User login
A Step-by-Step Guide for Diagnosing Cushing Syndrome
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
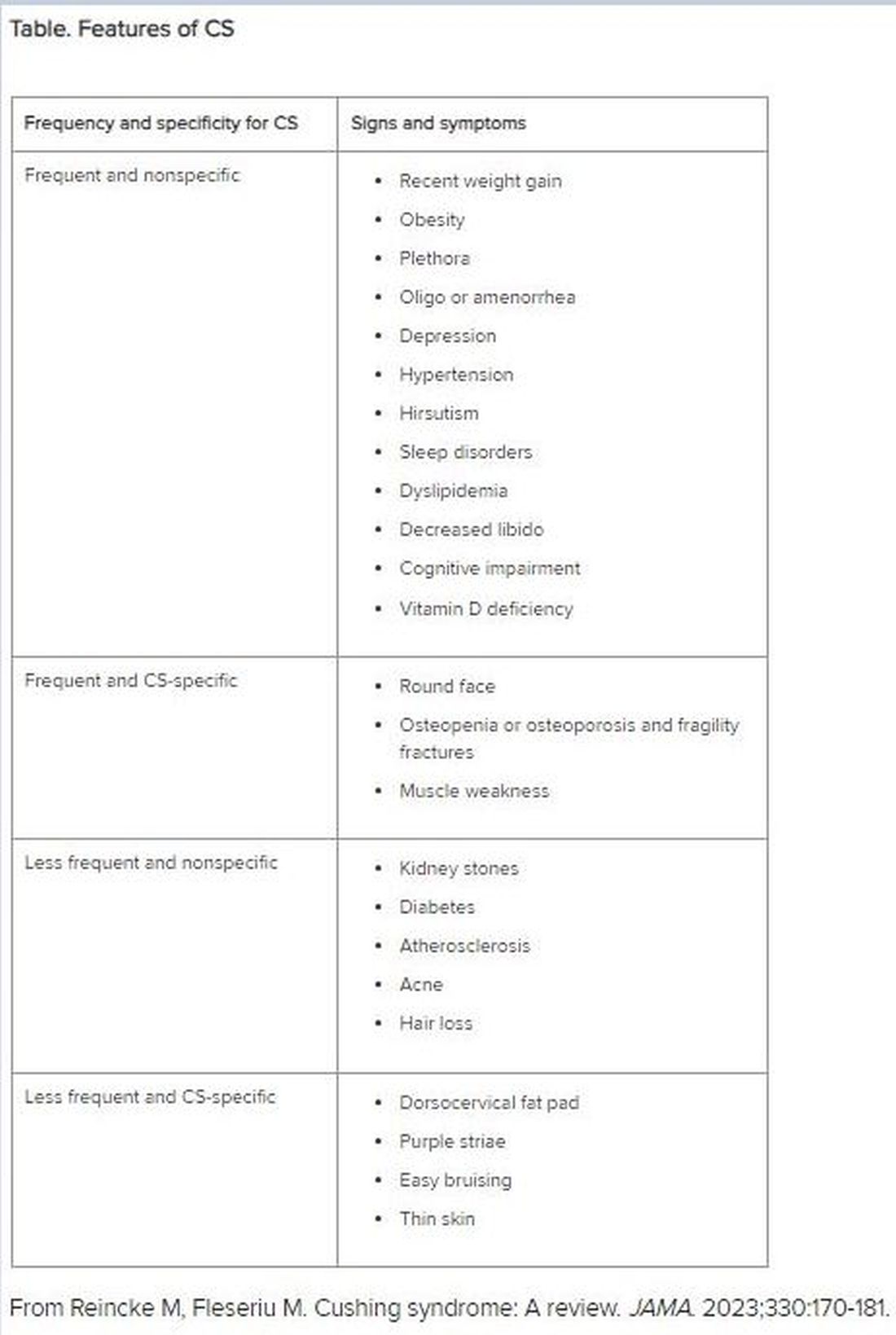
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
PTSD Needs a New Name, Experts Say — Here’s Why
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
‘Alarming’ Rise in Mental Health Hospital Admissions Involving Methamphetamine
new research showed. Investigators found that between 2008 and 2020, such admissions increased by more than 10-fold.
“Overall, our results show an alarming increase in mental health disorder–related hospitalizations with concurrent methamphetamine use from 2008 to 2020,” wrote the investigators, led by Diensn Xing, Department of Medicine, Louisiana State University Health Sciences Center, Shreveport.
“These results are especially concerning because these hospitalizations outpace hospitalizations for methamphetamine use alone or mental health disorders alone,” they added.
The study was published online in Nature Mental Health .
Action Needed
Mental illness and methamphetamine use are both growing health problems. The investigators pointed out that methamphetamine use can cause serious harm to an individual’s mental, emotional, and social well-being and can significantly alter the brain.
They added that long-term methamphetamine users can exhibit “extreme anxiety, confusion, troubled sleep, mood changes, and aggressive behavior.” In addition, use of the drug can cause psychotic side effects such as paranoia, hallucinations, delusions, and suicidality.
The investigators noted that, to date, no studies have examined the combined effects of both diseases or characterized national trends over more than 10 years.
The researchers analyzed US mental health–related trends in methamphetamine users from 2008 to 2020. In particular, they wanted to characterize which demographic and geographic groups might be affected by both of these diseases because people with mental illness and co-occurring methamphetamine use are an “intersectional group” that is “doubly vulnerable to suicide and overdose death due to the synergistic effects of methamphetamine and mental health disorders.”
The investigators evaluated US trends in mental health disorder–related hospital admissions (MHD-HAs) and compared them with mental health admissions that involved concurrent methamphetamine use (MHD-HA-MUs) between 2008 and 2020.
Using data from the largest US inpatient care database, which encompasses more than 7 million hospital stays annually, they examined close to 4 million weighted hospital admissions and found more than a 10-fold increase in MHD-HA-MUs, compared with a 1.4-fold increase in MHD-HAs.
MHD-HA-MUs increased significantly among men (13-fold), non-Hispanic Black patients (39-fold), and those aged 41-64 years (16-fold). In the southern United States, MHD-HA-MUs increased 24-fold, larger than in any other region in the United States.
“Overall, the data suggest that there are synergistic effects with methamphetamine use and mental health disorder, highlighting this patient group’s unique needs, requiring distinct action,” the researchers wrote.
They proposed several interventions, including public education about substance use disorders, mental illness, and the effects of stigma. They also suggested decreasing criminal penalties for those with substance use disorders and improving healthcare delivery for this patient population.
This work was supported by the National Institutes of Health and an award from the National Institute of General Medical Sciences. The study authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research showed. Investigators found that between 2008 and 2020, such admissions increased by more than 10-fold.
“Overall, our results show an alarming increase in mental health disorder–related hospitalizations with concurrent methamphetamine use from 2008 to 2020,” wrote the investigators, led by Diensn Xing, Department of Medicine, Louisiana State University Health Sciences Center, Shreveport.
“These results are especially concerning because these hospitalizations outpace hospitalizations for methamphetamine use alone or mental health disorders alone,” they added.
The study was published online in Nature Mental Health .
Action Needed
Mental illness and methamphetamine use are both growing health problems. The investigators pointed out that methamphetamine use can cause serious harm to an individual’s mental, emotional, and social well-being and can significantly alter the brain.
They added that long-term methamphetamine users can exhibit “extreme anxiety, confusion, troubled sleep, mood changes, and aggressive behavior.” In addition, use of the drug can cause psychotic side effects such as paranoia, hallucinations, delusions, and suicidality.
The investigators noted that, to date, no studies have examined the combined effects of both diseases or characterized national trends over more than 10 years.
The researchers analyzed US mental health–related trends in methamphetamine users from 2008 to 2020. In particular, they wanted to characterize which demographic and geographic groups might be affected by both of these diseases because people with mental illness and co-occurring methamphetamine use are an “intersectional group” that is “doubly vulnerable to suicide and overdose death due to the synergistic effects of methamphetamine and mental health disorders.”
The investigators evaluated US trends in mental health disorder–related hospital admissions (MHD-HAs) and compared them with mental health admissions that involved concurrent methamphetamine use (MHD-HA-MUs) between 2008 and 2020.
Using data from the largest US inpatient care database, which encompasses more than 7 million hospital stays annually, they examined close to 4 million weighted hospital admissions and found more than a 10-fold increase in MHD-HA-MUs, compared with a 1.4-fold increase in MHD-HAs.
MHD-HA-MUs increased significantly among men (13-fold), non-Hispanic Black patients (39-fold), and those aged 41-64 years (16-fold). In the southern United States, MHD-HA-MUs increased 24-fold, larger than in any other region in the United States.
“Overall, the data suggest that there are synergistic effects with methamphetamine use and mental health disorder, highlighting this patient group’s unique needs, requiring distinct action,” the researchers wrote.
They proposed several interventions, including public education about substance use disorders, mental illness, and the effects of stigma. They also suggested decreasing criminal penalties for those with substance use disorders and improving healthcare delivery for this patient population.
This work was supported by the National Institutes of Health and an award from the National Institute of General Medical Sciences. The study authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research showed. Investigators found that between 2008 and 2020, such admissions increased by more than 10-fold.
“Overall, our results show an alarming increase in mental health disorder–related hospitalizations with concurrent methamphetamine use from 2008 to 2020,” wrote the investigators, led by Diensn Xing, Department of Medicine, Louisiana State University Health Sciences Center, Shreveport.
“These results are especially concerning because these hospitalizations outpace hospitalizations for methamphetamine use alone or mental health disorders alone,” they added.
The study was published online in Nature Mental Health .
Action Needed
Mental illness and methamphetamine use are both growing health problems. The investigators pointed out that methamphetamine use can cause serious harm to an individual’s mental, emotional, and social well-being and can significantly alter the brain.
They added that long-term methamphetamine users can exhibit “extreme anxiety, confusion, troubled sleep, mood changes, and aggressive behavior.” In addition, use of the drug can cause psychotic side effects such as paranoia, hallucinations, delusions, and suicidality.
The investigators noted that, to date, no studies have examined the combined effects of both diseases or characterized national trends over more than 10 years.
The researchers analyzed US mental health–related trends in methamphetamine users from 2008 to 2020. In particular, they wanted to characterize which demographic and geographic groups might be affected by both of these diseases because people with mental illness and co-occurring methamphetamine use are an “intersectional group” that is “doubly vulnerable to suicide and overdose death due to the synergistic effects of methamphetamine and mental health disorders.”
The investigators evaluated US trends in mental health disorder–related hospital admissions (MHD-HAs) and compared them with mental health admissions that involved concurrent methamphetamine use (MHD-HA-MUs) between 2008 and 2020.
Using data from the largest US inpatient care database, which encompasses more than 7 million hospital stays annually, they examined close to 4 million weighted hospital admissions and found more than a 10-fold increase in MHD-HA-MUs, compared with a 1.4-fold increase in MHD-HAs.
MHD-HA-MUs increased significantly among men (13-fold), non-Hispanic Black patients (39-fold), and those aged 41-64 years (16-fold). In the southern United States, MHD-HA-MUs increased 24-fold, larger than in any other region in the United States.
“Overall, the data suggest that there are synergistic effects with methamphetamine use and mental health disorder, highlighting this patient group’s unique needs, requiring distinct action,” the researchers wrote.
They proposed several interventions, including public education about substance use disorders, mental illness, and the effects of stigma. They also suggested decreasing criminal penalties for those with substance use disorders and improving healthcare delivery for this patient population.
This work was supported by the National Institutes of Health and an award from the National Institute of General Medical Sciences. The study authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MENTAL HEALTH
Atogepant May Prevent Rebound Headache From Medication Overuse in Chronic Migraine
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Is Anxiety a Prodromal Feature of Parkinson’s Disease?
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF GENERAL PRACTICE
Two-Drug Combo Promising for Methamphetamine Use Disorder
Extended-release injectable naltrexone combined with extended-release oral bupropion (NTX + BUPN) for moderate or severe methamphetamine use disorder was associated with a significant decrease in use of the drug, a new study showed.
Investigators leading the randomized clinical trial found a 27% increase in negative methamphetamine urine tests in the treatment group — indicating reduced use — compared with an 11% increase in negative urine tests in control participants.
“These findings have important implications for pharmacological treatment for methamphetamine use disorder. There is no FDA-approved medication for it, yet methamphetamine-involved overdoses have greatly increased over the past decade,” lead author Michael Li, MD, assistant professor-in-residence of family medicine at the David Geffen School of Medicine at UCLA, Los Angeles, said in a news release.
The study was published online in Addiction.
Methamphetamine use has increased worldwide, from 33 million users in 2010 to 34 million in 2020, with overdose deaths rising fivefold in the United States over the past decade, the authors wrote.
A previous open-label study of NTX + BUPN showed efficacy for treating severe methamphetamine use disorder, and NTX and BUPN have each shown efficacy separately for this indication.
This new study is the second phase of the multicenter ADAPT-2 trial, conducted between 2017 and 2019 in 403 participants with methamphetamine use disorder. In the first stage, 109 people received NTX + BUPN and 294 received placebo.
The treatment group received extended-release NTX (380 mg) or placebo as an intramuscular injection on weeks 1, 4, 7, and 10. Extended-release BUPN or placebo tablets were administered weekly, with BUPN doses starting at 150 mg on day 1 and increasing to 450 mg by day 3. At week 13, participants received a tapering dose for 4 days before discontinuing.
As previously reported by this news organization, the two-drug combo was effective at reducing methamphetamine use at 6 weeks. The current analysis measured change in methamphetamine use during weeks 7-12 of the trial and in posttreatment weeks 13-16.
Participants in the intervention group during stage 1 showed an additional 9.2% increase (P = .038) during stage 2 in their probability of testing negative for methamphetamine. This represented a total increase of 27.1% in negative urine tests across the complete 12 weeks of treatment, compared with a total 11.4% increase in negative tests in the placebo group.
The 12-week increase in methamphetamine-negative urine tests in the intervention group was 15.8% greater (P = .006) than the increase in the placebo group.
There was no significant change in either group at posttreatment follow-up in weeks 13-16.
“Our findings suggest that ongoing NTX + BUPN treatment yields statistically significant reductions in methamphetamine use that continue from weeks 7 to 12,” the authors wrote. The lack of change in methamphetamine use from weeks 13-16 corresponds to the conclusion of treatment in week 12, they added.
It remains to be determined “whether continued use of NTX + BUPN treatment past 12 weeks would yield further reductions in use,” the authors wrote, noting that prior stimulant use disorder trials suggest that change in use is gradual and that sustained abstinence is unlikely in merely 12 weeks of a trial. Rather, it is dependent on treatment duration.
“This warrants future clinical trials to quantify changes in methamphetamine use beyond 12 weeks and to identify the optimal duration of treatment with this medication,” they concluded.
The study was funded by awards from the National Institute on Drug Abuse (NIDA), the US Department of Health and Human Services, the National Institute of Mental Health, and the O’Donnell Clinical Neuroscience Scholar Award from the University of Texas Southwestern Medical Center. Alkermes provided Vivitrol (naltrexone for extended-release injectable suspension) and matched placebo free of charge for use in this trial under a written agreement with NIDA. Dr. Li reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
Extended-release injectable naltrexone combined with extended-release oral bupropion (NTX + BUPN) for moderate or severe methamphetamine use disorder was associated with a significant decrease in use of the drug, a new study showed.
Investigators leading the randomized clinical trial found a 27% increase in negative methamphetamine urine tests in the treatment group — indicating reduced use — compared with an 11% increase in negative urine tests in control participants.
“These findings have important implications for pharmacological treatment for methamphetamine use disorder. There is no FDA-approved medication for it, yet methamphetamine-involved overdoses have greatly increased over the past decade,” lead author Michael Li, MD, assistant professor-in-residence of family medicine at the David Geffen School of Medicine at UCLA, Los Angeles, said in a news release.
The study was published online in Addiction.
Methamphetamine use has increased worldwide, from 33 million users in 2010 to 34 million in 2020, with overdose deaths rising fivefold in the United States over the past decade, the authors wrote.
A previous open-label study of NTX + BUPN showed efficacy for treating severe methamphetamine use disorder, and NTX and BUPN have each shown efficacy separately for this indication.
This new study is the second phase of the multicenter ADAPT-2 trial, conducted between 2017 and 2019 in 403 participants with methamphetamine use disorder. In the first stage, 109 people received NTX + BUPN and 294 received placebo.
The treatment group received extended-release NTX (380 mg) or placebo as an intramuscular injection on weeks 1, 4, 7, and 10. Extended-release BUPN or placebo tablets were administered weekly, with BUPN doses starting at 150 mg on day 1 and increasing to 450 mg by day 3. At week 13, participants received a tapering dose for 4 days before discontinuing.
As previously reported by this news organization, the two-drug combo was effective at reducing methamphetamine use at 6 weeks. The current analysis measured change in methamphetamine use during weeks 7-12 of the trial and in posttreatment weeks 13-16.
Participants in the intervention group during stage 1 showed an additional 9.2% increase (P = .038) during stage 2 in their probability of testing negative for methamphetamine. This represented a total increase of 27.1% in negative urine tests across the complete 12 weeks of treatment, compared with a total 11.4% increase in negative tests in the placebo group.
The 12-week increase in methamphetamine-negative urine tests in the intervention group was 15.8% greater (P = .006) than the increase in the placebo group.
There was no significant change in either group at posttreatment follow-up in weeks 13-16.
“Our findings suggest that ongoing NTX + BUPN treatment yields statistically significant reductions in methamphetamine use that continue from weeks 7 to 12,” the authors wrote. The lack of change in methamphetamine use from weeks 13-16 corresponds to the conclusion of treatment in week 12, they added.
It remains to be determined “whether continued use of NTX + BUPN treatment past 12 weeks would yield further reductions in use,” the authors wrote, noting that prior stimulant use disorder trials suggest that change in use is gradual and that sustained abstinence is unlikely in merely 12 weeks of a trial. Rather, it is dependent on treatment duration.
“This warrants future clinical trials to quantify changes in methamphetamine use beyond 12 weeks and to identify the optimal duration of treatment with this medication,” they concluded.
The study was funded by awards from the National Institute on Drug Abuse (NIDA), the US Department of Health and Human Services, the National Institute of Mental Health, and the O’Donnell Clinical Neuroscience Scholar Award from the University of Texas Southwestern Medical Center. Alkermes provided Vivitrol (naltrexone for extended-release injectable suspension) and matched placebo free of charge for use in this trial under a written agreement with NIDA. Dr. Li reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
Extended-release injectable naltrexone combined with extended-release oral bupropion (NTX + BUPN) for moderate or severe methamphetamine use disorder was associated with a significant decrease in use of the drug, a new study showed.
Investigators leading the randomized clinical trial found a 27% increase in negative methamphetamine urine tests in the treatment group — indicating reduced use — compared with an 11% increase in negative urine tests in control participants.
“These findings have important implications for pharmacological treatment for methamphetamine use disorder. There is no FDA-approved medication for it, yet methamphetamine-involved overdoses have greatly increased over the past decade,” lead author Michael Li, MD, assistant professor-in-residence of family medicine at the David Geffen School of Medicine at UCLA, Los Angeles, said in a news release.
The study was published online in Addiction.
Methamphetamine use has increased worldwide, from 33 million users in 2010 to 34 million in 2020, with overdose deaths rising fivefold in the United States over the past decade, the authors wrote.
A previous open-label study of NTX + BUPN showed efficacy for treating severe methamphetamine use disorder, and NTX and BUPN have each shown efficacy separately for this indication.
This new study is the second phase of the multicenter ADAPT-2 trial, conducted between 2017 and 2019 in 403 participants with methamphetamine use disorder. In the first stage, 109 people received NTX + BUPN and 294 received placebo.
The treatment group received extended-release NTX (380 mg) or placebo as an intramuscular injection on weeks 1, 4, 7, and 10. Extended-release BUPN or placebo tablets were administered weekly, with BUPN doses starting at 150 mg on day 1 and increasing to 450 mg by day 3. At week 13, participants received a tapering dose for 4 days before discontinuing.
As previously reported by this news organization, the two-drug combo was effective at reducing methamphetamine use at 6 weeks. The current analysis measured change in methamphetamine use during weeks 7-12 of the trial and in posttreatment weeks 13-16.
Participants in the intervention group during stage 1 showed an additional 9.2% increase (P = .038) during stage 2 in their probability of testing negative for methamphetamine. This represented a total increase of 27.1% in negative urine tests across the complete 12 weeks of treatment, compared with a total 11.4% increase in negative tests in the placebo group.
The 12-week increase in methamphetamine-negative urine tests in the intervention group was 15.8% greater (P = .006) than the increase in the placebo group.
There was no significant change in either group at posttreatment follow-up in weeks 13-16.
“Our findings suggest that ongoing NTX + BUPN treatment yields statistically significant reductions in methamphetamine use that continue from weeks 7 to 12,” the authors wrote. The lack of change in methamphetamine use from weeks 13-16 corresponds to the conclusion of treatment in week 12, they added.
It remains to be determined “whether continued use of NTX + BUPN treatment past 12 weeks would yield further reductions in use,” the authors wrote, noting that prior stimulant use disorder trials suggest that change in use is gradual and that sustained abstinence is unlikely in merely 12 weeks of a trial. Rather, it is dependent on treatment duration.
“This warrants future clinical trials to quantify changes in methamphetamine use beyond 12 weeks and to identify the optimal duration of treatment with this medication,” they concluded.
The study was funded by awards from the National Institute on Drug Abuse (NIDA), the US Department of Health and Human Services, the National Institute of Mental Health, and the O’Donnell Clinical Neuroscience Scholar Award from the University of Texas Southwestern Medical Center. Alkermes provided Vivitrol (naltrexone for extended-release injectable suspension) and matched placebo free of charge for use in this trial under a written agreement with NIDA. Dr. Li reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
New Clues on How Blast Exposure May Lead to Alzheimer’s Disease
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
Early-Life Excess Weight Tied to Subsequent Stroke Risk
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sharp Rise in US Pediatric ADHD Diagnoses
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
Early Memory Problems Linked to Increased Tau
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.