User login
Single-incision sling may help stress urinary incontinence
NATIONAL HARBOR, MD. – After 12 months, an adjustable, single-incision sling significantly improved clinical and quality of life measures associated with stress urinary incontinence in women.
The Altis sling decreased the Urinary Distress Index (UDI) from a mean of 55 to a mean of 10, Dr. Douglas Van Drie said at a meeting sponsored by the AAGL. The Incontinence Impact Questionnaire (ILQ-7) showed similar improvements at the interim assessment of the device’s 2-year safety and efficacy study.
The study was sponsored by Coloplast, which makes the sling, with input and monitoring by the Food and Drug Administration. Altis was approved in November 2012 based on an investigational device exemption study, which included implant data. The FDA efficacy requirement was a 50% decrease in pad weight by 6 months.
According to the company website, "Altis is a unique, minimally invasive sling that combines integrated two-way tensioning with lightweight sling material to provide strength, security, and adjustability."
Physicians are divided on their thoughts about a single-incision sling, said Dr. Van Drie, a urogynecologist in group practice in Grand Rapids, Mich. "Those [physicians] who use them are advocates for their safety and simplicity, and the ability to insert them in the office. They have been adopted in different areas of the world as an option for doing simplified, less costly incontinence surgery. The argument against is questions about their staying power – will the effect hold up long term?" he said.
Even though the Altis is a single-incision sling, it has a "very secure" anchoring system, Dr. Van Drie said. The anchor not only goes into the obturator internus, but into the membrane and the obturator externus.
The study involved 113 women, with a mean age of 54 years. Their histories included stress incontinence with hypermobility (81%), without hypermobility (19%), mixed incontinence (37%), and overactive bladder (5%).
Most of the procedures were performed during an inpatient hospital stay (59%); however, 24% were performed at an ambulatory surgical center and 17%, in the physician’s office. General anesthesia was used in 52%, spinal in 3%, and local in 45%.
At 12 months, 90% of patients had at least a 50% reduction in pad weight, and 90% had a negative cough stress test result. The UDI decreased by a mean of 46 points, and the IIQ-7 score, by a mean of 47 points.
There were 11 device-related failures in eight patients. These included one each of urinary retention, urinary tract infection, decreased urine stream, dyspareunia, inflammation, worsening of overactive bladder, and voiding dysfunction. There were four mesh extrusions (3.5%), all less than 3 cm. Two patients with extrusion were smokers, and one was diabetic.
Serious adverse events occurred in three patients: One with a hematoma, one patient who needed transfer to the operating room because of anxiety during repair of a 2-mm mesh extrusion, and one patient whose adverse event was changed to a severe adverse event when she moved out of the study. There were no unanticipated device effects, Dr. Van Drie noted.
Dr. Van Drie is a consultant for Coloplast and has received research money and grants from the company.
NATIONAL HARBOR, MD. – After 12 months, an adjustable, single-incision sling significantly improved clinical and quality of life measures associated with stress urinary incontinence in women.
The Altis sling decreased the Urinary Distress Index (UDI) from a mean of 55 to a mean of 10, Dr. Douglas Van Drie said at a meeting sponsored by the AAGL. The Incontinence Impact Questionnaire (ILQ-7) showed similar improvements at the interim assessment of the device’s 2-year safety and efficacy study.
The study was sponsored by Coloplast, which makes the sling, with input and monitoring by the Food and Drug Administration. Altis was approved in November 2012 based on an investigational device exemption study, which included implant data. The FDA efficacy requirement was a 50% decrease in pad weight by 6 months.
According to the company website, "Altis is a unique, minimally invasive sling that combines integrated two-way tensioning with lightweight sling material to provide strength, security, and adjustability."
Physicians are divided on their thoughts about a single-incision sling, said Dr. Van Drie, a urogynecologist in group practice in Grand Rapids, Mich. "Those [physicians] who use them are advocates for their safety and simplicity, and the ability to insert them in the office. They have been adopted in different areas of the world as an option for doing simplified, less costly incontinence surgery. The argument against is questions about their staying power – will the effect hold up long term?" he said.
Even though the Altis is a single-incision sling, it has a "very secure" anchoring system, Dr. Van Drie said. The anchor not only goes into the obturator internus, but into the membrane and the obturator externus.
The study involved 113 women, with a mean age of 54 years. Their histories included stress incontinence with hypermobility (81%), without hypermobility (19%), mixed incontinence (37%), and overactive bladder (5%).
Most of the procedures were performed during an inpatient hospital stay (59%); however, 24% were performed at an ambulatory surgical center and 17%, in the physician’s office. General anesthesia was used in 52%, spinal in 3%, and local in 45%.
At 12 months, 90% of patients had at least a 50% reduction in pad weight, and 90% had a negative cough stress test result. The UDI decreased by a mean of 46 points, and the IIQ-7 score, by a mean of 47 points.
There were 11 device-related failures in eight patients. These included one each of urinary retention, urinary tract infection, decreased urine stream, dyspareunia, inflammation, worsening of overactive bladder, and voiding dysfunction. There were four mesh extrusions (3.5%), all less than 3 cm. Two patients with extrusion were smokers, and one was diabetic.
Serious adverse events occurred in three patients: One with a hematoma, one patient who needed transfer to the operating room because of anxiety during repair of a 2-mm mesh extrusion, and one patient whose adverse event was changed to a severe adverse event when she moved out of the study. There were no unanticipated device effects, Dr. Van Drie noted.
Dr. Van Drie is a consultant for Coloplast and has received research money and grants from the company.
NATIONAL HARBOR, MD. – After 12 months, an adjustable, single-incision sling significantly improved clinical and quality of life measures associated with stress urinary incontinence in women.
The Altis sling decreased the Urinary Distress Index (UDI) from a mean of 55 to a mean of 10, Dr. Douglas Van Drie said at a meeting sponsored by the AAGL. The Incontinence Impact Questionnaire (ILQ-7) showed similar improvements at the interim assessment of the device’s 2-year safety and efficacy study.
The study was sponsored by Coloplast, which makes the sling, with input and monitoring by the Food and Drug Administration. Altis was approved in November 2012 based on an investigational device exemption study, which included implant data. The FDA efficacy requirement was a 50% decrease in pad weight by 6 months.
According to the company website, "Altis is a unique, minimally invasive sling that combines integrated two-way tensioning with lightweight sling material to provide strength, security, and adjustability."
Physicians are divided on their thoughts about a single-incision sling, said Dr. Van Drie, a urogynecologist in group practice in Grand Rapids, Mich. "Those [physicians] who use them are advocates for their safety and simplicity, and the ability to insert them in the office. They have been adopted in different areas of the world as an option for doing simplified, less costly incontinence surgery. The argument against is questions about their staying power – will the effect hold up long term?" he said.
Even though the Altis is a single-incision sling, it has a "very secure" anchoring system, Dr. Van Drie said. The anchor not only goes into the obturator internus, but into the membrane and the obturator externus.
The study involved 113 women, with a mean age of 54 years. Their histories included stress incontinence with hypermobility (81%), without hypermobility (19%), mixed incontinence (37%), and overactive bladder (5%).
Most of the procedures were performed during an inpatient hospital stay (59%); however, 24% were performed at an ambulatory surgical center and 17%, in the physician’s office. General anesthesia was used in 52%, spinal in 3%, and local in 45%.
At 12 months, 90% of patients had at least a 50% reduction in pad weight, and 90% had a negative cough stress test result. The UDI decreased by a mean of 46 points, and the IIQ-7 score, by a mean of 47 points.
There were 11 device-related failures in eight patients. These included one each of urinary retention, urinary tract infection, decreased urine stream, dyspareunia, inflammation, worsening of overactive bladder, and voiding dysfunction. There were four mesh extrusions (3.5%), all less than 3 cm. Two patients with extrusion were smokers, and one was diabetic.
Serious adverse events occurred in three patients: One with a hematoma, one patient who needed transfer to the operating room because of anxiety during repair of a 2-mm mesh extrusion, and one patient whose adverse event was changed to a severe adverse event when she moved out of the study. There were no unanticipated device effects, Dr. Van Drie noted.
Dr. Van Drie is a consultant for Coloplast and has received research money and grants from the company.
AT THE AAGL GLOBAL CONGRESS
Major finding: After receiving a single-incision incontinence sling, 90% of women had significant improvements in clinical and quality of life measures related to stress urinary incontinence.
Data source: A prospective study of 113 women.
Disclosures: Coloplast sponsored the study. Dr. Van Drie is a consultant for Coloplast and has received research money and grants from the company.
Prostate cancer stage has declined more than Gleason score
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
Major finding: Over 22 years, the number of prostate cancers stage T3 or higher fell by 85%, while the number with a Gleason score of 8 or higher fell by 30% – a significant difference.
Data source: Study of 1,207 men who underwent radical prostatectomy from 1982 to 2004.
Disclosures: The Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute provided funding. Dr. Penney had no disclosures.
Prolaris test eyed as predictor of prostate cancer outcomes
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
AT THE ASCO ANNUAL MEETING 2013
Major finding: In conservatively managed prostate cancer patients, the cell cycle progression score in tissue samples was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy).
Data source: A retrospective study of tissue samples from more than 1,600 patients in five patient cohorts who were either managed conservatively, underwent prostatectomy, or received external beam radiotherapy.
Disclosures: The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
New test beats PSA in predicting significant prostate Ca
SAN DIEGO – A blood test that detects the –2proPSA isoform of prostate-specific antigen may provide a way to reduce the number of unneeded prostate biopsies, results from a multicenter study showed.
Using a Prostate Health Index (phi) level of 27 as a threshold for selecting men for prostate cancer could eliminate unnecessary biopsies in 26% of men when total PSA is 4-10 ng/mL, said Dr. Martin G. Sanda, chief of urology at Emory University in Atlanta, during a press briefing at the annual meeting of the American Urological Association.
"This is a substantial portion of the population who may undergo PSA testing. [The index] would allow the ability to detect aggressive prostate cancer while having an acceptable false-negative rate. The Prostate Health Index has the potential to mitigate harms of overdetection/overtreatment of indolent cancers while retaining benefits of detecting aggressive prostate cancer which warrants treatment," he said.
The Prostate Health Index (phi), developed by Beckman Coulter and granted premarket approval from the Food and Drug Administration in June 2012, is a simple, noninvasive blood test that is 2.5 times more specific in detecting prostate cancer than PSA in patients with PSA values in the 4- to 10-ng/mL range and is shown to reduce the number of prostate biopsies.
"The Achilles’ heel of PSA detection in its current form is the overdetection and subsequently the downstream overtreatment of indolent prostate cancers," Dr. Sanda said. "The phi is a manner of reporting the detection of the –2proPSA isoform of PSA. This is a small subset of the PSA molecules, as opposed to the routine total PSA test that we are familiar with."
For the current study Dr. Sanda and his associates investigated whether the use of phi, compared with total PSA and the ratio of free to total PSA (%fPSA), could reduce unnecessary biopsies and overdetection of indolent prostate cancer while improving the detection of aggressive prostate cancer. He reported results from 658 men whose PSA was 4-10 ng/mL. Of these 658 men, 324 had prostate cancer. Among these 324 cancers, 160 were aggressive (meaning a Gleason score of 7 or greater) and 164 were indolent cancers.
Dr. Sanda reported that at 90% sensitivity, the specificity of phi was 31.1%, compared with 19.8% for %fPSA (P = .024) and 10.8% for PSA (P less than .001). When the phi ranged from 0 to 26.9, the probability of significant prostate cancer was 3.9% and rose sequentially with increasing range of phi. Specifically, the probability of significant prostate cancer was 8.5% for those with a phi of 27.0-35.9, 14.4% for those in the range of 36.0-54.9, and 28.9% for those with a phi level of 55 or higher.
"When phi is less than 27, the probability of one of these cancers being a Gleason score of 7 or higher was under 4%," said Dr. Sanda, who also directs the university’s Prostate Cancer Center. "With that particular threshold, we would be able to retain the benefits of being able to detect aggressive cancers in patients who had a biopsy when their phi was higher than 27 while avoiding unnecessary [biopsies] in about 26% of the men, substantially reducing the number of indolent cancers diagnosed and the number of unnecessary biopsies performed."
The false-positive rate was "in an acceptable range," he added. Only 4 out of 109 Gleason 3 + 4 cancers were missed (3.7%), and only 1 out of 35 Gleason 4 + 3 cancers was missed (2.9%).
"Because this is a straightforward serum assay, phi does have the potential to have a favorable cost profile relative to some of the genetic marker testing that’s coming down the pipeline," Dr. Sanda commented. "The next step is to validate these findings in a larger and separate cohort." That effort is currently underway with the Early Detection Research Network, a cohort study funded by the National Cancer Institute.
The study was funded by Beckman Coulter. Dr. Sanda disclosed that he is an investigator for the company. He also reported affiliations with Medicametrix, Accuray, and other companies.
SAN DIEGO – A blood test that detects the –2proPSA isoform of prostate-specific antigen may provide a way to reduce the number of unneeded prostate biopsies, results from a multicenter study showed.
Using a Prostate Health Index (phi) level of 27 as a threshold for selecting men for prostate cancer could eliminate unnecessary biopsies in 26% of men when total PSA is 4-10 ng/mL, said Dr. Martin G. Sanda, chief of urology at Emory University in Atlanta, during a press briefing at the annual meeting of the American Urological Association.
"This is a substantial portion of the population who may undergo PSA testing. [The index] would allow the ability to detect aggressive prostate cancer while having an acceptable false-negative rate. The Prostate Health Index has the potential to mitigate harms of overdetection/overtreatment of indolent cancers while retaining benefits of detecting aggressive prostate cancer which warrants treatment," he said.
The Prostate Health Index (phi), developed by Beckman Coulter and granted premarket approval from the Food and Drug Administration in June 2012, is a simple, noninvasive blood test that is 2.5 times more specific in detecting prostate cancer than PSA in patients with PSA values in the 4- to 10-ng/mL range and is shown to reduce the number of prostate biopsies.
"The Achilles’ heel of PSA detection in its current form is the overdetection and subsequently the downstream overtreatment of indolent prostate cancers," Dr. Sanda said. "The phi is a manner of reporting the detection of the –2proPSA isoform of PSA. This is a small subset of the PSA molecules, as opposed to the routine total PSA test that we are familiar with."
For the current study Dr. Sanda and his associates investigated whether the use of phi, compared with total PSA and the ratio of free to total PSA (%fPSA), could reduce unnecessary biopsies and overdetection of indolent prostate cancer while improving the detection of aggressive prostate cancer. He reported results from 658 men whose PSA was 4-10 ng/mL. Of these 658 men, 324 had prostate cancer. Among these 324 cancers, 160 were aggressive (meaning a Gleason score of 7 or greater) and 164 were indolent cancers.
Dr. Sanda reported that at 90% sensitivity, the specificity of phi was 31.1%, compared with 19.8% for %fPSA (P = .024) and 10.8% for PSA (P less than .001). When the phi ranged from 0 to 26.9, the probability of significant prostate cancer was 3.9% and rose sequentially with increasing range of phi. Specifically, the probability of significant prostate cancer was 8.5% for those with a phi of 27.0-35.9, 14.4% for those in the range of 36.0-54.9, and 28.9% for those with a phi level of 55 or higher.
"When phi is less than 27, the probability of one of these cancers being a Gleason score of 7 or higher was under 4%," said Dr. Sanda, who also directs the university’s Prostate Cancer Center. "With that particular threshold, we would be able to retain the benefits of being able to detect aggressive cancers in patients who had a biopsy when their phi was higher than 27 while avoiding unnecessary [biopsies] in about 26% of the men, substantially reducing the number of indolent cancers diagnosed and the number of unnecessary biopsies performed."
The false-positive rate was "in an acceptable range," he added. Only 4 out of 109 Gleason 3 + 4 cancers were missed (3.7%), and only 1 out of 35 Gleason 4 + 3 cancers was missed (2.9%).
"Because this is a straightforward serum assay, phi does have the potential to have a favorable cost profile relative to some of the genetic marker testing that’s coming down the pipeline," Dr. Sanda commented. "The next step is to validate these findings in a larger and separate cohort." That effort is currently underway with the Early Detection Research Network, a cohort study funded by the National Cancer Institute.
The study was funded by Beckman Coulter. Dr. Sanda disclosed that he is an investigator for the company. He also reported affiliations with Medicametrix, Accuray, and other companies.
SAN DIEGO – A blood test that detects the –2proPSA isoform of prostate-specific antigen may provide a way to reduce the number of unneeded prostate biopsies, results from a multicenter study showed.
Using a Prostate Health Index (phi) level of 27 as a threshold for selecting men for prostate cancer could eliminate unnecessary biopsies in 26% of men when total PSA is 4-10 ng/mL, said Dr. Martin G. Sanda, chief of urology at Emory University in Atlanta, during a press briefing at the annual meeting of the American Urological Association.
"This is a substantial portion of the population who may undergo PSA testing. [The index] would allow the ability to detect aggressive prostate cancer while having an acceptable false-negative rate. The Prostate Health Index has the potential to mitigate harms of overdetection/overtreatment of indolent cancers while retaining benefits of detecting aggressive prostate cancer which warrants treatment," he said.
The Prostate Health Index (phi), developed by Beckman Coulter and granted premarket approval from the Food and Drug Administration in June 2012, is a simple, noninvasive blood test that is 2.5 times more specific in detecting prostate cancer than PSA in patients with PSA values in the 4- to 10-ng/mL range and is shown to reduce the number of prostate biopsies.
"The Achilles’ heel of PSA detection in its current form is the overdetection and subsequently the downstream overtreatment of indolent prostate cancers," Dr. Sanda said. "The phi is a manner of reporting the detection of the –2proPSA isoform of PSA. This is a small subset of the PSA molecules, as opposed to the routine total PSA test that we are familiar with."
For the current study Dr. Sanda and his associates investigated whether the use of phi, compared with total PSA and the ratio of free to total PSA (%fPSA), could reduce unnecessary biopsies and overdetection of indolent prostate cancer while improving the detection of aggressive prostate cancer. He reported results from 658 men whose PSA was 4-10 ng/mL. Of these 658 men, 324 had prostate cancer. Among these 324 cancers, 160 were aggressive (meaning a Gleason score of 7 or greater) and 164 were indolent cancers.
Dr. Sanda reported that at 90% sensitivity, the specificity of phi was 31.1%, compared with 19.8% for %fPSA (P = .024) and 10.8% for PSA (P less than .001). When the phi ranged from 0 to 26.9, the probability of significant prostate cancer was 3.9% and rose sequentially with increasing range of phi. Specifically, the probability of significant prostate cancer was 8.5% for those with a phi of 27.0-35.9, 14.4% for those in the range of 36.0-54.9, and 28.9% for those with a phi level of 55 or higher.
"When phi is less than 27, the probability of one of these cancers being a Gleason score of 7 or higher was under 4%," said Dr. Sanda, who also directs the university’s Prostate Cancer Center. "With that particular threshold, we would be able to retain the benefits of being able to detect aggressive cancers in patients who had a biopsy when their phi was higher than 27 while avoiding unnecessary [biopsies] in about 26% of the men, substantially reducing the number of indolent cancers diagnosed and the number of unnecessary biopsies performed."
The false-positive rate was "in an acceptable range," he added. Only 4 out of 109 Gleason 3 + 4 cancers were missed (3.7%), and only 1 out of 35 Gleason 4 + 3 cancers was missed (2.9%).
"Because this is a straightforward serum assay, phi does have the potential to have a favorable cost profile relative to some of the genetic marker testing that’s coming down the pipeline," Dr. Sanda commented. "The next step is to validate these findings in a larger and separate cohort." That effort is currently underway with the Early Detection Research Network, a cohort study funded by the National Cancer Institute.
The study was funded by Beckman Coulter. Dr. Sanda disclosed that he is an investigator for the company. He also reported affiliations with Medicametrix, Accuray, and other companies.
AT THE AUA ANNUAL MEETING
Major finding: The probability of significant prostate cancer was 8.5% for men with a Prostate Health Index (phi) level of 27.0-35.9, 14.4% for those with a phi level of 36.0-54.9, and 28.9% for those with a level of 55 or higher.
Data source: A multicenter study of 658 men whose PSA was 4-10 ng/mL.
Disclosures: The study was funded by Beckman Coulter. Dr. Sanda disclosed that he is an investigator for the company. He also reported affiliations with Medicametrix, Accuray, and other companies.
Consequences of not screening for prostate cancer prove dire
SAN DIEGO – The mean survival of men who initially presented with a prostate-specific antigen score of 100 ng/mL or greater was just 18 months, results from a single-center study showed.
In an effort to provide insight into the consequences of not screening for prostate cancer, researchers at Santa Clara Valley Medical Center, San Jose, Calif., – a county hospital affiliated with the Stanford (Calif.) University that serves a large underinsured population – evaluated the impact of initial prostate-specific antigen (PSA) levels of 100 ng/mL or greater on patient morbidity and mortality. "What we hypothesized is that they would do pretty well because with newer forms of treatment, and once they get into our system, we have comprehensive care that we can provide to them," Dr. Jeffrey H. Reese, chief of the division of urology at Santa Clara Valley Medical Center, said during a press briefing at the annual meeting of the American Urological Association. However, "what we found is that they did not do well at all."
Dr. Reese reported results from 71 men with a mean age of 67 years who presented with a mean PSA score of 100 ng/mL or greater between 1998 and 2008 – none of whom had received a prior prostate cancer screening at the medical center. The median PSA at presentation was 399 ng/mL, and the median survival was 18 months. "These patients did profoundly worse than what we would have expected," Dr. Reese said. Only 9.6% of the patients survived beyond 3 years.
About 80% of patients had chronic pain from their disease. Common comorbidities included hospitalization (64%), chronic catheterization (29%), spinal cord compression (19%), and compression fracture (17%).
"There are a variety of reasons why [these men] are not being screened," Dr. Reese said. "This is a population that either has no health insurance or minimal health insurance. Some were brought in by concerned family members. Some were immigrants. I think our public hospitals represent a snapshot of what prostate cancer was like before PSA screening. It was not uncommon to have these patients come in with widely metastatic disease. There would be consequences to not screening for PSA, if we were just to abandon it entirely."
He described death from prostate cancer as "a really bad way to die. It’s painful and prolonged. There’s a profound price to pay for this disease."
Study coauthor Dr. Winifred Adams, a urology fellow at the Stanford University, acknowledged that the relatively small sample of 71 patients was a limitation. "We wonder: Is this just a problem of metastatic disease, or is it more of a PSA issue of over 100? So we need to go back and look at all patients with metastatic disease versus those who have PSA over 100 and see if the outcome is the same," Dr. Adams said.
The researchers reported having no relevant financial conflicts to disclose.
SAN DIEGO – The mean survival of men who initially presented with a prostate-specific antigen score of 100 ng/mL or greater was just 18 months, results from a single-center study showed.
In an effort to provide insight into the consequences of not screening for prostate cancer, researchers at Santa Clara Valley Medical Center, San Jose, Calif., – a county hospital affiliated with the Stanford (Calif.) University that serves a large underinsured population – evaluated the impact of initial prostate-specific antigen (PSA) levels of 100 ng/mL or greater on patient morbidity and mortality. "What we hypothesized is that they would do pretty well because with newer forms of treatment, and once they get into our system, we have comprehensive care that we can provide to them," Dr. Jeffrey H. Reese, chief of the division of urology at Santa Clara Valley Medical Center, said during a press briefing at the annual meeting of the American Urological Association. However, "what we found is that they did not do well at all."
Dr. Reese reported results from 71 men with a mean age of 67 years who presented with a mean PSA score of 100 ng/mL or greater between 1998 and 2008 – none of whom had received a prior prostate cancer screening at the medical center. The median PSA at presentation was 399 ng/mL, and the median survival was 18 months. "These patients did profoundly worse than what we would have expected," Dr. Reese said. Only 9.6% of the patients survived beyond 3 years.
About 80% of patients had chronic pain from their disease. Common comorbidities included hospitalization (64%), chronic catheterization (29%), spinal cord compression (19%), and compression fracture (17%).
"There are a variety of reasons why [these men] are not being screened," Dr. Reese said. "This is a population that either has no health insurance or minimal health insurance. Some were brought in by concerned family members. Some were immigrants. I think our public hospitals represent a snapshot of what prostate cancer was like before PSA screening. It was not uncommon to have these patients come in with widely metastatic disease. There would be consequences to not screening for PSA, if we were just to abandon it entirely."
He described death from prostate cancer as "a really bad way to die. It’s painful and prolonged. There’s a profound price to pay for this disease."
Study coauthor Dr. Winifred Adams, a urology fellow at the Stanford University, acknowledged that the relatively small sample of 71 patients was a limitation. "We wonder: Is this just a problem of metastatic disease, or is it more of a PSA issue of over 100? So we need to go back and look at all patients with metastatic disease versus those who have PSA over 100 and see if the outcome is the same," Dr. Adams said.
The researchers reported having no relevant financial conflicts to disclose.
SAN DIEGO – The mean survival of men who initially presented with a prostate-specific antigen score of 100 ng/mL or greater was just 18 months, results from a single-center study showed.
In an effort to provide insight into the consequences of not screening for prostate cancer, researchers at Santa Clara Valley Medical Center, San Jose, Calif., – a county hospital affiliated with the Stanford (Calif.) University that serves a large underinsured population – evaluated the impact of initial prostate-specific antigen (PSA) levels of 100 ng/mL or greater on patient morbidity and mortality. "What we hypothesized is that they would do pretty well because with newer forms of treatment, and once they get into our system, we have comprehensive care that we can provide to them," Dr. Jeffrey H. Reese, chief of the division of urology at Santa Clara Valley Medical Center, said during a press briefing at the annual meeting of the American Urological Association. However, "what we found is that they did not do well at all."
Dr. Reese reported results from 71 men with a mean age of 67 years who presented with a mean PSA score of 100 ng/mL or greater between 1998 and 2008 – none of whom had received a prior prostate cancer screening at the medical center. The median PSA at presentation was 399 ng/mL, and the median survival was 18 months. "These patients did profoundly worse than what we would have expected," Dr. Reese said. Only 9.6% of the patients survived beyond 3 years.
About 80% of patients had chronic pain from their disease. Common comorbidities included hospitalization (64%), chronic catheterization (29%), spinal cord compression (19%), and compression fracture (17%).
"There are a variety of reasons why [these men] are not being screened," Dr. Reese said. "This is a population that either has no health insurance or minimal health insurance. Some were brought in by concerned family members. Some were immigrants. I think our public hospitals represent a snapshot of what prostate cancer was like before PSA screening. It was not uncommon to have these patients come in with widely metastatic disease. There would be consequences to not screening for PSA, if we were just to abandon it entirely."
He described death from prostate cancer as "a really bad way to die. It’s painful and prolonged. There’s a profound price to pay for this disease."
Study coauthor Dr. Winifred Adams, a urology fellow at the Stanford University, acknowledged that the relatively small sample of 71 patients was a limitation. "We wonder: Is this just a problem of metastatic disease, or is it more of a PSA issue of over 100? So we need to go back and look at all patients with metastatic disease versus those who have PSA over 100 and see if the outcome is the same," Dr. Adams said.
The researchers reported having no relevant financial conflicts to disclose.
AT THE AUA ANNUAL MEETING
Major finding: Men who first presented with a PSA score of 100 ng/mL or greater survived a median of just 18 months.
Data source: A study of 71 men with a mean age of 67 years who presented to Santa Clara Valley Medical Center between 1998 and 2008.
Disclosures: The researchers reported having no relevant financial conflicts to disclose.
Forecast warns of urologist shortage
SAN DIEGO – The number of urologists practicing in the United States is expected to decrease by 29% between 2009 and 2025, according to a new analysis.
"It’s one thing if the demand for urologists is going up and the supply is stable, but to have the demand go up and the supply almost falling off of a cliff is worrisome," Dr. Raj S. Pruthi said in an interview at the annual meeting of the American Urological Association. "The people who will be hardest hit by this are ones who already struggle with access: those who live in rural communities."
Dr. Pruthi and his colleagues used stock and flow models, starting with the supply of urologists in 2009. They added new entrants from the graduate medical education (GME) pipeline and subtracted attrition from training and from the workforce due to retirement or breaks from practice. The forecast model estimates a 29% head count reduction and a 25% decrease in the full-time equivalent (FTE) supply of urologists between 2009 and 2025. The projected decrease is more than four times greater than the Health Resources and Services Administration’s Physician Supply Model, which estimated a 7% decrease over the same time period.
Dr. Pruthi warned that none of the proposed changes to GME (recommendations from the Council of Graduate Medical Education’s 16th report or a recent proposal to Congress) will increase GME enough to offset the projected decline in head count. "GME funding has been capped since 1996," he said. "We’re setting forth a recipe for a very big problem that we’re going to have for future generations in terms of who’s going to take care of" a rapidly aging population.
As the Affordable Health Care Act takes shape, "one thing that’s not been considered adequately is physician supply," added Dr. Pruthi, chief of urologic surgery at the University of North Carolina at Chapel Hill. "Are there enough of us to help care for the population? That needs to be part of the calculus. We need to do efficient, appropriate care. We need to cut health care costs, but we have to remember our physician supply."
The shrinking number of urologists could affect mortality rates, as research has demonstrated an association between a higher density of urologists in a defined area and lower mortality from prostate, bladder, and kidney cancer. "As supply contracts, rural areas are likely to have an even greater loss of urologic surgeons since these areas have a higher percentage of surgeons closer to retirement age than urban areas," the researchers noted in their abstract. "The result may decrease access to screening, medical and surgical treatment for urologic conditions."
Dr. Pruthi acknowledged that the ability to predict physician demand is an imprecise science. However, "there is indirect data to suggest that our demand isn’t going to go away. It’s only going to go up with that rising incidence and with the rising number of aging baby boomers. Second, we don’t know the appropriate ratio of supply and demand. If you have limited supply, you have limited access. Is our culture accepting of that? Some of these limitations to access may have health consequences."
He and his associates plan to conduct more detailed work on the projection model, including the impact of increasing numbers of women entering the urology field. "About 5% of urologists are female, but in current residency matching the numbers are about 25% female," he said. "What impact will that have? We don’t know yet."
Dr. Pruthi said that he had no relevant financial conflicts to disclose.
SAN DIEGO – The number of urologists practicing in the United States is expected to decrease by 29% between 2009 and 2025, according to a new analysis.
"It’s one thing if the demand for urologists is going up and the supply is stable, but to have the demand go up and the supply almost falling off of a cliff is worrisome," Dr. Raj S. Pruthi said in an interview at the annual meeting of the American Urological Association. "The people who will be hardest hit by this are ones who already struggle with access: those who live in rural communities."
Dr. Pruthi and his colleagues used stock and flow models, starting with the supply of urologists in 2009. They added new entrants from the graduate medical education (GME) pipeline and subtracted attrition from training and from the workforce due to retirement or breaks from practice. The forecast model estimates a 29% head count reduction and a 25% decrease in the full-time equivalent (FTE) supply of urologists between 2009 and 2025. The projected decrease is more than four times greater than the Health Resources and Services Administration’s Physician Supply Model, which estimated a 7% decrease over the same time period.
Dr. Pruthi warned that none of the proposed changes to GME (recommendations from the Council of Graduate Medical Education’s 16th report or a recent proposal to Congress) will increase GME enough to offset the projected decline in head count. "GME funding has been capped since 1996," he said. "We’re setting forth a recipe for a very big problem that we’re going to have for future generations in terms of who’s going to take care of" a rapidly aging population.
As the Affordable Health Care Act takes shape, "one thing that’s not been considered adequately is physician supply," added Dr. Pruthi, chief of urologic surgery at the University of North Carolina at Chapel Hill. "Are there enough of us to help care for the population? That needs to be part of the calculus. We need to do efficient, appropriate care. We need to cut health care costs, but we have to remember our physician supply."
The shrinking number of urologists could affect mortality rates, as research has demonstrated an association between a higher density of urologists in a defined area and lower mortality from prostate, bladder, and kidney cancer. "As supply contracts, rural areas are likely to have an even greater loss of urologic surgeons since these areas have a higher percentage of surgeons closer to retirement age than urban areas," the researchers noted in their abstract. "The result may decrease access to screening, medical and surgical treatment for urologic conditions."
Dr. Pruthi acknowledged that the ability to predict physician demand is an imprecise science. However, "there is indirect data to suggest that our demand isn’t going to go away. It’s only going to go up with that rising incidence and with the rising number of aging baby boomers. Second, we don’t know the appropriate ratio of supply and demand. If you have limited supply, you have limited access. Is our culture accepting of that? Some of these limitations to access may have health consequences."
He and his associates plan to conduct more detailed work on the projection model, including the impact of increasing numbers of women entering the urology field. "About 5% of urologists are female, but in current residency matching the numbers are about 25% female," he said. "What impact will that have? We don’t know yet."
Dr. Pruthi said that he had no relevant financial conflicts to disclose.
SAN DIEGO – The number of urologists practicing in the United States is expected to decrease by 29% between 2009 and 2025, according to a new analysis.
"It’s one thing if the demand for urologists is going up and the supply is stable, but to have the demand go up and the supply almost falling off of a cliff is worrisome," Dr. Raj S. Pruthi said in an interview at the annual meeting of the American Urological Association. "The people who will be hardest hit by this are ones who already struggle with access: those who live in rural communities."
Dr. Pruthi and his colleagues used stock and flow models, starting with the supply of urologists in 2009. They added new entrants from the graduate medical education (GME) pipeline and subtracted attrition from training and from the workforce due to retirement or breaks from practice. The forecast model estimates a 29% head count reduction and a 25% decrease in the full-time equivalent (FTE) supply of urologists between 2009 and 2025. The projected decrease is more than four times greater than the Health Resources and Services Administration’s Physician Supply Model, which estimated a 7% decrease over the same time period.
Dr. Pruthi warned that none of the proposed changes to GME (recommendations from the Council of Graduate Medical Education’s 16th report or a recent proposal to Congress) will increase GME enough to offset the projected decline in head count. "GME funding has been capped since 1996," he said. "We’re setting forth a recipe for a very big problem that we’re going to have for future generations in terms of who’s going to take care of" a rapidly aging population.
As the Affordable Health Care Act takes shape, "one thing that’s not been considered adequately is physician supply," added Dr. Pruthi, chief of urologic surgery at the University of North Carolina at Chapel Hill. "Are there enough of us to help care for the population? That needs to be part of the calculus. We need to do efficient, appropriate care. We need to cut health care costs, but we have to remember our physician supply."
The shrinking number of urologists could affect mortality rates, as research has demonstrated an association between a higher density of urologists in a defined area and lower mortality from prostate, bladder, and kidney cancer. "As supply contracts, rural areas are likely to have an even greater loss of urologic surgeons since these areas have a higher percentage of surgeons closer to retirement age than urban areas," the researchers noted in their abstract. "The result may decrease access to screening, medical and surgical treatment for urologic conditions."
Dr. Pruthi acknowledged that the ability to predict physician demand is an imprecise science. However, "there is indirect data to suggest that our demand isn’t going to go away. It’s only going to go up with that rising incidence and with the rising number of aging baby boomers. Second, we don’t know the appropriate ratio of supply and demand. If you have limited supply, you have limited access. Is our culture accepting of that? Some of these limitations to access may have health consequences."
He and his associates plan to conduct more detailed work on the projection model, including the impact of increasing numbers of women entering the urology field. "About 5% of urologists are female, but in current residency matching the numbers are about 25% female," he said. "What impact will that have? We don’t know yet."
Dr. Pruthi said that he had no relevant financial conflicts to disclose.
AT THE AUA ANNUAL MEETING
Major finding: The forecast model estimates a 29% head count reduction and a 25% decrease in the full-time equivalent (FTE) supply of urologists between 2009 and 2025.
Data source: An analysis that used stock and flow models, starting with the supply of urologists in 2009.
Disclosures: Dr. Pruthi said that he had no relevant disclosures.
FDA advisory panel decides tivozanib falls short for advanced renal cell carcinoma
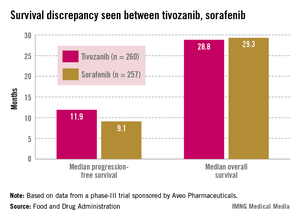
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).
"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
AT AN FDA ADVISORY PANEL MEETING
Abiraterone reduced morbidity in mCRPC patients
When patients with metastatic castration-resistant prostate cancer without prior chemotherapy were treated with abiraterone acetate plus prednisone, their risk of disease progression was reduced by almost half, and their risk of death was decreased by almost 20% compared with patients given prednisone alone, based on data from an interim analysis of the phase III trial COU-AA-302.
Treatment with both drugs also delayed times to opiate use and chemotherapy, was associated with improved quality of life measures, and remained safe and well tolerated, said Dr. Dana E. Rathkopf, the study’s lead author, who presented the results at the annual gastrointestinal cancers symposium sponsored by the American Society of Clinical Oncology. Results on quality of life measures were reported at last year’s European Society for Medical Oncology congress.
This was the third planned interim analysis for the randomized trial COU-AA-302, which is designed to evaluate the clinical benefit of abiraterone (Zytiga) and prednisone compared with prednisone alone in mildly symptomatic or asymptomatic patients with progressive metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy.
Similar to the findings at a previous interim analysis (43% of total overall survival events) conducted in December 2011, the third analysis (55% of overall survival events) continued to show that the radiographic progression-free survival (rPFS) in the abiraterone plus prednisone arm remained significant. Although overall survival continued to favor the abiraterone plus prednisone arm, it didn’t cross the boundary for significance.
"We may never get the answer to the question of whether prechemotherapy abiraterone can improve overall survival," commented Dr. William Oh, chief of hematology and medical oncology at the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York. "To me, that is okay as long as we still have evidence that [the drug has] meaningful value to patients" from a quality of life perspective.
For the COU-AA-302 trial, 1,088 patients were randomized to 1,000 mg/day of abiraterone plus 5 mg twice per day of prednisone or to the control arm of placebo plus prednisone. The treatment arms were evenly matched.
Coprimary endpoints were rPFS and overall survival. Secondary endpoints included time to opiate use, time to chemotherapy initiation, time to ECOG-PS (Eastern Cooperative Oncology Group–performance status) deterioration, and time to prostate-specific antigen (PSA) progression
At the third interim analysis, primary endpoints and all secondary endpoints favored the abiraterone and prednisone arm. The time to rPFS was doubled relative to the control arm (16.5 months and 8.3 months; HR = 0.53; P = .0001).
Overall survival was 35.3 months with abiraterone and prednisone versus 30.1 months with placebo and prednisone. The difference was not significantly different (HR = 0.79; P = .0151). Dr. Rathkopf and his colleagues pointed out that the median overall survival of 35.3 months is the longest reported for this mCRPC population.
Abiraterone plus prednisone also doubled the maximal decline in PSA compared with placebo and prednisone (11.1 months and 5.6 months; HR = 0.50; P = .0001). Prednisone is an active control therapy, as 29% of the patients in the control arm had a decline in PSA of greater than or equal to 50%, Dr. Rathkopf said.
The most common adverse event was fatigue. Beyond the 2 years since the study began, no new safety signals have emerged with abiraterone.
At a median follow-up of 27 months, 77% of the patients in the abiraterone and prednisone arm and 89% in the placebo and prednisone arm had discontinued therapy, mainly because of disease progression.
Dr. Rathkopf said he had no financial relationships to disclose. Dr. Oh has been a consultant or adviser to several drug makers including Janssen Biotech, the maker of Zytiga.
When patients with metastatic castration-resistant prostate cancer without prior chemotherapy were treated with abiraterone acetate plus prednisone, their risk of disease progression was reduced by almost half, and their risk of death was decreased by almost 20% compared with patients given prednisone alone, based on data from an interim analysis of the phase III trial COU-AA-302.
Treatment with both drugs also delayed times to opiate use and chemotherapy, was associated with improved quality of life measures, and remained safe and well tolerated, said Dr. Dana E. Rathkopf, the study’s lead author, who presented the results at the annual gastrointestinal cancers symposium sponsored by the American Society of Clinical Oncology. Results on quality of life measures were reported at last year’s European Society for Medical Oncology congress.
This was the third planned interim analysis for the randomized trial COU-AA-302, which is designed to evaluate the clinical benefit of abiraterone (Zytiga) and prednisone compared with prednisone alone in mildly symptomatic or asymptomatic patients with progressive metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy.
Similar to the findings at a previous interim analysis (43% of total overall survival events) conducted in December 2011, the third analysis (55% of overall survival events) continued to show that the radiographic progression-free survival (rPFS) in the abiraterone plus prednisone arm remained significant. Although overall survival continued to favor the abiraterone plus prednisone arm, it didn’t cross the boundary for significance.
"We may never get the answer to the question of whether prechemotherapy abiraterone can improve overall survival," commented Dr. William Oh, chief of hematology and medical oncology at the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York. "To me, that is okay as long as we still have evidence that [the drug has] meaningful value to patients" from a quality of life perspective.
For the COU-AA-302 trial, 1,088 patients were randomized to 1,000 mg/day of abiraterone plus 5 mg twice per day of prednisone or to the control arm of placebo plus prednisone. The treatment arms were evenly matched.
Coprimary endpoints were rPFS and overall survival. Secondary endpoints included time to opiate use, time to chemotherapy initiation, time to ECOG-PS (Eastern Cooperative Oncology Group–performance status) deterioration, and time to prostate-specific antigen (PSA) progression
At the third interim analysis, primary endpoints and all secondary endpoints favored the abiraterone and prednisone arm. The time to rPFS was doubled relative to the control arm (16.5 months and 8.3 months; HR = 0.53; P = .0001).
Overall survival was 35.3 months with abiraterone and prednisone versus 30.1 months with placebo and prednisone. The difference was not significantly different (HR = 0.79; P = .0151). Dr. Rathkopf and his colleagues pointed out that the median overall survival of 35.3 months is the longest reported for this mCRPC population.
Abiraterone plus prednisone also doubled the maximal decline in PSA compared with placebo and prednisone (11.1 months and 5.6 months; HR = 0.50; P = .0001). Prednisone is an active control therapy, as 29% of the patients in the control arm had a decline in PSA of greater than or equal to 50%, Dr. Rathkopf said.
The most common adverse event was fatigue. Beyond the 2 years since the study began, no new safety signals have emerged with abiraterone.
At a median follow-up of 27 months, 77% of the patients in the abiraterone and prednisone arm and 89% in the placebo and prednisone arm had discontinued therapy, mainly because of disease progression.
Dr. Rathkopf said he had no financial relationships to disclose. Dr. Oh has been a consultant or adviser to several drug makers including Janssen Biotech, the maker of Zytiga.
When patients with metastatic castration-resistant prostate cancer without prior chemotherapy were treated with abiraterone acetate plus prednisone, their risk of disease progression was reduced by almost half, and their risk of death was decreased by almost 20% compared with patients given prednisone alone, based on data from an interim analysis of the phase III trial COU-AA-302.
Treatment with both drugs also delayed times to opiate use and chemotherapy, was associated with improved quality of life measures, and remained safe and well tolerated, said Dr. Dana E. Rathkopf, the study’s lead author, who presented the results at the annual gastrointestinal cancers symposium sponsored by the American Society of Clinical Oncology. Results on quality of life measures were reported at last year’s European Society for Medical Oncology congress.
This was the third planned interim analysis for the randomized trial COU-AA-302, which is designed to evaluate the clinical benefit of abiraterone (Zytiga) and prednisone compared with prednisone alone in mildly symptomatic or asymptomatic patients with progressive metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy.
Similar to the findings at a previous interim analysis (43% of total overall survival events) conducted in December 2011, the third analysis (55% of overall survival events) continued to show that the radiographic progression-free survival (rPFS) in the abiraterone plus prednisone arm remained significant. Although overall survival continued to favor the abiraterone plus prednisone arm, it didn’t cross the boundary for significance.
"We may never get the answer to the question of whether prechemotherapy abiraterone can improve overall survival," commented Dr. William Oh, chief of hematology and medical oncology at the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York. "To me, that is okay as long as we still have evidence that [the drug has] meaningful value to patients" from a quality of life perspective.
For the COU-AA-302 trial, 1,088 patients were randomized to 1,000 mg/day of abiraterone plus 5 mg twice per day of prednisone or to the control arm of placebo plus prednisone. The treatment arms were evenly matched.
Coprimary endpoints were rPFS and overall survival. Secondary endpoints included time to opiate use, time to chemotherapy initiation, time to ECOG-PS (Eastern Cooperative Oncology Group–performance status) deterioration, and time to prostate-specific antigen (PSA) progression
At the third interim analysis, primary endpoints and all secondary endpoints favored the abiraterone and prednisone arm. The time to rPFS was doubled relative to the control arm (16.5 months and 8.3 months; HR = 0.53; P = .0001).
Overall survival was 35.3 months with abiraterone and prednisone versus 30.1 months with placebo and prednisone. The difference was not significantly different (HR = 0.79; P = .0151). Dr. Rathkopf and his colleagues pointed out that the median overall survival of 35.3 months is the longest reported for this mCRPC population.
Abiraterone plus prednisone also doubled the maximal decline in PSA compared with placebo and prednisone (11.1 months and 5.6 months; HR = 0.50; P = .0001). Prednisone is an active control therapy, as 29% of the patients in the control arm had a decline in PSA of greater than or equal to 50%, Dr. Rathkopf said.
The most common adverse event was fatigue. Beyond the 2 years since the study began, no new safety signals have emerged with abiraterone.
At a median follow-up of 27 months, 77% of the patients in the abiraterone and prednisone arm and 89% in the placebo and prednisone arm had discontinued therapy, mainly because of disease progression.
Dr. Rathkopf said he had no financial relationships to disclose. Dr. Oh has been a consultant or adviser to several drug makers including Janssen Biotech, the maker of Zytiga.
AT THE ASCO GENITOURINARY CANCERS SYMPOSIUM
Major finding: The time of radiographic progression-free survival was doubled in patients given abiraterone and prednisone relative to those given placebo and prednisone, 16.5 months and 8.3 months, respectively (HR = 0.53; P = 0.0001).
Data source: Phase III trial of 1,088 patients with metastatic castration-resistant prostate cancer who had not received prior chemotherapy.
Disclosures: Dr. Rathkopf said he had no financial relationships to disclose. Dr. Oh has been a consultant or adviser to several drug makers including Janssen Biotech, the maker of Zytiga.
Survival higher with surveillance of small kidney tumors
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Data Source: Mortality data from the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm.
Disclosures: Dr. Huang said he had no relevant financial disclosures.
Shorter androgen blockade okay in localized prostate cancer
Men who underwent radiotherapy for localized high-risk prostate cancer and had 18 months of androgen blockade did just as well as comparable men who had 3 years of androgen blockade.
The finding of nearly identical overall and disease-free survival with traditional and shortened hormone therapy is "practice changing," Dr. Bruce Roth said during a press briefing at the 2013 Genitourinary Cancers Symposium.
"I would be willing to change the way I treat my patients," based on the results of the phase III study, said Dr. Roth of Washington University, St. Louis. "The consequences of long-term androgen deprivation are often more striking than the positive aspects of prolonging it. If treatment time can be safely halved, patients will enjoy a much better quality of life."
Dr. Abdenour Nabid of the Centre Hospitalier Universitaire de Sherbrooke, Canada, headed the study. It randomized 630 men with node-negative high-risk prostate cancer to radiation therapy plus either 36 or 18 months of androgen blockade. The hormone therapy consisted of bicalutamide 50 mg/day for 1 month plus goserelin 10.8 mg given as an injection once every 3 months.
At baseline, the patients were a median of 71 years old; the median prostate specific antigen (PSA) level was 16 ng/mL, and the median Gleason score was 8. Most had T2-T3 disease.
The median follow-up period was about 77 months. At that point, 23% of patients in the extended therapy group and 24% in the abbreviated therapy group had died. Of these 147 patients, 116 (79%) had died from causes other than prostate cancer.
Fifty patients developed bone metastases, Dr. Nabid said, but there was not a significant between-group difference in this outcome. Nor were there differences in biochemical failure, regional or distant recurrence, pelvic metastases, or the need for a second course of androgen blockade.
When Dr. Nabid broke the data out by 5- and 10-year follow-up points, the survival results were similar. At 5 years, overall survival was 92% in the 36-month group and 87% in the 18-month group; disease specific survival was 98% and 96%, respectively. At 10 years, overall survival was 64% vs. 63%, and disease-specific survival was 87% in both groups.
He said the study will follow all patients through 10 years, adding "I am convinced this finding won’t change."
Decreasing the length of androgen blockade could avoid some cases of permanent castration syndrome, Dr. Roth said. "The longer the hormone therapy, the less chance that testosterone will recover when it’s finished. With 3 years of hormone therapy, you might be inducing a lifetime of hormone suppression" and a host of problems including sexual dysfunction, loss of muscle tone, and increased visceral fat. These consequences are in turn associated with significantly increased rates of metabolic syndrome, diabetes, myocardial infarction, and coronary heart disease.
"If a patient has several decades left of life and his testosterone levels are lowered to the point of castration syndrome, there is a price to pay," Dr. Roth said. "If this patient dies of a heart attack 10 years before he would have died of prostate cancer, we have not really provided good anticancer therapy."
This is the first study showing that short-term androgen blockade is both safe and effective. "Could we get by with less?" Dr. Nabid asked. "We don’t know. Previous trials have shown that 6 months of hormone treatment is inferior to 24 months, but we don’t know what the effect of 12 months would be. Still, we are excited to see this, and our hope is that this will change the standard of care for this stage of disease."
Dr. Nabid had no financial disclosures.
Men who underwent radiotherapy for localized high-risk prostate cancer and had 18 months of androgen blockade did just as well as comparable men who had 3 years of androgen blockade.
The finding of nearly identical overall and disease-free survival with traditional and shortened hormone therapy is "practice changing," Dr. Bruce Roth said during a press briefing at the 2013 Genitourinary Cancers Symposium.
"I would be willing to change the way I treat my patients," based on the results of the phase III study, said Dr. Roth of Washington University, St. Louis. "The consequences of long-term androgen deprivation are often more striking than the positive aspects of prolonging it. If treatment time can be safely halved, patients will enjoy a much better quality of life."
Dr. Abdenour Nabid of the Centre Hospitalier Universitaire de Sherbrooke, Canada, headed the study. It randomized 630 men with node-negative high-risk prostate cancer to radiation therapy plus either 36 or 18 months of androgen blockade. The hormone therapy consisted of bicalutamide 50 mg/day for 1 month plus goserelin 10.8 mg given as an injection once every 3 months.
At baseline, the patients were a median of 71 years old; the median prostate specific antigen (PSA) level was 16 ng/mL, and the median Gleason score was 8. Most had T2-T3 disease.
The median follow-up period was about 77 months. At that point, 23% of patients in the extended therapy group and 24% in the abbreviated therapy group had died. Of these 147 patients, 116 (79%) had died from causes other than prostate cancer.
Fifty patients developed bone metastases, Dr. Nabid said, but there was not a significant between-group difference in this outcome. Nor were there differences in biochemical failure, regional or distant recurrence, pelvic metastases, or the need for a second course of androgen blockade.
When Dr. Nabid broke the data out by 5- and 10-year follow-up points, the survival results were similar. At 5 years, overall survival was 92% in the 36-month group and 87% in the 18-month group; disease specific survival was 98% and 96%, respectively. At 10 years, overall survival was 64% vs. 63%, and disease-specific survival was 87% in both groups.
He said the study will follow all patients through 10 years, adding "I am convinced this finding won’t change."
Decreasing the length of androgen blockade could avoid some cases of permanent castration syndrome, Dr. Roth said. "The longer the hormone therapy, the less chance that testosterone will recover when it’s finished. With 3 years of hormone therapy, you might be inducing a lifetime of hormone suppression" and a host of problems including sexual dysfunction, loss of muscle tone, and increased visceral fat. These consequences are in turn associated with significantly increased rates of metabolic syndrome, diabetes, myocardial infarction, and coronary heart disease.
"If a patient has several decades left of life and his testosterone levels are lowered to the point of castration syndrome, there is a price to pay," Dr. Roth said. "If this patient dies of a heart attack 10 years before he would have died of prostate cancer, we have not really provided good anticancer therapy."
This is the first study showing that short-term androgen blockade is both safe and effective. "Could we get by with less?" Dr. Nabid asked. "We don’t know. Previous trials have shown that 6 months of hormone treatment is inferior to 24 months, but we don’t know what the effect of 12 months would be. Still, we are excited to see this, and our hope is that this will change the standard of care for this stage of disease."
Dr. Nabid had no financial disclosures.
Men who underwent radiotherapy for localized high-risk prostate cancer and had 18 months of androgen blockade did just as well as comparable men who had 3 years of androgen blockade.
The finding of nearly identical overall and disease-free survival with traditional and shortened hormone therapy is "practice changing," Dr. Bruce Roth said during a press briefing at the 2013 Genitourinary Cancers Symposium.
"I would be willing to change the way I treat my patients," based on the results of the phase III study, said Dr. Roth of Washington University, St. Louis. "The consequences of long-term androgen deprivation are often more striking than the positive aspects of prolonging it. If treatment time can be safely halved, patients will enjoy a much better quality of life."
Dr. Abdenour Nabid of the Centre Hospitalier Universitaire de Sherbrooke, Canada, headed the study. It randomized 630 men with node-negative high-risk prostate cancer to radiation therapy plus either 36 or 18 months of androgen blockade. The hormone therapy consisted of bicalutamide 50 mg/day for 1 month plus goserelin 10.8 mg given as an injection once every 3 months.
At baseline, the patients were a median of 71 years old; the median prostate specific antigen (PSA) level was 16 ng/mL, and the median Gleason score was 8. Most had T2-T3 disease.
The median follow-up period was about 77 months. At that point, 23% of patients in the extended therapy group and 24% in the abbreviated therapy group had died. Of these 147 patients, 116 (79%) had died from causes other than prostate cancer.
Fifty patients developed bone metastases, Dr. Nabid said, but there was not a significant between-group difference in this outcome. Nor were there differences in biochemical failure, regional or distant recurrence, pelvic metastases, or the need for a second course of androgen blockade.
When Dr. Nabid broke the data out by 5- and 10-year follow-up points, the survival results were similar. At 5 years, overall survival was 92% in the 36-month group and 87% in the 18-month group; disease specific survival was 98% and 96%, respectively. At 10 years, overall survival was 64% vs. 63%, and disease-specific survival was 87% in both groups.
He said the study will follow all patients through 10 years, adding "I am convinced this finding won’t change."
Decreasing the length of androgen blockade could avoid some cases of permanent castration syndrome, Dr. Roth said. "The longer the hormone therapy, the less chance that testosterone will recover when it’s finished. With 3 years of hormone therapy, you might be inducing a lifetime of hormone suppression" and a host of problems including sexual dysfunction, loss of muscle tone, and increased visceral fat. These consequences are in turn associated with significantly increased rates of metabolic syndrome, diabetes, myocardial infarction, and coronary heart disease.
"If a patient has several decades left of life and his testosterone levels are lowered to the point of castration syndrome, there is a price to pay," Dr. Roth said. "If this patient dies of a heart attack 10 years before he would have died of prostate cancer, we have not really provided good anticancer therapy."
This is the first study showing that short-term androgen blockade is both safe and effective. "Could we get by with less?" Dr. Nabid asked. "We don’t know. Previous trials have shown that 6 months of hormone treatment is inferior to 24 months, but we don’t know what the effect of 12 months would be. Still, we are excited to see this, and our hope is that this will change the standard of care for this stage of disease."
Dr. Nabid had no financial disclosures.
FROM THE 2013 GENITOURINARY CANCERS SYMPOSIUM
Major Finding: At 5 years, overall survival was 92% in the 36-month group and 87% in the 18-month group; disease specific survival was 98% and 96%. At 10 years, overall survival was 64% vs. 63%, and disease-specific survival was 87% in both groups.
Data Source: The study randomized 630 men with node-negative high-risk prostate cancer to radiation therapy plus either 36 or 18 months of androgen blockade.
Disclosures: Dr. Nabid had no financial disclosures.