heart disease and diabetes,” she said, and when surveys ask about AD, “they often don’t ask specific enough questions or ask about severity.” The clinical impacts of adverse climatic changes and extreme weather events — sudden therapy interruption, particularly of systemic agents, and delayed treatment, for instance — should be reflected in the planning and provision of dermatology services, Dr. Abuabara and her coauthors wrote.
There are currently no evidence-based recommendations for what patients with AD can do differently when faced with wildfire smoke or other climatic hazards, other than general recommendations, for instance, to reduce exposure to wildfire smoke and aeroallergens, she said in the interview. But “overall, the field has moved to more proactive treatment patterns ... toward providing anticipatory guidance and having individualized treatment plans that give people the tools to be ready to step things up or counteract [flares or worsening] if they need to.”
She and her San Francisco–based coauthors have already experienced the impact of wildfires firsthand. “It was amazing — in the period right after a major wildfire hundreds of miles away from the Bay area, we saw a huge spike in visits for itch and for eczema,” she said, referring to research on AD clinic visits after the 2018 California Camp Fire. “It showed up dramatically in the data,” said Dr. Abuabara, one of the authors of that study.
The new review adds to a growing body of literature documenting health impacts of climate change and advocating for action. In September 2021, more than 230 medical journals, including the New England Journal of Medicine — though not any dermatology journals — published an editorial calling for emergency action to limit global warming and protect health.
The following year, a commentary published across four dermatology journals discussed current and future impacts of climate change and urged dermatologists to become more engaged in finding solutions to help mitigate and adapt to climate change.
More recently, dermatologists have published about the environmental impact of professional practices such as print journals and meeting samples using single-use plastics.
Dr. Abuabara disclosed to Allergy that she is a consultant for TARGET RWE and Amgen and that her institution receives grants for research from Pfizer and LaRoche Posay. Dr. Eichenfield reported serving as a scientific adviser, consultant, and/or study investigator for Pfizer, AbbVie, Amgen and other companies. Dr. Wang disclosed that she is an International Eczema Council Fellow with financial support from Abbvie. Other authors had multiple disclosures.
FROM ALLERGY
Glycemic control in pregnancy: The role of CGM for T1D and T2D, and intrapartum management
WASHINGTON — Continuous glucose monitoring (CGM) is widely used during pregnancy for individuals with type 1 diabetes — with pregnancy-specific target metrics now chosen and benefits on perinatal outcomes demonstrated — but more research is needed to elucidate its role in the growing population of pregnant people with type 2 diabetes and gestational diabetes (GDM). And overall, there are still “many more questions unanswered about CGM use in pregnancy than what we have answered,” Celeste Durnwald, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
There’s much to learn about how to best interpret “the detailed and complex data that CGM provides,” and what targets in addition to time in range (TIR) are most important, said Dr. Durnwald, director of the perinatal diabetes program and associate professor of ob.gyn. at the Hospital of the University of Pennsylvania, Philadelphia, in a presentation on CGM.
Among other questions are whether fasting glucose is “as important in the era of CGM,” and whether there should be different glycemic targets for nocturnal versus daytime TIR, she said. Moreover, questions justifiably remain about whether the TIR targets for type 1 diabetes in pregnancy are indeed optimal, she said in a discussion period.
Ongoing research is looking at whether CGM can motivate and guide patients with GDM through diet and lifestyle changes such that “we can see changes in amounts of medication we use,” Dr. Durnwald noted in her presentation. “There’s a whole breadth of research looking at whether CGM can help predict diagnosis of GDM, large for gestational age, or preeclampsia, and what are the targets.”
Maternal hypoglycemia during pregnancy — a time when strict glycemic control is recommended to reduce the risk of congenital malformations and other fetal and neonatal morbidity — remains a concern in type 1 diabetes, even with widespread use of CGM in this population, said Barak Rosenn, MD, during a presentation on glycemic control in type 1 diabetes.
A pilot study of a newly designed pregnancy-specific closed-loop insulin delivery system, published last year (Diabetes Care. 2023;46:1425-31), has offered the first “really encouraging information about the ability to use our most up-to-date technology to help our type 1 patients maintain strict control and at the same time decrease their risk of severe hypoglycemia,” said Dr. Rosenn, a maternal-fetal medicine specialist at the Jersey City Medical Center, Jersey City, New Jersey.
Guidance for tight intrapartum glucose control, meanwhile, has been backed by little evidence, said Michal Fishel Bartal, MD, MS, and some recent studies and reviews have shown little to no effect of such tight control on neonatal hypoglycemia, which is the aim of the guidance.
“We need to reexamine current recommendations,” said Dr. Bartal, assistant professor in the division of maternal-fetal medicine at the University of Texas Health Science Center, Houston, during a presentation on intrapartum care. “There’s very limited evidence-based data for the way we manage people with diabetes [during labor and delivery].”
The Knowns And Unknowns of CGM in Pregnancy
The multicenter, international CONCEPTT trial (Continuous Glucose Monitoring in Pregnant Women With Type 1 Diabetes), published in 2017, was the first trial to demonstrate improvements in perinatal outcomes, and it “brought CGM to the forefront in terms of widespread use,” Dr. Durnwald said.
The trial randomized more than 300 patients with type 1 diabetes who were pregnant or planning pregnancy (both users of insulin pumps and users of multiple insulin injections) to continuous, real-time CGM in addition to finger-stick glucose monitoring, or standard finger-stick glucose tests alone. In addition to small improvements in A1c and 7% more TIR (without an increase in hypoglycemia), pregnant CGM users had reductions in large-for-gestational age (LGA) births (53% vs 69%, P = .0489), neonatal intensive care admissions lasting more than 24 hours, and severe neonatal hypoglycemia.
Numbers needed to treat to prevent adverse outcomes in the CONCEPTT trial were six for LGA, six for NICU admission, and eight for neonatal hypoglycemia.
Data from the CONCEPTT trial featured prominently in the development of consensus recommendations for CGM targets in pregnancy by an international expert panel endorsed by the American Diabetes Association. In its 2019 report, the group recommended a target range of 63-140 mg/dL for type 1 and type 2 diabetes during pregnancy (compared with 70-180 mg/dL outside of pregnancy), and a TIR > 70% for pregnant people with type 1 diabetes. (Targets for time below range and time above range are also defined for type 1.)
More data are needed, the group said, in order to recommend TIR targets for type 2 diabetes in pregnancy or GDM (Diabetes Care. 2019;42:1593-603). “Many argue,” Dr. Durnwald said, “that there could be more stringent targets for those at less risk for [maternal] hypoglycemia, especially our GDM population.”
There’s a question of whether even higher TIR would further improve perinatal outcomes, she said, “or will we reach a threshold where higher TIR doesn’t get us a [further] reduction in LGA or preeclampsia.”
And while TIR is “certainly our buzzword,” lower mean glucose levels have also been associated with a lower risk of LGA and other adverse neonatal outcomes. A 2019 retrospective study from Sweden, for instance, analyzed patterns of CGM data from 186 pregnant women with type 1 diabetes and found significant associations between elevated mean glucose levels (in the second and third trimesters) and both LGA and an adverse neonatal composite outcome (Diabetologia. 2019;62:1143-53).
Elevated TIR was also associated with LGA, but “mean glucose had the strongest association with the rate of LGA,” Dr. Durnwald said.
Similarly, a 2020 subanalysis of the CONCEPTT trial data found that a higher mean glucose at both 24 and 34 weeks of gestation was significantly associated with a greater risk of LGA (Diabetes Care. 2020;43:1178-84), and a smaller 2015 analysis of data from two randomized controlled trials of CGM in pregnant women with type 1 and type 2 diabetes found this association in trimesters 2 and 3 (Diabetes Care. 2015;38;1319-25).
The ADA’s Standards of Care in Diabetes (Diabetes Care. 2024;47:S282-S294) endorse CGM as an adjunctive tool in pregnancy — not as a replacement for all traditional blood glucose monitoring — and advise that the use of CGM-reported mean glucose is superior to the use of estimated A1c, glucose management indicator, and other calculations to estimate A1c. Changes occur in pregnancy, Dr. Durnwald pointed out. “Most experts will identify a [target] mean glucose < 120 mg/dL in those with type 1, but there’s potential to have a mean glucose closer to 100 in certainly our patients with GDM and some of our patients with type 2,” she said. To a lesser extent, researchers have also looked at the effect of CMG-reported glycemic variability on outcomes such as LGA, with at least two studies finding some association, and there has been some research on nocturnal glucose and LGA, Dr. Durnwald said. CGM “gives us the opportunity,” she said, “to think about nocturnal glucose as a possible target” for further optimizing diabetes management during pregnancy.
CGM in Type 2, GDM
CGM in type 2 diabetes in pregnancy was addressed in a recently published systematic review and meta-analysis, which found only three qualifying randomized controlled trials and concluded that CGM use was not associated with improvements in perinatal outcomes, as assessed by LGA and preeclampsia (Am J Obstet Gynecol MFM. 2023;5:100969). “It’s very limited by the small sample size and the fact that most [patients] were using intermittent CGM,” Dr. Durnwald said. “It highlights how important it is to perform larger studies with continuous CGM.”
While the 2024 ADA standards say there are insufficient data to support the use of CGM in all patients with type 2 diabetes or GDM — and that the decision should be individualized “based on treatment regimen, circumstance, preferences, and needs” — real-world access to CGM for type 2, and even a bit for GDM, is improving, she said.
Some insurers require patients to be on insulin, but the trends are such that “we certainly talk about CGM to all our patients with type 2 diabetes and even our patients with GDM,” Dr. Durnwald said in a later interview. “CGMs are being advertised so we definitely have people who ask about them upon diagnosis, and we try to make it work for them.”
Is Preventing Maternal Hypoglycemia Possible?
Advancements in technology and pharmacology aimed at optimizing glycemic control — increased adoption of CGM, the use of insulin pump therapy, and the use of more rapid insulin analogs — appear to have had little to no impact on rates of severe maternal hypoglycemia in type 1 diabetes in pregnancy, said Dr. Rosenn, referring to several published studies.
The CONCEPTT study in type 1 diabetes, for instance, “gave us the best data we have on the use of CGM,” but differences in the percentage of patients with severe hypoglycemia and the total number of severe hypoglycemia episodes were basically the same whether patients used CGM or not, he said.
Closed-loop insulin delivery systems have been found in nonpregnant patients with type 1 diabetes to “be helpful in keeping people in range and also possibly [decreasing nocturnal hypoglycemia],” but the systems are not approved for use in pregnancy. “There’s not enough data on use in pregnancy, but probably more important, the algorithms used in the closed-loop systems are not directed to the targets we consider ideal for pregnancy,” Dr. Rosenn said.
In a pilot study of a closed-loop delivery system customized for pregnancies complicated by type 1 diabetes, 10 pregnant women were recruited at 14-32 weeks and, after a 1- to 2-week run-in period using a regular CGM-augmented pump, they used the closed-loop system targeting a daytime glucose of 80-110 mg/dL and nocturnal glucose of 80-100 mg/dL.
Mean TIR (a target range of 63-140 mg/dL) increased from 65% during the run-in period to 79% on the closed-loop system, and there were significant decreases in both time above range and time in the hypoglycemic ranges of < 63 mg/dL and < 54 mg/dL. Hypoglycemic events per week (defined as < 54 mg/dL for over 15 minutes) decreased from 4 to 0.7 (Diabetes Care. 2023;46:1425-31).
The investigators are continuing their research, and there are currently two randomized controlled trials underway examining use of closed-loop systems designed for pregnancy, said Dr. Rosenn, who was involved in feasibility research leading up to the pilot study. “So I’m hopeful we’ll see some encouraging information in the future.”
Maternal hypoglycemia during pregnancy is more common in type 1 diabetes, but it also affects pregnancies complicated by type 2 diabetes and GDM. In addition to the strict glycemic control imposed to improve maternal and fetal outcomes, pregnancy itself plays a role.
Research several decades ago from the Diabetes in Pregnancy Program Project, a prospective cohort in Cincinnati which Dr. Rosenn co-led, documented impaired counterregulatory physiology in pregnancy. Even in nondiabetic patients, there are declines in secretion of glucagon and growth hormone in response to hypoglycemia, for instance. In patients with type 1 diabetes, the diminishment in counterregulatory response is more severe.
Rethinking Intrapartum Care
Guidance for tight blood glucose control during labor and delivery for insulin-treated individuals — as reflected in the American College of Obstetricians and Gynecologists Practice Bulletin No. 201 on Pregestational Diabetes and in recommendations from the United Kingdom’s National Institute for Health and Care Excellence (NICE) — is based on small case series and overall “poor-quality” evidence that more recent research has failed to back up, Dr. Bartal said.
A systematic review published in 2018, for example, concluded there is a paucity of high-quality data supporting the association of glucose during labor and delivery with neonatal hypoglycemia in pregnancies complicated by diabetes (Diabet Med. 2018;35:173-83). And in a subsequent retrospective cohort study of pregnant women with type 1/type 2/GDM and their neonates, the same investigators reported no difference in the target glucose in labor between those with and without neonatal hypotension, after adjustment for important neonatal factors such as LGA and preterm delivery (Diabet Med. 2020;37:138-46).
Also exemplifying the body of research, Dr. Bartal said, is another single-center retrospective study published in 2020 that evaluated outcomes in the years before and after the institution of a formal intrapartum insulin regimen (a standardized protocol for titration of insulin and glucose infusions) for women with pregestational or gestational diabetes. The protocol was associated with improved maternal glucose control, but an increased frequency of neonatal hypoglycemia (Obstet Gynecol. 2020;136:411-6).
Her own group at the University of Texas in Houston looked retrospectively at 233 insulin-treated pregnancies complicated by type 2 diabetes and found no significant difference in the rate of neonatal hypoglycemia between those placed on a drip and those who were not, Dr. Bartal said. Over 40% of the newborns had hypoglycemia; it occurred irrespective of the route of delivery as well (J Matern Fetal Neonatal Med. 2022;35:7445-51).
Only two published randomized controlled trials have evaluated blood sugar control in labor, she said. The first, published in 2006, compared a continuous insulin drip with a rotation of glucose and non–glucose-containing fluids in insulin-requiring diabetes and found no differences in maternal blood glucose (the primary outcome) and a similar risk of neonatal hypoglycemia (Am J Obstet Gynecol. 2006;195;1095-9).
The second RCT, published in 2019, evaluated tight versus liberalized control (60-100 mg/dL, checking every hour, versus 60-120 mg/dL, checking every 4 hours) in laboring women with GDM. The first neonatal blood glucose level was similar in both groups, while the mean neonatal blood glucose level in the first 24 hours of life was lower with tight control (54 vs 58 mg/dL, P = .49) (Obstet Gynecol. 2019;133:1171-7). Findings from a new RCT conducted at the University of Texas in Houston of usual care versus more permissive glucose control will be presented at the SMFM Pregnancy Meeting in February 2024, she said.
Neonatal hypoglycemia is associated with increased risk of NICU admission, “but it’s also associated with possible long-term developmental deficit,” Dr. Bartal said, with the risk highest in children exposed to severe, recurrent, or clinically undetected hypoglycemia. Research has documented significantly increased risks of low executive function and visual motor function, for instance, in children who experienced neonatal hypoglycemia.
The risk of neonatal hypoglycemia has been linked to a variety of factors outside of the intrapartum period such as diabetes control and weight gain during pregnancy, neonatal birth weight/LGA, neonatal adiposity, gestational age at delivery, maternal body mass index, smoking, and diabetes control prior to pregnancy, Dr. Bartal noted. Also challenging is the reality that neonatal hypoglycemia as a research outcome is not standardized; definitions have varied across studies.
Tight intrapartum control comes with “costs,” from close monitoring of labor to increased resource utilization, and it may affect the labor experience/satisfaction, Dr. Bartal said. “But furthermore,” she said, “there are studies coming out, especially in the anesthesiology journals, that show there may be possible harm,” such as the risk of maternal and neonatal hyponatremia, and maternal hypoglycemia. A 2016 editorial in Anaesthesia (2016;71:750) describes these concerns, she noted.
“I do think we need to rethink our current recommendations,” she said.
Dr. Durnwald reported serving on the Dexcom GDM advisory board and receiving funding from United Health Group and the Helmsley Charitable Trust. Dr. Bartal and Dr. Rosenn reported no conflicts of interest.
WASHINGTON — Continuous glucose monitoring (CGM) is widely used during pregnancy for individuals with type 1 diabetes — with pregnancy-specific target metrics now chosen and benefits on perinatal outcomes demonstrated — but more research is needed to elucidate its role in the growing population of pregnant people with type 2 diabetes and gestational diabetes (GDM). And overall, there are still “many more questions unanswered about CGM use in pregnancy than what we have answered,” Celeste Durnwald, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
There’s much to learn about how to best interpret “the detailed and complex data that CGM provides,” and what targets in addition to time in range (TIR) are most important, said Dr. Durnwald, director of the perinatal diabetes program and associate professor of ob.gyn. at the Hospital of the University of Pennsylvania, Philadelphia, in a presentation on CGM.
Among other questions are whether fasting glucose is “as important in the era of CGM,” and whether there should be different glycemic targets for nocturnal versus daytime TIR, she said. Moreover, questions justifiably remain about whether the TIR targets for type 1 diabetes in pregnancy are indeed optimal, she said in a discussion period.
Ongoing research is looking at whether CGM can motivate and guide patients with GDM through diet and lifestyle changes such that “we can see changes in amounts of medication we use,” Dr. Durnwald noted in her presentation. “There’s a whole breadth of research looking at whether CGM can help predict diagnosis of GDM, large for gestational age, or preeclampsia, and what are the targets.”
Maternal hypoglycemia during pregnancy — a time when strict glycemic control is recommended to reduce the risk of congenital malformations and other fetal and neonatal morbidity — remains a concern in type 1 diabetes, even with widespread use of CGM in this population, said Barak Rosenn, MD, during a presentation on glycemic control in type 1 diabetes.
A pilot study of a newly designed pregnancy-specific closed-loop insulin delivery system, published last year (Diabetes Care. 2023;46:1425-31), has offered the first “really encouraging information about the ability to use our most up-to-date technology to help our type 1 patients maintain strict control and at the same time decrease their risk of severe hypoglycemia,” said Dr. Rosenn, a maternal-fetal medicine specialist at the Jersey City Medical Center, Jersey City, New Jersey.
Guidance for tight intrapartum glucose control, meanwhile, has been backed by little evidence, said Michal Fishel Bartal, MD, MS, and some recent studies and reviews have shown little to no effect of such tight control on neonatal hypoglycemia, which is the aim of the guidance.
“We need to reexamine current recommendations,” said Dr. Bartal, assistant professor in the division of maternal-fetal medicine at the University of Texas Health Science Center, Houston, during a presentation on intrapartum care. “There’s very limited evidence-based data for the way we manage people with diabetes [during labor and delivery].”
The Knowns And Unknowns of CGM in Pregnancy
The multicenter, international CONCEPTT trial (Continuous Glucose Monitoring in Pregnant Women With Type 1 Diabetes), published in 2017, was the first trial to demonstrate improvements in perinatal outcomes, and it “brought CGM to the forefront in terms of widespread use,” Dr. Durnwald said.
The trial randomized more than 300 patients with type 1 diabetes who were pregnant or planning pregnancy (both users of insulin pumps and users of multiple insulin injections) to continuous, real-time CGM in addition to finger-stick glucose monitoring, or standard finger-stick glucose tests alone. In addition to small improvements in A1c and 7% more TIR (without an increase in hypoglycemia), pregnant CGM users had reductions in large-for-gestational age (LGA) births (53% vs 69%, P = .0489), neonatal intensive care admissions lasting more than 24 hours, and severe neonatal hypoglycemia.
Numbers needed to treat to prevent adverse outcomes in the CONCEPTT trial were six for LGA, six for NICU admission, and eight for neonatal hypoglycemia.
Data from the CONCEPTT trial featured prominently in the development of consensus recommendations for CGM targets in pregnancy by an international expert panel endorsed by the American Diabetes Association. In its 2019 report, the group recommended a target range of 63-140 mg/dL for type 1 and type 2 diabetes during pregnancy (compared with 70-180 mg/dL outside of pregnancy), and a TIR > 70% for pregnant people with type 1 diabetes. (Targets for time below range and time above range are also defined for type 1.)
More data are needed, the group said, in order to recommend TIR targets for type 2 diabetes in pregnancy or GDM (Diabetes Care. 2019;42:1593-603). “Many argue,” Dr. Durnwald said, “that there could be more stringent targets for those at less risk for [maternal] hypoglycemia, especially our GDM population.”
There’s a question of whether even higher TIR would further improve perinatal outcomes, she said, “or will we reach a threshold where higher TIR doesn’t get us a [further] reduction in LGA or preeclampsia.”
And while TIR is “certainly our buzzword,” lower mean glucose levels have also been associated with a lower risk of LGA and other adverse neonatal outcomes. A 2019 retrospective study from Sweden, for instance, analyzed patterns of CGM data from 186 pregnant women with type 1 diabetes and found significant associations between elevated mean glucose levels (in the second and third trimesters) and both LGA and an adverse neonatal composite outcome (Diabetologia. 2019;62:1143-53).
Elevated TIR was also associated with LGA, but “mean glucose had the strongest association with the rate of LGA,” Dr. Durnwald said.
Similarly, a 2020 subanalysis of the CONCEPTT trial data found that a higher mean glucose at both 24 and 34 weeks of gestation was significantly associated with a greater risk of LGA (Diabetes Care. 2020;43:1178-84), and a smaller 2015 analysis of data from two randomized controlled trials of CGM in pregnant women with type 1 and type 2 diabetes found this association in trimesters 2 and 3 (Diabetes Care. 2015;38;1319-25).
The ADA’s Standards of Care in Diabetes (Diabetes Care. 2024;47:S282-S294) endorse CGM as an adjunctive tool in pregnancy — not as a replacement for all traditional blood glucose monitoring — and advise that the use of CGM-reported mean glucose is superior to the use of estimated A1c, glucose management indicator, and other calculations to estimate A1c. Changes occur in pregnancy, Dr. Durnwald pointed out. “Most experts will identify a [target] mean glucose < 120 mg/dL in those with type 1, but there’s potential to have a mean glucose closer to 100 in certainly our patients with GDM and some of our patients with type 2,” she said. To a lesser extent, researchers have also looked at the effect of CMG-reported glycemic variability on outcomes such as LGA, with at least two studies finding some association, and there has been some research on nocturnal glucose and LGA, Dr. Durnwald said. CGM “gives us the opportunity,” she said, “to think about nocturnal glucose as a possible target” for further optimizing diabetes management during pregnancy.
CGM in Type 2, GDM
CGM in type 2 diabetes in pregnancy was addressed in a recently published systematic review and meta-analysis, which found only three qualifying randomized controlled trials and concluded that CGM use was not associated with improvements in perinatal outcomes, as assessed by LGA and preeclampsia (Am J Obstet Gynecol MFM. 2023;5:100969). “It’s very limited by the small sample size and the fact that most [patients] were using intermittent CGM,” Dr. Durnwald said. “It highlights how important it is to perform larger studies with continuous CGM.”
While the 2024 ADA standards say there are insufficient data to support the use of CGM in all patients with type 2 diabetes or GDM — and that the decision should be individualized “based on treatment regimen, circumstance, preferences, and needs” — real-world access to CGM for type 2, and even a bit for GDM, is improving, she said.
Some insurers require patients to be on insulin, but the trends are such that “we certainly talk about CGM to all our patients with type 2 diabetes and even our patients with GDM,” Dr. Durnwald said in a later interview. “CGMs are being advertised so we definitely have people who ask about them upon diagnosis, and we try to make it work for them.”
Is Preventing Maternal Hypoglycemia Possible?
Advancements in technology and pharmacology aimed at optimizing glycemic control — increased adoption of CGM, the use of insulin pump therapy, and the use of more rapid insulin analogs — appear to have had little to no impact on rates of severe maternal hypoglycemia in type 1 diabetes in pregnancy, said Dr. Rosenn, referring to several published studies.
The CONCEPTT study in type 1 diabetes, for instance, “gave us the best data we have on the use of CGM,” but differences in the percentage of patients with severe hypoglycemia and the total number of severe hypoglycemia episodes were basically the same whether patients used CGM or not, he said.
Closed-loop insulin delivery systems have been found in nonpregnant patients with type 1 diabetes to “be helpful in keeping people in range and also possibly [decreasing nocturnal hypoglycemia],” but the systems are not approved for use in pregnancy. “There’s not enough data on use in pregnancy, but probably more important, the algorithms used in the closed-loop systems are not directed to the targets we consider ideal for pregnancy,” Dr. Rosenn said.
In a pilot study of a closed-loop delivery system customized for pregnancies complicated by type 1 diabetes, 10 pregnant women were recruited at 14-32 weeks and, after a 1- to 2-week run-in period using a regular CGM-augmented pump, they used the closed-loop system targeting a daytime glucose of 80-110 mg/dL and nocturnal glucose of 80-100 mg/dL.
Mean TIR (a target range of 63-140 mg/dL) increased from 65% during the run-in period to 79% on the closed-loop system, and there were significant decreases in both time above range and time in the hypoglycemic ranges of < 63 mg/dL and < 54 mg/dL. Hypoglycemic events per week (defined as < 54 mg/dL for over 15 minutes) decreased from 4 to 0.7 (Diabetes Care. 2023;46:1425-31).
The investigators are continuing their research, and there are currently two randomized controlled trials underway examining use of closed-loop systems designed for pregnancy, said Dr. Rosenn, who was involved in feasibility research leading up to the pilot study. “So I’m hopeful we’ll see some encouraging information in the future.”
Maternal hypoglycemia during pregnancy is more common in type 1 diabetes, but it also affects pregnancies complicated by type 2 diabetes and GDM. In addition to the strict glycemic control imposed to improve maternal and fetal outcomes, pregnancy itself plays a role.
Research several decades ago from the Diabetes in Pregnancy Program Project, a prospective cohort in Cincinnati which Dr. Rosenn co-led, documented impaired counterregulatory physiology in pregnancy. Even in nondiabetic patients, there are declines in secretion of glucagon and growth hormone in response to hypoglycemia, for instance. In patients with type 1 diabetes, the diminishment in counterregulatory response is more severe.
Rethinking Intrapartum Care
Guidance for tight blood glucose control during labor and delivery for insulin-treated individuals — as reflected in the American College of Obstetricians and Gynecologists Practice Bulletin No. 201 on Pregestational Diabetes and in recommendations from the United Kingdom’s National Institute for Health and Care Excellence (NICE) — is based on small case series and overall “poor-quality” evidence that more recent research has failed to back up, Dr. Bartal said.
A systematic review published in 2018, for example, concluded there is a paucity of high-quality data supporting the association of glucose during labor and delivery with neonatal hypoglycemia in pregnancies complicated by diabetes (Diabet Med. 2018;35:173-83). And in a subsequent retrospective cohort study of pregnant women with type 1/type 2/GDM and their neonates, the same investigators reported no difference in the target glucose in labor between those with and without neonatal hypotension, after adjustment for important neonatal factors such as LGA and preterm delivery (Diabet Med. 2020;37:138-46).
Also exemplifying the body of research, Dr. Bartal said, is another single-center retrospective study published in 2020 that evaluated outcomes in the years before and after the institution of a formal intrapartum insulin regimen (a standardized protocol for titration of insulin and glucose infusions) for women with pregestational or gestational diabetes. The protocol was associated with improved maternal glucose control, but an increased frequency of neonatal hypoglycemia (Obstet Gynecol. 2020;136:411-6).
Her own group at the University of Texas in Houston looked retrospectively at 233 insulin-treated pregnancies complicated by type 2 diabetes and found no significant difference in the rate of neonatal hypoglycemia between those placed on a drip and those who were not, Dr. Bartal said. Over 40% of the newborns had hypoglycemia; it occurred irrespective of the route of delivery as well (J Matern Fetal Neonatal Med. 2022;35:7445-51).
Only two published randomized controlled trials have evaluated blood sugar control in labor, she said. The first, published in 2006, compared a continuous insulin drip with a rotation of glucose and non–glucose-containing fluids in insulin-requiring diabetes and found no differences in maternal blood glucose (the primary outcome) and a similar risk of neonatal hypoglycemia (Am J Obstet Gynecol. 2006;195;1095-9).
The second RCT, published in 2019, evaluated tight versus liberalized control (60-100 mg/dL, checking every hour, versus 60-120 mg/dL, checking every 4 hours) in laboring women with GDM. The first neonatal blood glucose level was similar in both groups, while the mean neonatal blood glucose level in the first 24 hours of life was lower with tight control (54 vs 58 mg/dL, P = .49) (Obstet Gynecol. 2019;133:1171-7). Findings from a new RCT conducted at the University of Texas in Houston of usual care versus more permissive glucose control will be presented at the SMFM Pregnancy Meeting in February 2024, she said.
Neonatal hypoglycemia is associated with increased risk of NICU admission, “but it’s also associated with possible long-term developmental deficit,” Dr. Bartal said, with the risk highest in children exposed to severe, recurrent, or clinically undetected hypoglycemia. Research has documented significantly increased risks of low executive function and visual motor function, for instance, in children who experienced neonatal hypoglycemia.
The risk of neonatal hypoglycemia has been linked to a variety of factors outside of the intrapartum period such as diabetes control and weight gain during pregnancy, neonatal birth weight/LGA, neonatal adiposity, gestational age at delivery, maternal body mass index, smoking, and diabetes control prior to pregnancy, Dr. Bartal noted. Also challenging is the reality that neonatal hypoglycemia as a research outcome is not standardized; definitions have varied across studies.
Tight intrapartum control comes with “costs,” from close monitoring of labor to increased resource utilization, and it may affect the labor experience/satisfaction, Dr. Bartal said. “But furthermore,” she said, “there are studies coming out, especially in the anesthesiology journals, that show there may be possible harm,” such as the risk of maternal and neonatal hyponatremia, and maternal hypoglycemia. A 2016 editorial in Anaesthesia (2016;71:750) describes these concerns, she noted.
“I do think we need to rethink our current recommendations,” she said.
Dr. Durnwald reported serving on the Dexcom GDM advisory board and receiving funding from United Health Group and the Helmsley Charitable Trust. Dr. Bartal and Dr. Rosenn reported no conflicts of interest.
WASHINGTON — Continuous glucose monitoring (CGM) is widely used during pregnancy for individuals with type 1 diabetes — with pregnancy-specific target metrics now chosen and benefits on perinatal outcomes demonstrated — but more research is needed to elucidate its role in the growing population of pregnant people with type 2 diabetes and gestational diabetes (GDM). And overall, there are still “many more questions unanswered about CGM use in pregnancy than what we have answered,” Celeste Durnwald, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
There’s much to learn about how to best interpret “the detailed and complex data that CGM provides,” and what targets in addition to time in range (TIR) are most important, said Dr. Durnwald, director of the perinatal diabetes program and associate professor of ob.gyn. at the Hospital of the University of Pennsylvania, Philadelphia, in a presentation on CGM.
Among other questions are whether fasting glucose is “as important in the era of CGM,” and whether there should be different glycemic targets for nocturnal versus daytime TIR, she said. Moreover, questions justifiably remain about whether the TIR targets for type 1 diabetes in pregnancy are indeed optimal, she said in a discussion period.
Ongoing research is looking at whether CGM can motivate and guide patients with GDM through diet and lifestyle changes such that “we can see changes in amounts of medication we use,” Dr. Durnwald noted in her presentation. “There’s a whole breadth of research looking at whether CGM can help predict diagnosis of GDM, large for gestational age, or preeclampsia, and what are the targets.”
Maternal hypoglycemia during pregnancy — a time when strict glycemic control is recommended to reduce the risk of congenital malformations and other fetal and neonatal morbidity — remains a concern in type 1 diabetes, even with widespread use of CGM in this population, said Barak Rosenn, MD, during a presentation on glycemic control in type 1 diabetes.
A pilot study of a newly designed pregnancy-specific closed-loop insulin delivery system, published last year (Diabetes Care. 2023;46:1425-31), has offered the first “really encouraging information about the ability to use our most up-to-date technology to help our type 1 patients maintain strict control and at the same time decrease their risk of severe hypoglycemia,” said Dr. Rosenn, a maternal-fetal medicine specialist at the Jersey City Medical Center, Jersey City, New Jersey.
Guidance for tight intrapartum glucose control, meanwhile, has been backed by little evidence, said Michal Fishel Bartal, MD, MS, and some recent studies and reviews have shown little to no effect of such tight control on neonatal hypoglycemia, which is the aim of the guidance.
“We need to reexamine current recommendations,” said Dr. Bartal, assistant professor in the division of maternal-fetal medicine at the University of Texas Health Science Center, Houston, during a presentation on intrapartum care. “There’s very limited evidence-based data for the way we manage people with diabetes [during labor and delivery].”
The Knowns And Unknowns of CGM in Pregnancy
The multicenter, international CONCEPTT trial (Continuous Glucose Monitoring in Pregnant Women With Type 1 Diabetes), published in 2017, was the first trial to demonstrate improvements in perinatal outcomes, and it “brought CGM to the forefront in terms of widespread use,” Dr. Durnwald said.
The trial randomized more than 300 patients with type 1 diabetes who were pregnant or planning pregnancy (both users of insulin pumps and users of multiple insulin injections) to continuous, real-time CGM in addition to finger-stick glucose monitoring, or standard finger-stick glucose tests alone. In addition to small improvements in A1c and 7% more TIR (without an increase in hypoglycemia), pregnant CGM users had reductions in large-for-gestational age (LGA) births (53% vs 69%, P = .0489), neonatal intensive care admissions lasting more than 24 hours, and severe neonatal hypoglycemia.
Numbers needed to treat to prevent adverse outcomes in the CONCEPTT trial were six for LGA, six for NICU admission, and eight for neonatal hypoglycemia.
Data from the CONCEPTT trial featured prominently in the development of consensus recommendations for CGM targets in pregnancy by an international expert panel endorsed by the American Diabetes Association. In its 2019 report, the group recommended a target range of 63-140 mg/dL for type 1 and type 2 diabetes during pregnancy (compared with 70-180 mg/dL outside of pregnancy), and a TIR > 70% for pregnant people with type 1 diabetes. (Targets for time below range and time above range are also defined for type 1.)
More data are needed, the group said, in order to recommend TIR targets for type 2 diabetes in pregnancy or GDM (Diabetes Care. 2019;42:1593-603). “Many argue,” Dr. Durnwald said, “that there could be more stringent targets for those at less risk for [maternal] hypoglycemia, especially our GDM population.”
There’s a question of whether even higher TIR would further improve perinatal outcomes, she said, “or will we reach a threshold where higher TIR doesn’t get us a [further] reduction in LGA or preeclampsia.”
And while TIR is “certainly our buzzword,” lower mean glucose levels have also been associated with a lower risk of LGA and other adverse neonatal outcomes. A 2019 retrospective study from Sweden, for instance, analyzed patterns of CGM data from 186 pregnant women with type 1 diabetes and found significant associations between elevated mean glucose levels (in the second and third trimesters) and both LGA and an adverse neonatal composite outcome (Diabetologia. 2019;62:1143-53).
Elevated TIR was also associated with LGA, but “mean glucose had the strongest association with the rate of LGA,” Dr. Durnwald said.
Similarly, a 2020 subanalysis of the CONCEPTT trial data found that a higher mean glucose at both 24 and 34 weeks of gestation was significantly associated with a greater risk of LGA (Diabetes Care. 2020;43:1178-84), and a smaller 2015 analysis of data from two randomized controlled trials of CGM in pregnant women with type 1 and type 2 diabetes found this association in trimesters 2 and 3 (Diabetes Care. 2015;38;1319-25).
The ADA’s Standards of Care in Diabetes (Diabetes Care. 2024;47:S282-S294) endorse CGM as an adjunctive tool in pregnancy — not as a replacement for all traditional blood glucose monitoring — and advise that the use of CGM-reported mean glucose is superior to the use of estimated A1c, glucose management indicator, and other calculations to estimate A1c. Changes occur in pregnancy, Dr. Durnwald pointed out. “Most experts will identify a [target] mean glucose < 120 mg/dL in those with type 1, but there’s potential to have a mean glucose closer to 100 in certainly our patients with GDM and some of our patients with type 2,” she said. To a lesser extent, researchers have also looked at the effect of CMG-reported glycemic variability on outcomes such as LGA, with at least two studies finding some association, and there has been some research on nocturnal glucose and LGA, Dr. Durnwald said. CGM “gives us the opportunity,” she said, “to think about nocturnal glucose as a possible target” for further optimizing diabetes management during pregnancy.
CGM in Type 2, GDM
CGM in type 2 diabetes in pregnancy was addressed in a recently published systematic review and meta-analysis, which found only three qualifying randomized controlled trials and concluded that CGM use was not associated with improvements in perinatal outcomes, as assessed by LGA and preeclampsia (Am J Obstet Gynecol MFM. 2023;5:100969). “It’s very limited by the small sample size and the fact that most [patients] were using intermittent CGM,” Dr. Durnwald said. “It highlights how important it is to perform larger studies with continuous CGM.”
While the 2024 ADA standards say there are insufficient data to support the use of CGM in all patients with type 2 diabetes or GDM — and that the decision should be individualized “based on treatment regimen, circumstance, preferences, and needs” — real-world access to CGM for type 2, and even a bit for GDM, is improving, she said.
Some insurers require patients to be on insulin, but the trends are such that “we certainly talk about CGM to all our patients with type 2 diabetes and even our patients with GDM,” Dr. Durnwald said in a later interview. “CGMs are being advertised so we definitely have people who ask about them upon diagnosis, and we try to make it work for them.”
Is Preventing Maternal Hypoglycemia Possible?
Advancements in technology and pharmacology aimed at optimizing glycemic control — increased adoption of CGM, the use of insulin pump therapy, and the use of more rapid insulin analogs — appear to have had little to no impact on rates of severe maternal hypoglycemia in type 1 diabetes in pregnancy, said Dr. Rosenn, referring to several published studies.
The CONCEPTT study in type 1 diabetes, for instance, “gave us the best data we have on the use of CGM,” but differences in the percentage of patients with severe hypoglycemia and the total number of severe hypoglycemia episodes were basically the same whether patients used CGM or not, he said.
Closed-loop insulin delivery systems have been found in nonpregnant patients with type 1 diabetes to “be helpful in keeping people in range and also possibly [decreasing nocturnal hypoglycemia],” but the systems are not approved for use in pregnancy. “There’s not enough data on use in pregnancy, but probably more important, the algorithms used in the closed-loop systems are not directed to the targets we consider ideal for pregnancy,” Dr. Rosenn said.
In a pilot study of a closed-loop delivery system customized for pregnancies complicated by type 1 diabetes, 10 pregnant women were recruited at 14-32 weeks and, after a 1- to 2-week run-in period using a regular CGM-augmented pump, they used the closed-loop system targeting a daytime glucose of 80-110 mg/dL and nocturnal glucose of 80-100 mg/dL.
Mean TIR (a target range of 63-140 mg/dL) increased from 65% during the run-in period to 79% on the closed-loop system, and there were significant decreases in both time above range and time in the hypoglycemic ranges of < 63 mg/dL and < 54 mg/dL. Hypoglycemic events per week (defined as < 54 mg/dL for over 15 minutes) decreased from 4 to 0.7 (Diabetes Care. 2023;46:1425-31).
The investigators are continuing their research, and there are currently two randomized controlled trials underway examining use of closed-loop systems designed for pregnancy, said Dr. Rosenn, who was involved in feasibility research leading up to the pilot study. “So I’m hopeful we’ll see some encouraging information in the future.”
Maternal hypoglycemia during pregnancy is more common in type 1 diabetes, but it also affects pregnancies complicated by type 2 diabetes and GDM. In addition to the strict glycemic control imposed to improve maternal and fetal outcomes, pregnancy itself plays a role.
Research several decades ago from the Diabetes in Pregnancy Program Project, a prospective cohort in Cincinnati which Dr. Rosenn co-led, documented impaired counterregulatory physiology in pregnancy. Even in nondiabetic patients, there are declines in secretion of glucagon and growth hormone in response to hypoglycemia, for instance. In patients with type 1 diabetes, the diminishment in counterregulatory response is more severe.
Rethinking Intrapartum Care
Guidance for tight blood glucose control during labor and delivery for insulin-treated individuals — as reflected in the American College of Obstetricians and Gynecologists Practice Bulletin No. 201 on Pregestational Diabetes and in recommendations from the United Kingdom’s National Institute for Health and Care Excellence (NICE) — is based on small case series and overall “poor-quality” evidence that more recent research has failed to back up, Dr. Bartal said.
A systematic review published in 2018, for example, concluded there is a paucity of high-quality data supporting the association of glucose during labor and delivery with neonatal hypoglycemia in pregnancies complicated by diabetes (Diabet Med. 2018;35:173-83). And in a subsequent retrospective cohort study of pregnant women with type 1/type 2/GDM and their neonates, the same investigators reported no difference in the target glucose in labor between those with and without neonatal hypotension, after adjustment for important neonatal factors such as LGA and preterm delivery (Diabet Med. 2020;37:138-46).
Also exemplifying the body of research, Dr. Bartal said, is another single-center retrospective study published in 2020 that evaluated outcomes in the years before and after the institution of a formal intrapartum insulin regimen (a standardized protocol for titration of insulin and glucose infusions) for women with pregestational or gestational diabetes. The protocol was associated with improved maternal glucose control, but an increased frequency of neonatal hypoglycemia (Obstet Gynecol. 2020;136:411-6).
Her own group at the University of Texas in Houston looked retrospectively at 233 insulin-treated pregnancies complicated by type 2 diabetes and found no significant difference in the rate of neonatal hypoglycemia between those placed on a drip and those who were not, Dr. Bartal said. Over 40% of the newborns had hypoglycemia; it occurred irrespective of the route of delivery as well (J Matern Fetal Neonatal Med. 2022;35:7445-51).
Only two published randomized controlled trials have evaluated blood sugar control in labor, she said. The first, published in 2006, compared a continuous insulin drip with a rotation of glucose and non–glucose-containing fluids in insulin-requiring diabetes and found no differences in maternal blood glucose (the primary outcome) and a similar risk of neonatal hypoglycemia (Am J Obstet Gynecol. 2006;195;1095-9).
The second RCT, published in 2019, evaluated tight versus liberalized control (60-100 mg/dL, checking every hour, versus 60-120 mg/dL, checking every 4 hours) in laboring women with GDM. The first neonatal blood glucose level was similar in both groups, while the mean neonatal blood glucose level in the first 24 hours of life was lower with tight control (54 vs 58 mg/dL, P = .49) (Obstet Gynecol. 2019;133:1171-7). Findings from a new RCT conducted at the University of Texas in Houston of usual care versus more permissive glucose control will be presented at the SMFM Pregnancy Meeting in February 2024, she said.
Neonatal hypoglycemia is associated with increased risk of NICU admission, “but it’s also associated with possible long-term developmental deficit,” Dr. Bartal said, with the risk highest in children exposed to severe, recurrent, or clinically undetected hypoglycemia. Research has documented significantly increased risks of low executive function and visual motor function, for instance, in children who experienced neonatal hypoglycemia.
The risk of neonatal hypoglycemia has been linked to a variety of factors outside of the intrapartum period such as diabetes control and weight gain during pregnancy, neonatal birth weight/LGA, neonatal adiposity, gestational age at delivery, maternal body mass index, smoking, and diabetes control prior to pregnancy, Dr. Bartal noted. Also challenging is the reality that neonatal hypoglycemia as a research outcome is not standardized; definitions have varied across studies.
Tight intrapartum control comes with “costs,” from close monitoring of labor to increased resource utilization, and it may affect the labor experience/satisfaction, Dr. Bartal said. “But furthermore,” she said, “there are studies coming out, especially in the anesthesiology journals, that show there may be possible harm,” such as the risk of maternal and neonatal hyponatremia, and maternal hypoglycemia. A 2016 editorial in Anaesthesia (2016;71:750) describes these concerns, she noted.
“I do think we need to rethink our current recommendations,” she said.
Dr. Durnwald reported serving on the Dexcom GDM advisory board and receiving funding from United Health Group and the Helmsley Charitable Trust. Dr. Bartal and Dr. Rosenn reported no conflicts of interest.
FROM DPSG-NA 2024
Gestational Diabetes Treatment Moves Forward With Uncertainty And Hope
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FROM DPSG-NA 2023
The Knowns and Unknowns About Delivery Timing in Diabetes
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FROM DPSG-NA 2023
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.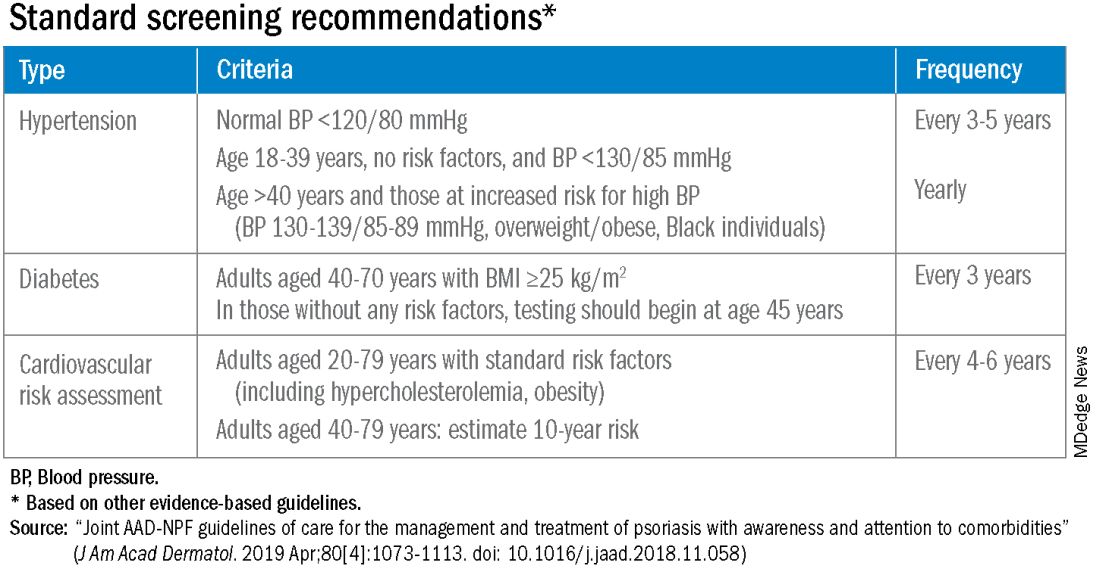
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Dietary supplements may play a role in managing vitiligo
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
FROM IDS 2023
Study: CBD provides symptom relief and improvements in gastroparesis
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
The challenges of palmoplantar pustulosis and other acral psoriatic disease
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
AT THE NPF RESEARCH SYMPOSIUM 2023