User login
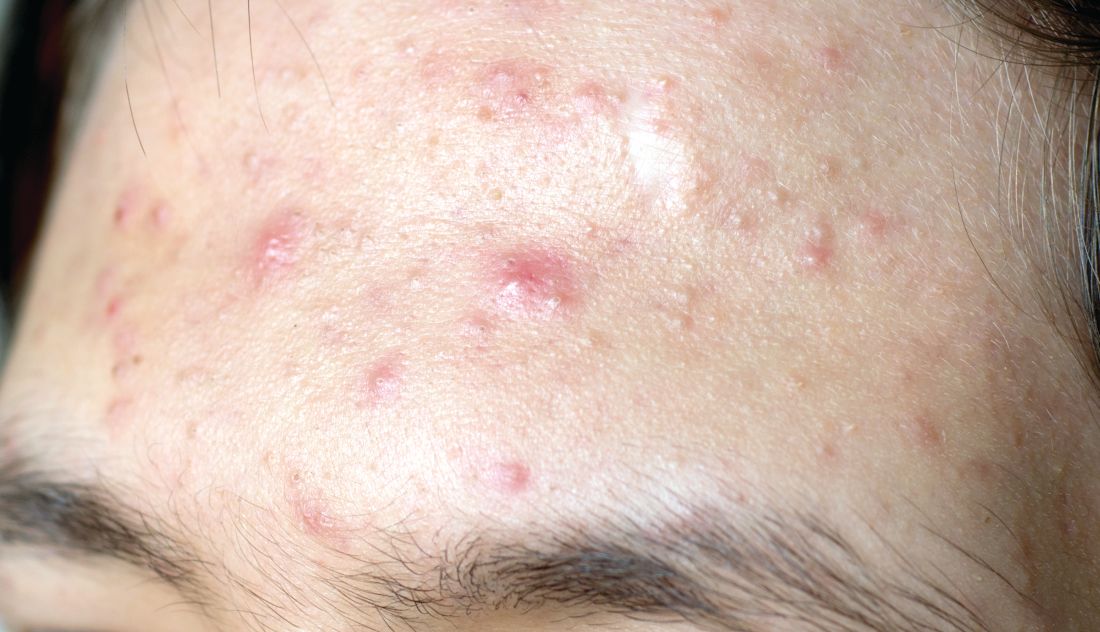
Face masks can aggravate rosacea
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
FROM THE EADV CONGRESS
Adjuvant nivolumab plus ipilimumab shows strong results in resected stage IV melanoma
Results of the
IMMUNED was a multicenter German double-blind, placebo-controlled, phase 2 trial conducted by the Dermatologic Cooperative Oncology Group. It included 167 patients with resected stage IV melanoma and no evidence of disease who were randomized to adjuvant nivolumab (Opdivo) plus placebo, nivolumab plus ipilimumab (Yervoy), or double placebo, with relapse-free survival as the primary outcome, Merrick I. Ross, MD, explained at a forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“The patients who received adjuvant ipilimumab and nivolumab had amazing 24-month outcomes: a relapse-free survival of 70% versus 42% with nivolumab and 14% with placebo,” observed Dr. Ross, professor of surgical oncology and chief of the melanoma section at the University of Texas M.D. Anderson Cancer Center, Houston.
“It’s not a long-term survival outcome, but we’ll see what happens long term. This could be a very interesting approach to move forward with,” he commented.
By way of background, the cancer surgeon noted that nivolumab has achieved standard-of-care status as adjuvant immunotherapy in patients with resected stage IIIB-C and stage IV melanoma, largely on the strength of the CheckMate-238 trial, which randomized 906 such patients at 130 academic centers in 25 countries to 1 year of adjuvant therapy with either intravenous nivolumab or ipilimumab. In the study, nivolumab emerged as the clear winner, with a 4-year recurrence-free survival of 51.7%, compared with 41.2% for ipilimumab, for a 29% relative risk reduction. Ipilimumab was associated with greater toxicity.
The between-group difference in relapse-free survival in the overall study population also held true in the subgroup comprised of 169 CheckMate 238 participants with resected stage IV melanoma and no evidence of disease at enrollment, Dr. Ross noted.
In the IMMUNED trial, the superior outcome achieved with adjuvant nivolumab plus ipilimumab came at the cost of significantly greater toxicity than with nivolumab alone. Treatment-related adverse events led to medication discontinuation in 62% of the dual-adjuvant therapy group, compared with 13% of those on adjuvant nivolumab.
IMMUNED was funded by Bristol-Myers Squibb.
Dr. Ross reported having no financial conflicts regarding his presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Results of the
IMMUNED was a multicenter German double-blind, placebo-controlled, phase 2 trial conducted by the Dermatologic Cooperative Oncology Group. It included 167 patients with resected stage IV melanoma and no evidence of disease who were randomized to adjuvant nivolumab (Opdivo) plus placebo, nivolumab plus ipilimumab (Yervoy), or double placebo, with relapse-free survival as the primary outcome, Merrick I. Ross, MD, explained at a forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“The patients who received adjuvant ipilimumab and nivolumab had amazing 24-month outcomes: a relapse-free survival of 70% versus 42% with nivolumab and 14% with placebo,” observed Dr. Ross, professor of surgical oncology and chief of the melanoma section at the University of Texas M.D. Anderson Cancer Center, Houston.
“It’s not a long-term survival outcome, but we’ll see what happens long term. This could be a very interesting approach to move forward with,” he commented.
By way of background, the cancer surgeon noted that nivolumab has achieved standard-of-care status as adjuvant immunotherapy in patients with resected stage IIIB-C and stage IV melanoma, largely on the strength of the CheckMate-238 trial, which randomized 906 such patients at 130 academic centers in 25 countries to 1 year of adjuvant therapy with either intravenous nivolumab or ipilimumab. In the study, nivolumab emerged as the clear winner, with a 4-year recurrence-free survival of 51.7%, compared with 41.2% for ipilimumab, for a 29% relative risk reduction. Ipilimumab was associated with greater toxicity.
The between-group difference in relapse-free survival in the overall study population also held true in the subgroup comprised of 169 CheckMate 238 participants with resected stage IV melanoma and no evidence of disease at enrollment, Dr. Ross noted.
In the IMMUNED trial, the superior outcome achieved with adjuvant nivolumab plus ipilimumab came at the cost of significantly greater toxicity than with nivolumab alone. Treatment-related adverse events led to medication discontinuation in 62% of the dual-adjuvant therapy group, compared with 13% of those on adjuvant nivolumab.
IMMUNED was funded by Bristol-Myers Squibb.
Dr. Ross reported having no financial conflicts regarding his presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Results of the
IMMUNED was a multicenter German double-blind, placebo-controlled, phase 2 trial conducted by the Dermatologic Cooperative Oncology Group. It included 167 patients with resected stage IV melanoma and no evidence of disease who were randomized to adjuvant nivolumab (Opdivo) plus placebo, nivolumab plus ipilimumab (Yervoy), or double placebo, with relapse-free survival as the primary outcome, Merrick I. Ross, MD, explained at a forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“The patients who received adjuvant ipilimumab and nivolumab had amazing 24-month outcomes: a relapse-free survival of 70% versus 42% with nivolumab and 14% with placebo,” observed Dr. Ross, professor of surgical oncology and chief of the melanoma section at the University of Texas M.D. Anderson Cancer Center, Houston.
“It’s not a long-term survival outcome, but we’ll see what happens long term. This could be a very interesting approach to move forward with,” he commented.
By way of background, the cancer surgeon noted that nivolumab has achieved standard-of-care status as adjuvant immunotherapy in patients with resected stage IIIB-C and stage IV melanoma, largely on the strength of the CheckMate-238 trial, which randomized 906 such patients at 130 academic centers in 25 countries to 1 year of adjuvant therapy with either intravenous nivolumab or ipilimumab. In the study, nivolumab emerged as the clear winner, with a 4-year recurrence-free survival of 51.7%, compared with 41.2% for ipilimumab, for a 29% relative risk reduction. Ipilimumab was associated with greater toxicity.
The between-group difference in relapse-free survival in the overall study population also held true in the subgroup comprised of 169 CheckMate 238 participants with resected stage IV melanoma and no evidence of disease at enrollment, Dr. Ross noted.
In the IMMUNED trial, the superior outcome achieved with adjuvant nivolumab plus ipilimumab came at the cost of significantly greater toxicity than with nivolumab alone. Treatment-related adverse events led to medication discontinuation in 62% of the dual-adjuvant therapy group, compared with 13% of those on adjuvant nivolumab.
IMMUNED was funded by Bristol-Myers Squibb.
Dr. Ross reported having no financial conflicts regarding his presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Deucravacitinib offers biologic-like psoriasis efficacy in oral form
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Expert offers clinical pearls on leg ulcer therapy
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
FROM THE EADV CONGRESS
Telltale dermoscopic features of melanomas lacking pigment reviewed
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
by familiarity with a handful of dermoscopic features specific to melanomas lacking significant pigment, Steven Q. Wang, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
These features emerged from a major study conducted on five continents by members of the International Dermoscopy Society. The investigators developed a simple, eight-variable model, which demonstrated a sensitivity of 70% and specificity of 56% for diagnosis of melanoma. And while that’s a markedly worse performance than when dermoscopy is used for detection of pigmented melanomas, where sensitivities in excess of 90% and specificities greater than 70% are typical, it’s nonetheless a significant improvement over naked-eye evaluation of these challenging pigment-deprived melanomas, noted Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan Kettering Basking Ridge (N.J.)
Using the predictive model developed in the international study to evaluate lesions lacking pigment, a diagnosis of melanoma is made provided two conditions are met: The lesion can have no more than three milia-like cysts, and it has to possess one or more of seven positive dermoscopic findings. The strongest predictor of melanoma in the study was the presence of a blue-white veil, which in univariate analysis was associated with a 13-fold increased likelihood of melanoma.
The other positive predictors were irregularly shaped depigmentation, more than one shade of pink, predominant central vessels, irregularly sized or distributed brown dots or globules, multiple blue-gray dots, and dotted and linear irregular vessels.
Dr. Wang emphasized that, when dermoscopy and clinical skin examination of a featureless hypomelanotic or amelanotic lesion yield ambiguous findings, frequent vigilant follow-up is a viable strategy to detect early melanoma – provided the lesion is superficial.
“The reality is not all melanomas are the same. The superficial spreading melanomas and lentigo melanomas grow very, very slowly: less than 0.1 mm per month. Those are the types of lesions you can monitor. But there is one type of lesion you should never, ever monitor: nodular lesions. They are the type of lesions that can do your patient harm because nodular melanomas can grow really fast. So my key takeaway message is, if you see a nodule and you don’t know what it is, take it off,” the dermatologist said.
Dermoscopy in the hands of experienced users has repeatedly been shown to improve diagnostic accuracy by more than 25%. But there is an additional very important reason to embrace dermoscopy in daily clinical practice, according to Dr. Wang: “When you put the scope on an individual, you slow down the exam and patients feels like you’re paying more attention to them.”
That’s worthwhile because the No. 1 complaint voiced by patients who make their way to Sloan Kettering for a second opinion is that their prior skin examination by an outside physician wasn’t thorough. They’re often angry about it. And while it’s true that incorporating dermoscopy does make for a lengthier skin examination, the additional time involved is actually minimal. Dr. Wang cited a randomized, prospective, multicenter study which documented that the median time required to conduct a thorough complete skin examination without dermoscopy was 70 seconds versus 142 seconds with dermoscopy.
Dr. Wang reported having no financial conflicts regarding his presentation.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Oral JAK inhibitor for alopecia areata advances to phase 3
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
FROM THE EADV CONGRESS
Why a mycosis fungoides diagnosis takes so long
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Home phototherapy never looked better, expert says
Kenneth B. Gordon, MD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“In my practice, I’m using more and more home UVB, and there are a number of reasons for that. It’s more convenient and easier for the patient, as it’s getting more difficult for patients to give up time from work to come to the office. And I might add that, in this time of COVID-19, people don’t want to come to the office. It’s generally less expensive for patients because of copays, which increase the cost of UVB. And believe it or not, I believe it’s easier for the clinician as well. I write a prescription, the patient gets a number of treatments, and I don’t lose any sleep because I think it’s very difficult for patients to get into trouble with narrow-band UVB at home,” explained Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“There’s all sorts of insurance company silliness in getting this paid for, but if you do get it paid for, I think it’s a really effective way to treat psoriasis,” the dermatologist added.
A Dutch multicenter randomized trial demonstrated that home UVB phototherapy for psoriasis was equally safe and effective as outpatient UVB phototherapy, and with greater patient satisfaction.
Surveys show most dermatologists consider phototherapy their preferred treatment for patients with extensive psoriasis because its side effect profile is so benign, compared with that of systemic therapies, be they biologic agents or older drugs such as methotrexate or acitretin. Phototherapy is particularly popular for use in women of childbearing potential, since it’s a nonsystemic therapy.
And speaking of side effects, Dr. Gordon declared, “The risks of narrow-band UVB are sometimes, I believe, exaggerated.” Indeed, he considers the No. 1 side effect of office-based phototherapy to be the loss of productive time.
“Simply put, phototherapy in the office is very easy for me. I write a prescription, the tech takes care of it, and if there’s a problem I’m handy to see the patient. But for the patient, it’s very difficult. Whereas it might take only a few minutes to get the treatment in-office, it takes a lot of time to get to the office, and many patients don’t have transportation. So I think the loss of productive time with phototherapy has to be considered a side effect,” Dr. Gordon said.
Turning to the therapy’s other side effects, he said that although there is some degree of photoaging associated with narrow-band UVB – which is far and away the most commonly used form of phototherapy in the United States – it’s nothing close to the photoaging caused by PUVA.
“I don’t believe that PUVA, with all the destruction of the skin that you see with it, is a significant part of our treatment modalities today,” Dr. Gordon said.
Sunburn is a risk with narrow-band UVB, especially if the dose is ramped up too quickly. Reactivation of herpes simplex virus infection is a frequent problem, and one patients find especially concerning when it manifests as eruptions of cold sores on the face.
The side effect of narrow-band UVB of greatest interest to most patients and physicians is skin cancer. “This is an extremely controversial area,” the dermatologist observed.
Unlike with PUVA, there has never been a convincing study to show that narrow-band UVB is associated with significantly increased risks of keratinocyte carcinomas or melanoma. A large Scottish study found no significantly increased risk, but a modestly increased trend for more squamous cell carcinomas. How modest? The investigators calculated that it would require 50,000 psoriasis patients with a minimum of 100 narrow-band UVB treatments to be followed for 5 years in order to demonstrate a twofold increased risk of the malignancy.
“In other words, it takes an incredible number of patients to be able to see a difference in a skin cancer that we can relatively easily treat. That’s why when I see patients, I don’t emphasize the risk of skin cancer,” Dr. Gordon said.
Similarly reassuring was a Swedish study, which showed the skin cancer rate in UVB-treated psoriasis patients was no different than in the general population.
Guideline recommendations regarding UVB phototherapy and skin cancer risk are all over the map. French guidelines advise a maximum of 230 narrow-band UVB treatments. British guidelines recommend reducing narrow-band UVB exposure to skin areas with significant sun exposure. American guidelines leave the topic untouched, Dr. Gordon noted.
He reported having no financial conflicts of interest regarding his presentation, as neither he, the Medical College of Wisconsin, or its department of dermatology receive any payment for phototherapy services he prescribes. Those payments go to the hospital system where he works. MedscapeLive and this news organization are owned by the same parent company.
Kenneth B. Gordon, MD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“In my practice, I’m using more and more home UVB, and there are a number of reasons for that. It’s more convenient and easier for the patient, as it’s getting more difficult for patients to give up time from work to come to the office. And I might add that, in this time of COVID-19, people don’t want to come to the office. It’s generally less expensive for patients because of copays, which increase the cost of UVB. And believe it or not, I believe it’s easier for the clinician as well. I write a prescription, the patient gets a number of treatments, and I don’t lose any sleep because I think it’s very difficult for patients to get into trouble with narrow-band UVB at home,” explained Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“There’s all sorts of insurance company silliness in getting this paid for, but if you do get it paid for, I think it’s a really effective way to treat psoriasis,” the dermatologist added.
A Dutch multicenter randomized trial demonstrated that home UVB phototherapy for psoriasis was equally safe and effective as outpatient UVB phototherapy, and with greater patient satisfaction.
Surveys show most dermatologists consider phototherapy their preferred treatment for patients with extensive psoriasis because its side effect profile is so benign, compared with that of systemic therapies, be they biologic agents or older drugs such as methotrexate or acitretin. Phototherapy is particularly popular for use in women of childbearing potential, since it’s a nonsystemic therapy.
And speaking of side effects, Dr. Gordon declared, “The risks of narrow-band UVB are sometimes, I believe, exaggerated.” Indeed, he considers the No. 1 side effect of office-based phototherapy to be the loss of productive time.
“Simply put, phototherapy in the office is very easy for me. I write a prescription, the tech takes care of it, and if there’s a problem I’m handy to see the patient. But for the patient, it’s very difficult. Whereas it might take only a few minutes to get the treatment in-office, it takes a lot of time to get to the office, and many patients don’t have transportation. So I think the loss of productive time with phototherapy has to be considered a side effect,” Dr. Gordon said.
Turning to the therapy’s other side effects, he said that although there is some degree of photoaging associated with narrow-band UVB – which is far and away the most commonly used form of phototherapy in the United States – it’s nothing close to the photoaging caused by PUVA.
“I don’t believe that PUVA, with all the destruction of the skin that you see with it, is a significant part of our treatment modalities today,” Dr. Gordon said.
Sunburn is a risk with narrow-band UVB, especially if the dose is ramped up too quickly. Reactivation of herpes simplex virus infection is a frequent problem, and one patients find especially concerning when it manifests as eruptions of cold sores on the face.
The side effect of narrow-band UVB of greatest interest to most patients and physicians is skin cancer. “This is an extremely controversial area,” the dermatologist observed.
Unlike with PUVA, there has never been a convincing study to show that narrow-band UVB is associated with significantly increased risks of keratinocyte carcinomas or melanoma. A large Scottish study found no significantly increased risk, but a modestly increased trend for more squamous cell carcinomas. How modest? The investigators calculated that it would require 50,000 psoriasis patients with a minimum of 100 narrow-band UVB treatments to be followed for 5 years in order to demonstrate a twofold increased risk of the malignancy.
“In other words, it takes an incredible number of patients to be able to see a difference in a skin cancer that we can relatively easily treat. That’s why when I see patients, I don’t emphasize the risk of skin cancer,” Dr. Gordon said.
Similarly reassuring was a Swedish study, which showed the skin cancer rate in UVB-treated psoriasis patients was no different than in the general population.
Guideline recommendations regarding UVB phototherapy and skin cancer risk are all over the map. French guidelines advise a maximum of 230 narrow-band UVB treatments. British guidelines recommend reducing narrow-band UVB exposure to skin areas with significant sun exposure. American guidelines leave the topic untouched, Dr. Gordon noted.
He reported having no financial conflicts of interest regarding his presentation, as neither he, the Medical College of Wisconsin, or its department of dermatology receive any payment for phototherapy services he prescribes. Those payments go to the hospital system where he works. MedscapeLive and this news organization are owned by the same parent company.
Kenneth B. Gordon, MD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“In my practice, I’m using more and more home UVB, and there are a number of reasons for that. It’s more convenient and easier for the patient, as it’s getting more difficult for patients to give up time from work to come to the office. And I might add that, in this time of COVID-19, people don’t want to come to the office. It’s generally less expensive for patients because of copays, which increase the cost of UVB. And believe it or not, I believe it’s easier for the clinician as well. I write a prescription, the patient gets a number of treatments, and I don’t lose any sleep because I think it’s very difficult for patients to get into trouble with narrow-band UVB at home,” explained Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“There’s all sorts of insurance company silliness in getting this paid for, but if you do get it paid for, I think it’s a really effective way to treat psoriasis,” the dermatologist added.
A Dutch multicenter randomized trial demonstrated that home UVB phototherapy for psoriasis was equally safe and effective as outpatient UVB phototherapy, and with greater patient satisfaction.
Surveys show most dermatologists consider phototherapy their preferred treatment for patients with extensive psoriasis because its side effect profile is so benign, compared with that of systemic therapies, be they biologic agents or older drugs such as methotrexate or acitretin. Phototherapy is particularly popular for use in women of childbearing potential, since it’s a nonsystemic therapy.
And speaking of side effects, Dr. Gordon declared, “The risks of narrow-band UVB are sometimes, I believe, exaggerated.” Indeed, he considers the No. 1 side effect of office-based phototherapy to be the loss of productive time.
“Simply put, phototherapy in the office is very easy for me. I write a prescription, the tech takes care of it, and if there’s a problem I’m handy to see the patient. But for the patient, it’s very difficult. Whereas it might take only a few minutes to get the treatment in-office, it takes a lot of time to get to the office, and many patients don’t have transportation. So I think the loss of productive time with phototherapy has to be considered a side effect,” Dr. Gordon said.
Turning to the therapy’s other side effects, he said that although there is some degree of photoaging associated with narrow-band UVB – which is far and away the most commonly used form of phototherapy in the United States – it’s nothing close to the photoaging caused by PUVA.
“I don’t believe that PUVA, with all the destruction of the skin that you see with it, is a significant part of our treatment modalities today,” Dr. Gordon said.
Sunburn is a risk with narrow-band UVB, especially if the dose is ramped up too quickly. Reactivation of herpes simplex virus infection is a frequent problem, and one patients find especially concerning when it manifests as eruptions of cold sores on the face.
The side effect of narrow-band UVB of greatest interest to most patients and physicians is skin cancer. “This is an extremely controversial area,” the dermatologist observed.
Unlike with PUVA, there has never been a convincing study to show that narrow-band UVB is associated with significantly increased risks of keratinocyte carcinomas or melanoma. A large Scottish study found no significantly increased risk, but a modestly increased trend for more squamous cell carcinomas. How modest? The investigators calculated that it would require 50,000 psoriasis patients with a minimum of 100 narrow-band UVB treatments to be followed for 5 years in order to demonstrate a twofold increased risk of the malignancy.
“In other words, it takes an incredible number of patients to be able to see a difference in a skin cancer that we can relatively easily treat. That’s why when I see patients, I don’t emphasize the risk of skin cancer,” Dr. Gordon said.
Similarly reassuring was a Swedish study, which showed the skin cancer rate in UVB-treated psoriasis patients was no different than in the general population.
Guideline recommendations regarding UVB phototherapy and skin cancer risk are all over the map. French guidelines advise a maximum of 230 narrow-band UVB treatments. British guidelines recommend reducing narrow-band UVB exposure to skin areas with significant sun exposure. American guidelines leave the topic untouched, Dr. Gordon noted.
He reported having no financial conflicts of interest regarding his presentation, as neither he, the Medical College of Wisconsin, or its department of dermatology receive any payment for phototherapy services he prescribes. Those payments go to the hospital system where he works. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Acute-on-chronic itch is new frontier in atopic dermatitis
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Novel topical acne combo hits marks in phase 3 trials
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR