User login
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).
"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
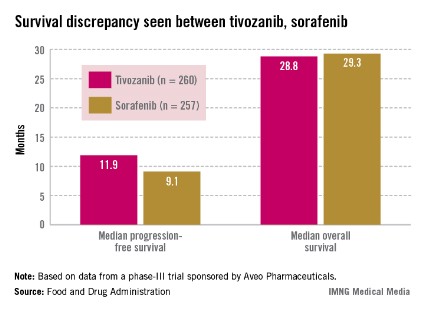
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
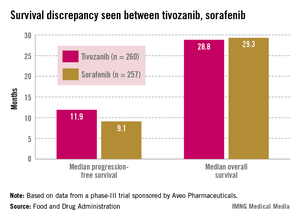
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
AT AN FDA ADVISORY PANEL MEETING