User login
Anticoagulation Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving treatment options for preventing stroke, acute coronary events, deep vein thrombosis, and pulmonary embolism in at-risk patients. The Anticoagulation Hub is powered by Frontline Medical Communications.
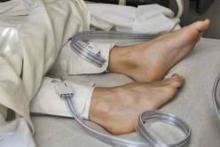
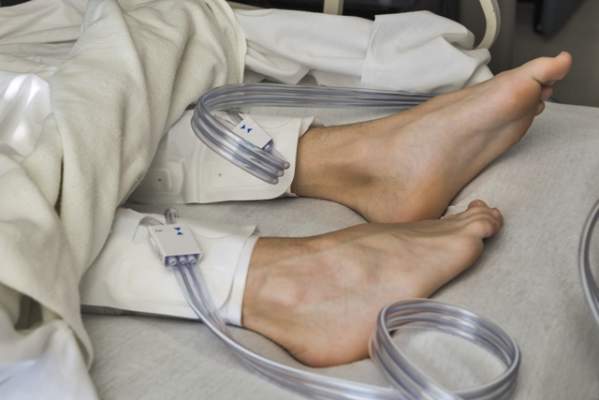
IPC maintains first-line status in preventing DVT in most surgical patients
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
FROM JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: IPC is efficacious as the sole prophylactic agent in low- or moderate-risk surgical patients and in patients with high risk of bleeding with drug therapy.
Major finding: In high-risk patients, IPC is an added modality for preventing DVT in combination with pharmacologic prophylaxis.
Data source: Researchers performed an assessment of the literature in English since 2011 in MEDLINE and the Cochrane libraries.
Disclosures: The authors reported that they had no conflicts of interest.
Low incidence of DVT seen in routine MRI of damaged knees
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
FROM JOURNAL OF CLINICAL IMAGING
EASD: SGLT2 inhibitor empagliflozin cuts CV risk in patients with type 2 diabetes
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
AT EASD 2015
Key clinical point: Empagliflozin reduced cardiovascular risk in patients with type 2 diabetes who were at high cardiovascular risk, making it the first antidiabetic drug to have been shown to have such an effect.
Major finding: This primary composite endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke was reached by 10.5% (N = 4,687) of patients treated with empagliflozin versus 12.1% (N = 2,333) of those given placebo.
Data source: EMPA-REG OUTCOME: a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin added to usual care versus usual care alone in more than 7,000 patients with type 2 diabetes at high cardiovascular risk.
Disclosures: Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
HIT risk rises with obesity
LAS VEGAS – High body mass index is strongly associated with increased rates of heparin-induced thrombocytopenia, based on findings from a review of prospectively collected data from surgical and cardiac intensive care unit patients presumed to have the condition.
Of 304 patients included in the review, 36 (12%) were positive for heparin-induced thrombocytopenia (HIT). The rates increased in tandem with BMI. For example, the rate was 0% among 9 underweight individuals (BMI less than 18.5 kg/m2), 8% among 119 normal-weight individuals (BMI of 18.5-24.9 kg/m2), 11% among 98 overweight individuals (BMI of 25-29.9 kg/m2), 18% among 67 obese individuals (BMI of 30-39.9 kg/m2), and 36% among 11 morbidly obese individuals (BMI of 40 kg/m2 or greater), Dr. Matthew B. Bloom reported at the annual meeting of the American Association for the Surgery of Trauma.
The odds of HIT were 170% greater among obese patients, compared with normal-weight patients (odds ratio, 2.67), and 600% greater among morbidly obese patients, compared with normal-weight patient (odds ratio, 6.98), said Dr. Bloom of Cedars-Sinai Medical Center, Los Angeles.
Logistic regression showed that each 1 unit increase in BMI was associated with a 7.7% increase in the odds of developing HIT, he noted.
Additionally, an anti-heparin/PF4 (platelet factor 4) antibody OD (optical density) value of 2.0 or greater, but not of 0.4 or greater or 0.8 or greater, was also significantly increased with BMI, and in-hospital mortality increased significantly with BMI above normal, he said.
Warkentin 4T scores used to differentiate HIT from other types of thrombocytopenia were not found to correlate with changes in BMI in this study, nor were deep vein thrombosis, pulmonary embolism, or stroke.
The increase in PF4 with increasing BMI may be a marker for overall increasing levels of circulating antibodies in the obese ICU population, but more biochemical studies are needed to tease this out, he said.
Patients included in the review were all those admitted to the surgical and cardiac ICUs at Cedars-Sinai over a more than 7 year period. They had a mean age of 62.1 years, 59% were men, and their mean BMI was 27 kg/m2.
The findings are among the first to show a strong association between BMI and HIT in ICU patients, Dr. Bloom said, noting that several other studies have shown that obesity is linked with increased incidence and increased severity of immune-mediated diseases, including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.
“And HIT is an immune-mediated disease,” he added.
“BMI may be an important new clinical variable for estimating the pre-test probability of HIT, and perhaps, in the future, patient ‘thickness’ could be considered a new ‘T’ in the 4T score, he concluded.
Dr. Bloom reported having no disclosures.
LAS VEGAS – High body mass index is strongly associated with increased rates of heparin-induced thrombocytopenia, based on findings from a review of prospectively collected data from surgical and cardiac intensive care unit patients presumed to have the condition.
Of 304 patients included in the review, 36 (12%) were positive for heparin-induced thrombocytopenia (HIT). The rates increased in tandem with BMI. For example, the rate was 0% among 9 underweight individuals (BMI less than 18.5 kg/m2), 8% among 119 normal-weight individuals (BMI of 18.5-24.9 kg/m2), 11% among 98 overweight individuals (BMI of 25-29.9 kg/m2), 18% among 67 obese individuals (BMI of 30-39.9 kg/m2), and 36% among 11 morbidly obese individuals (BMI of 40 kg/m2 or greater), Dr. Matthew B. Bloom reported at the annual meeting of the American Association for the Surgery of Trauma.
The odds of HIT were 170% greater among obese patients, compared with normal-weight patients (odds ratio, 2.67), and 600% greater among morbidly obese patients, compared with normal-weight patient (odds ratio, 6.98), said Dr. Bloom of Cedars-Sinai Medical Center, Los Angeles.
Logistic regression showed that each 1 unit increase in BMI was associated with a 7.7% increase in the odds of developing HIT, he noted.
Additionally, an anti-heparin/PF4 (platelet factor 4) antibody OD (optical density) value of 2.0 or greater, but not of 0.4 or greater or 0.8 or greater, was also significantly increased with BMI, and in-hospital mortality increased significantly with BMI above normal, he said.
Warkentin 4T scores used to differentiate HIT from other types of thrombocytopenia were not found to correlate with changes in BMI in this study, nor were deep vein thrombosis, pulmonary embolism, or stroke.
The increase in PF4 with increasing BMI may be a marker for overall increasing levels of circulating antibodies in the obese ICU population, but more biochemical studies are needed to tease this out, he said.
Patients included in the review were all those admitted to the surgical and cardiac ICUs at Cedars-Sinai over a more than 7 year period. They had a mean age of 62.1 years, 59% were men, and their mean BMI was 27 kg/m2.
The findings are among the first to show a strong association between BMI and HIT in ICU patients, Dr. Bloom said, noting that several other studies have shown that obesity is linked with increased incidence and increased severity of immune-mediated diseases, including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.
“And HIT is an immune-mediated disease,” he added.
“BMI may be an important new clinical variable for estimating the pre-test probability of HIT, and perhaps, in the future, patient ‘thickness’ could be considered a new ‘T’ in the 4T score, he concluded.
Dr. Bloom reported having no disclosures.
LAS VEGAS – High body mass index is strongly associated with increased rates of heparin-induced thrombocytopenia, based on findings from a review of prospectively collected data from surgical and cardiac intensive care unit patients presumed to have the condition.
Of 304 patients included in the review, 36 (12%) were positive for heparin-induced thrombocytopenia (HIT). The rates increased in tandem with BMI. For example, the rate was 0% among 9 underweight individuals (BMI less than 18.5 kg/m2), 8% among 119 normal-weight individuals (BMI of 18.5-24.9 kg/m2), 11% among 98 overweight individuals (BMI of 25-29.9 kg/m2), 18% among 67 obese individuals (BMI of 30-39.9 kg/m2), and 36% among 11 morbidly obese individuals (BMI of 40 kg/m2 or greater), Dr. Matthew B. Bloom reported at the annual meeting of the American Association for the Surgery of Trauma.
The odds of HIT were 170% greater among obese patients, compared with normal-weight patients (odds ratio, 2.67), and 600% greater among morbidly obese patients, compared with normal-weight patient (odds ratio, 6.98), said Dr. Bloom of Cedars-Sinai Medical Center, Los Angeles.
Logistic regression showed that each 1 unit increase in BMI was associated with a 7.7% increase in the odds of developing HIT, he noted.
Additionally, an anti-heparin/PF4 (platelet factor 4) antibody OD (optical density) value of 2.0 or greater, but not of 0.4 or greater or 0.8 or greater, was also significantly increased with BMI, and in-hospital mortality increased significantly with BMI above normal, he said.
Warkentin 4T scores used to differentiate HIT from other types of thrombocytopenia were not found to correlate with changes in BMI in this study, nor were deep vein thrombosis, pulmonary embolism, or stroke.
The increase in PF4 with increasing BMI may be a marker for overall increasing levels of circulating antibodies in the obese ICU population, but more biochemical studies are needed to tease this out, he said.
Patients included in the review were all those admitted to the surgical and cardiac ICUs at Cedars-Sinai over a more than 7 year period. They had a mean age of 62.1 years, 59% were men, and their mean BMI was 27 kg/m2.
The findings are among the first to show a strong association between BMI and HIT in ICU patients, Dr. Bloom said, noting that several other studies have shown that obesity is linked with increased incidence and increased severity of immune-mediated diseases, including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.
“And HIT is an immune-mediated disease,” he added.
“BMI may be an important new clinical variable for estimating the pre-test probability of HIT, and perhaps, in the future, patient ‘thickness’ could be considered a new ‘T’ in the 4T score, he concluded.
Dr. Bloom reported having no disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Higher body mass index is strongly associated with increased rates of heparin-induced thrombocytopenia.
Major finding: The HIT rate was 0%, 8%, 11%, 18,%, and 36% among underweight, normal-weight, overweight, obese, and morbidly obese individuals, respectively.
Data source: A review of prospectively collected data for 304 patients.
Disclosures: Dr. Bloom reported having no disclosures.
Lives saved with lower systolic BP: SPRINT trial
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
Deaths were reduced by nearly one-quarter when systolic blood pressure was treated to a target of 120 rather than 140 mm Hg, according to a large NIH-sponsored study comparing standard blood pressure treatment with more-intensive lowering of systolic blood pressure. The lower blood pressure group also saw a 30% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death.
The magnitude of the effect of the lower blood pressure target prompted the study’s data safety monitoring board to end the study early, said officials from several National Institutes of Health agencies at a telebriefing. The study was unblinded in August 2015, and a full report of the primary outcome measures will come in a paper due out by the end of the year, they said.
The Systolic Blood Pressure Intervention Trial, or SPRINT, is a 100-site trial that enrolled more than 9,300 people in the United States and Puerto Rico aged at least 50 years with high blood pressure and at risk for cardiovascular disease; those with diabetes were excluded. Patients were randomized to a standard treatment target of 140 mm Hg or less, or to a more intensive 120 mm Hg.
SPRINT participants received evidence-based treatment with a variety of antihypertensives, with the intervention arm requiring an average of almost three medications, compared with just under two for the less-intensive treatment arm.
Against a backdrop of uncertainty in the literature about what the target systolic blood pressure should be for those with hypertension and at risk for cardiovascular events or kidney disease, the study provides compelling evidence that more-aggressive blood pressure lowering is important. “More-intensive management of blood pressure can save lives,” said Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute. This is good news, he said, since about one in three Americans has high blood pressure, and only about half of those 70 million currently have their blood pressure under control.
Dr. Jackson T. Wright Jr., SPRINT study lead and director of the clinical hypertension program at Case Western Reserve University in Cleveland, also emphasized that intensive blood pressure management can prevent the cardiovascular complications of hypertension. Though subgroup analysis is ongoing, the effect seems robust and consistent across age groups, sex, and ethnicity, he said. SPRINT, he said, also “offers an excellent opportunity to examine the tolerability and safety of the lower target.” The first look at the safety data shows that the more-intensive treatment is well tolerated, though data analysis is ongoing, he said.
Dr. Suzanne Oparil, director of the vascular biology and hypertension program at the University of Alabama-Birmingham, said, “This is a time of enlightenment.” The previous absence of compelling data played a part in the debate surrounding blood pressure levels that should be used in guidance documents, and Dr. Gibbons and Dr. Wright both emphasized that they would expect the forthcoming primary outcomes paper to have an impact on guideline-writing bodies. Dr. Wright said, however, “We are not providing guidance for providers or patients right now. The study was just unblinded a little less than 3 weeks ago.”
In 2014, the group of experts who had constituted the JNC 8 panel, a team assembled in 2008 by NHLBI to update official U.S. hypertension management guidelines, set the target blood pressure for the general population aged 60 years or older to less than 150/90 mm Hg, a major break from long-standing practice to treat such patients to a target systolic pressure of less than 140 mm Hg (JAMA. 2014;311[5]:507-20). These guidelines, released after SPRINT began, remain controversial.
The SPRINT MIND trial, tracking the relationship between systolic blood pressure and cognitive impairment or dementia, is ongoing. The study is also still collecting data about kidney function in study participants.
The study was funded by the National Institutes of Health. Two drug companies, Takeda and Arbor, provided some medication for the trial.
On Twitter @karioakes
FROM AN NHLBI TELEBRIEFING
ESC: Atrial fibrillation accelerates brain atrophy
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
AT THE ESC CONGRESS 2015
Key clinical point: Atrial fibrillation appears to accelerate brain aging in the elderly independent of cerebral embolism.
Major finding: Both prevalent and incident atrial fibrillation were associated with accelerated loss of gray matter and total brain volume as well as key aspects of cognitive function.
Data source: This population-based study included 2,472 nondemented participants with a mean baseline age of 76 years who were prospectively followed with brain scans and structured cognitive function tests for a mean of 5.2 years.
Disclosures: The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. The presenter reported having no financial conflicts.
ESC: Too much TV boosts PE risk
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
AT THE ESC CONGRESS 2015
Key clinical point: Tuning in to the television for an average of 5 hours or more per day is independently associated with a sharply increased pulmonary embolism risk.
Major finding: Middle-aged Japanese adults who spent 5 or more hours per day watching television were at an adjusted 6.5-fold greater risk of fatal pulmonary embolism than were those who averaged less than 2.5 hours of viewing.
Data source: This prospective Japanese national study included 86,024 participants aged 40-79 years who were followed for a median of 18.4 years.
Disclosures: The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. The presenter reported having no financial conflicts.
ESC: Rivaroxaban safety highlighted in real-world setting
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
AT THE ESC CONGRESS 2015
Key clinical point: Data from routine clinical practice studies suggest a low risk of bleeding and stroke with rivaroxaban and other non–vitamin K antagonists.
Major finding: The incidence of major bleeding with rivaroxaban was 2.1% per year and the risk of stroke was 0.7% per year in the XANTUS study.
Data source: More than 45,000 patients with nonvalvular atrial fibrillation treated with rivaroxaban for stroke prevention in two, real-world observational studies and separate electronic medical record analyses of patients treated with apixaban, rivaroxaban, and dabigatran.
Disclosures: The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
Eculizumab benefited pregnant women with paroxysmal nocturnal hemoglobinuria
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: None of the pregnant women with paroxysmal nocturnal hemoglobinuria died on eculizumab therapy.
Major finding: The fetal death rate was 4%, and the premature birth rate was 29%.
Data source: A retrospective, survey-based study of 75 pregnancies among 61 women with PNH.
Disclosures: Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
The pros and cons of novel anticoagulants
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.