User login
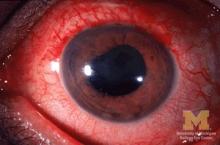
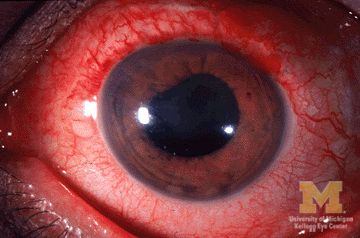
Many patients with acute anterior uveitis may have undiagnosed spondyloarthritis
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
FROM ARTHRITIS & RHEUMATOLOGY
Vitamin D supplements do not lower risk of fractures
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nordic walking bests other workouts on functional outcome in CVD
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Time-restricted eating may reduce CVD risk after breast cancer
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY: CARDIAC ONCO
Espresso coffee linked to increased total cholesterol
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Takotsubo syndrome also linked to happy life events
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
JACC: HEART FAILURE
Bariatric surgery cuts cardiovascular events, even in seniors
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Cannabis exposure in pregnancy linked with childhood obesity
There is a link between cannabis exposure during pregnancy and higher fasting glucose levels and adiposity in the offspring in early childhood, a new study suggests.
Research looking at the effect of prenatal exposure to cannabis on offspring is growing. It is known to affect childhood cognition and behavior; however, there is little work to date on how it affects metabolic outcomes, said lead author Brianna F. Moore, PhD.
“Officially, the American Academy of Obstetricians and Gynecologists recommends that women do not use cannabis during pregnancy or while breastfeeding to limit the effects on offspring. There’s really a lot we don’t know, but researchers across the country are starting to look into this more, and there are signs that it isn’t great for the offspring,” Dr. Moore, assistant professor at the Colorado School of Public Health in Aurora, said in an interview.
And she noted that while some women turn to cannabis to manage the challenging symptoms of pregnancy, “Clinicians should encourage pregnant women to refrain from using cannabis; it is best for these pregnant women to talk to their physicians about alternative ways of managing these symptoms.”
The findings were published online March 31 in the Journal of Clinical Endocrinology & Metabolism.
Study of mother and 5-year-old child pairs
The researchers assessed 103 sets of mothers and children from the Healthy Start study. At 27 weeks of gestation, the investigators assessed 12 metabolites of cannabis/cannabinoids in urine samples. Results from these samples were used to categorize fetal exposure to cannabis as either not exposed or exposed. They found that about 15% of the mothers had traceable amounts of cannabinoids, suggesting fetal cannabis exposure.
At follow-up, the study team assessed fat-free mass and fat mass using air displacement plethysmography among the offspring around age 5. They used generalized linear models to approximate the relationship between fetal exposure to cannabis with metabolic measures such as insulin, glucose, and homeostatic model assessment of insulin resistance (HOMA-IR), and adiposity measures such as body mass index, fat-free mass, fat mass, adiposity, and BMI z-scores.
The findings showed that, compared with nonexposed offspring, exposed offspring had greater:
- Fasting glucose (5.6 mg/dL; 95% confidence interval [CI], 0.8-10.3).
- Fat-free mass (1.2 kg; 95% CI, 0.4-2.0).
- Fat mass (1.0 kg; 95% CI, 0.3-1.7).
- Adiposity (2.6%; 95% CI, 0.1-5.2).
“This finding may suggest that fetal exposure to cannabis contributes to higher fasting glucose levels via a direct effect on the pancreatic β-cells. However, we cannot draw conclusions about β-cell response to glucose because we did not perform oral glucose tolerance tests,” the study authors wrote.
Notably, however, there was no relationship between BMI z-scores, BMI, or HOMA-IR and fasting insulin, the study team found.
Study limitations include the small sample size and lack of self-report data on cannabis use to differentiate between direct use and exposure to cannabis, Dr. Moore acknowledged.
Given the small sample size, the researchers were unable to look at dose-response, which future studies will focus on, Dr. Moore noted. Future efforts will also focus on comparing the effects of tetrahydrocannabinol (THC) and cannabidiol (CBD), Dr. Moore added.
“This is a relatively new field, so there’s still work to be done. This is just one study, and we need to study this more in other cohorts to confirm our findings,” she concluded.
Dr. Moore has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a link between cannabis exposure during pregnancy and higher fasting glucose levels and adiposity in the offspring in early childhood, a new study suggests.
Research looking at the effect of prenatal exposure to cannabis on offspring is growing. It is known to affect childhood cognition and behavior; however, there is little work to date on how it affects metabolic outcomes, said lead author Brianna F. Moore, PhD.
“Officially, the American Academy of Obstetricians and Gynecologists recommends that women do not use cannabis during pregnancy or while breastfeeding to limit the effects on offspring. There’s really a lot we don’t know, but researchers across the country are starting to look into this more, and there are signs that it isn’t great for the offspring,” Dr. Moore, assistant professor at the Colorado School of Public Health in Aurora, said in an interview.
And she noted that while some women turn to cannabis to manage the challenging symptoms of pregnancy, “Clinicians should encourage pregnant women to refrain from using cannabis; it is best for these pregnant women to talk to their physicians about alternative ways of managing these symptoms.”
The findings were published online March 31 in the Journal of Clinical Endocrinology & Metabolism.
Study of mother and 5-year-old child pairs
The researchers assessed 103 sets of mothers and children from the Healthy Start study. At 27 weeks of gestation, the investigators assessed 12 metabolites of cannabis/cannabinoids in urine samples. Results from these samples were used to categorize fetal exposure to cannabis as either not exposed or exposed. They found that about 15% of the mothers had traceable amounts of cannabinoids, suggesting fetal cannabis exposure.
At follow-up, the study team assessed fat-free mass and fat mass using air displacement plethysmography among the offspring around age 5. They used generalized linear models to approximate the relationship between fetal exposure to cannabis with metabolic measures such as insulin, glucose, and homeostatic model assessment of insulin resistance (HOMA-IR), and adiposity measures such as body mass index, fat-free mass, fat mass, adiposity, and BMI z-scores.
The findings showed that, compared with nonexposed offspring, exposed offspring had greater:
- Fasting glucose (5.6 mg/dL; 95% confidence interval [CI], 0.8-10.3).
- Fat-free mass (1.2 kg; 95% CI, 0.4-2.0).
- Fat mass (1.0 kg; 95% CI, 0.3-1.7).
- Adiposity (2.6%; 95% CI, 0.1-5.2).
“This finding may suggest that fetal exposure to cannabis contributes to higher fasting glucose levels via a direct effect on the pancreatic β-cells. However, we cannot draw conclusions about β-cell response to glucose because we did not perform oral glucose tolerance tests,” the study authors wrote.
Notably, however, there was no relationship between BMI z-scores, BMI, or HOMA-IR and fasting insulin, the study team found.
Study limitations include the small sample size and lack of self-report data on cannabis use to differentiate between direct use and exposure to cannabis, Dr. Moore acknowledged.
Given the small sample size, the researchers were unable to look at dose-response, which future studies will focus on, Dr. Moore noted. Future efforts will also focus on comparing the effects of tetrahydrocannabinol (THC) and cannabidiol (CBD), Dr. Moore added.
“This is a relatively new field, so there’s still work to be done. This is just one study, and we need to study this more in other cohorts to confirm our findings,” she concluded.
Dr. Moore has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a link between cannabis exposure during pregnancy and higher fasting glucose levels and adiposity in the offspring in early childhood, a new study suggests.
Research looking at the effect of prenatal exposure to cannabis on offspring is growing. It is known to affect childhood cognition and behavior; however, there is little work to date on how it affects metabolic outcomes, said lead author Brianna F. Moore, PhD.
“Officially, the American Academy of Obstetricians and Gynecologists recommends that women do not use cannabis during pregnancy or while breastfeeding to limit the effects on offspring. There’s really a lot we don’t know, but researchers across the country are starting to look into this more, and there are signs that it isn’t great for the offspring,” Dr. Moore, assistant professor at the Colorado School of Public Health in Aurora, said in an interview.
And she noted that while some women turn to cannabis to manage the challenging symptoms of pregnancy, “Clinicians should encourage pregnant women to refrain from using cannabis; it is best for these pregnant women to talk to their physicians about alternative ways of managing these symptoms.”
The findings were published online March 31 in the Journal of Clinical Endocrinology & Metabolism.
Study of mother and 5-year-old child pairs
The researchers assessed 103 sets of mothers and children from the Healthy Start study. At 27 weeks of gestation, the investigators assessed 12 metabolites of cannabis/cannabinoids in urine samples. Results from these samples were used to categorize fetal exposure to cannabis as either not exposed or exposed. They found that about 15% of the mothers had traceable amounts of cannabinoids, suggesting fetal cannabis exposure.
At follow-up, the study team assessed fat-free mass and fat mass using air displacement plethysmography among the offspring around age 5. They used generalized linear models to approximate the relationship between fetal exposure to cannabis with metabolic measures such as insulin, glucose, and homeostatic model assessment of insulin resistance (HOMA-IR), and adiposity measures such as body mass index, fat-free mass, fat mass, adiposity, and BMI z-scores.
The findings showed that, compared with nonexposed offspring, exposed offspring had greater:
- Fasting glucose (5.6 mg/dL; 95% confidence interval [CI], 0.8-10.3).
- Fat-free mass (1.2 kg; 95% CI, 0.4-2.0).
- Fat mass (1.0 kg; 95% CI, 0.3-1.7).
- Adiposity (2.6%; 95% CI, 0.1-5.2).
“This finding may suggest that fetal exposure to cannabis contributes to higher fasting glucose levels via a direct effect on the pancreatic β-cells. However, we cannot draw conclusions about β-cell response to glucose because we did not perform oral glucose tolerance tests,” the study authors wrote.
Notably, however, there was no relationship between BMI z-scores, BMI, or HOMA-IR and fasting insulin, the study team found.
Study limitations include the small sample size and lack of self-report data on cannabis use to differentiate between direct use and exposure to cannabis, Dr. Moore acknowledged.
Given the small sample size, the researchers were unable to look at dose-response, which future studies will focus on, Dr. Moore noted. Future efforts will also focus on comparing the effects of tetrahydrocannabinol (THC) and cannabidiol (CBD), Dr. Moore added.
“This is a relatively new field, so there’s still work to be done. This is just one study, and we need to study this more in other cohorts to confirm our findings,” she concluded.
Dr. Moore has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Boosting daily exercise after age 70 tied to lower CVD risk
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increasingly active patterns of physical activity were linked with reduced rates of overall mortality and cardiovascular disease (CVD), but early rather than later in late life, in a 20-year follow-up cohort study.
In this population of people older than 65 years, researchers found that physical activity overall was associated with lower rates of incident CVD, particularly among men, and the association was strongest in people 70 to 75 years of age, rather than in older age groups.
They also looked at “trajectories,” or changes in activity over time, and found that a stable-high trajectory of activity was associated with a significantly lower risk for cardiovascular outcomes in men than in those with a stable-low trajectory. For women, more physical activity was consistently associated with lower CVD outcomes, although not statistically significantly so, except for overall mortality, which did reach significance.
Notably, the greatest reduction in cardiovascular risk was reported in people who did more than 20 minutes of physical exercise each day, and it was more pronounced in those 70 years of age.
Physical activity was also associated with a lower incidence of heart failure and coronary heart disease in older people, again especially early on in late life, reported Claudio Barbiellini Amidei, MD, University of Padua, Italy, and colleagues.
The data suggest that physical activity is more effective in preventing CVD onset when implemented early rather than later in life, noted Dr. Amidei in an email.
“The findings of our study are suggestive of a protective effect of physical activity in late-life on cardiovascular health. WHO recommendations for adults and older adults are to practice at least 20 minutes of moderate to vigorous physical activity per day. I believe this is a realistic target, and policy makers should raise awareness on the importance of achieving this goal at all ages, including in late-life,” Dr. Amidei said.
The study was published online Feb. 14 in Heart.
Previous research has demonstrated that the most benefit of high physical activity, compared with low, begins at about 60 years of age, and that is because younger people are at much lower risk, noted Carl “Chip” Lavie MD, FACC, medical director of cardiac rehabilitation and prevention, Ochsner Clinical School–The University of Queensland School of Medicine, New Orleans, who was not involved in the study.
“At quite old ages, for example over age 80, resistance exercise or weight training and balance training may be even more important than aerobic training,” he added.
Activity ‘trajectories’
The benefits of physical activity on cardiovascular risk are well established, the researchers note. Less clear is the role that trajectories of activity over time play, although research to date suggests a reduction in risk with increasing activity from mid-life to early old age, they write.
For the current analysis, the researchers assessed 3,099 Italian participants. Mean age was about 75 years, and baseline data were collected from 1995 to 1997.
Follow-up visits were conducted after 4 years and again after 7 years. Using hospital medical records and mortality data, the researchers were able to collect surveillance data through 2018. Hospital records, surveys, and clinical assessments helped them identify incident and prevalent cardiovascular diseases, such as stroke, coronary heart disease, and heart failure.
Participants’ physical activity patterns were classified as stable-high, low-increasing, high-decreasing, and stable-low. Exposure was evaluated at 70, 75, 80, and 85 years of age.
“In our analyses, we focused on moderate to vigorous physical activity, and these include a broad range of exercises, such as walking very briskly, playing tennis, [and] jogging, but comprise also other activities, such as gardening or doing household chores,” said Dr. Amidei.
Patterns of stable-low physical activity were linked to a significantly greater risk for cardiovascular outcomes in men than patterns of stable-high physical activity (hazard ratio, 0.48; 95% confidence interval, 0.27-0.86; P for trend = .002).
No significant relation was found between physical activity and stroke, the researchers note.
“The benefits of physical activity seem to lessen above the age of 75 years and seem more important in men,” noted Dr. Lavie. “This may be partly due to the higher risk of CVD in men. Women typically lag 13 to 15 years behind men for CVD but start catching up in older years.”
Limitations of the study include lack of information regarding physical activity during mid-life, the limited number of stroke events, the relatively few participants older than 85 years, and potential recall bias, the researchers note.
Another limitation was that the physical activity data were based on patient surveys collected 3 years apart and did not involve the use of an accelerometer, the researchers add.
“Future observational studies are required to confirm our findings and pathophysiological studies are warranted to examine the underlying biological mechanisms. Physical activity is likely to be beneficial at any age, but to summarize our findings, we could say that when it comes to being physically active, the sooner the better,” concluded Dr. Amidei.
Dr. Amidei reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.