User login
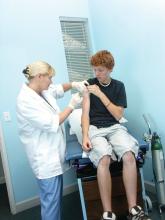
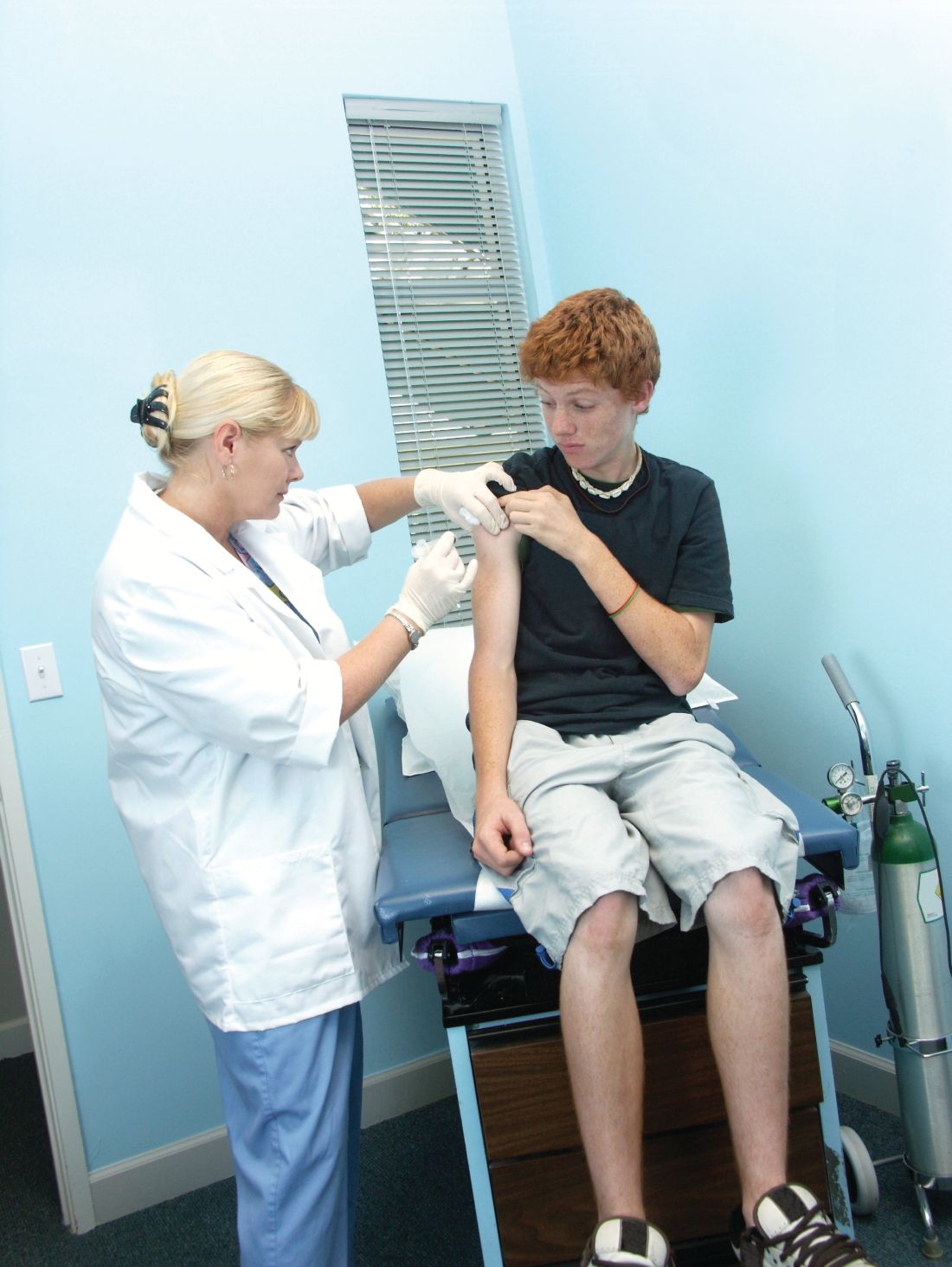
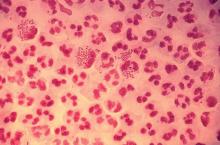
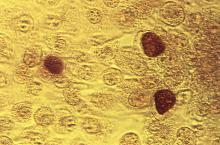
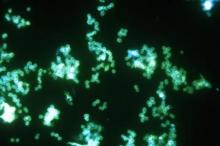
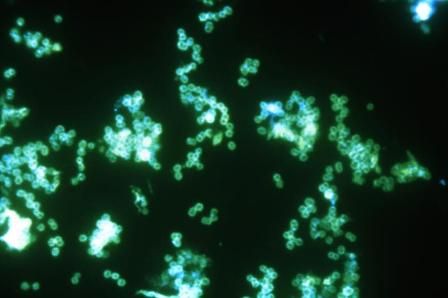
FDA grants marketing clearance for chlamydia and gonorrhea extragenital tests
The new tests, the Aptima Combo 2 Assay and Xpert CT/NG, use samples from the throat and rectum to test for chlamydia and gonorrhea, according to a statement from the FDA.
“It is best for patients if both [chlamydia and gonorrhea] are caught and treated right away, as significant complications can occur if left untreated,” noted Tim Stenzel, MD, in the statement.
“Today’s clearances provide a mechanism for more easily diagnosing these infections,” said Dr. Stenzel, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
The two tests were reviewed through the premarket notification – or 510(k) – pathway, which seeks to demonstrate to the FDA that the device to be marketed is equivalent or better in safety and effectiveness to the legally marketed device.
In the FDA’s evaluation of the tests, it reviewed clinical data from a multisite study of more than 2,500 patients. This study evaluated the diagnostic accuracy of multiple commercially available nucleic acid amplification tests for detection of Neisseria gonorrhoeae and Chlamydia trachomatis from throat and rectal sites. The results of this study and other information reviewed by the FDA demonstrated that the two tests “are safe and effective for extragenital testing for chlamydia and gonorrhea,” according to the statement.
The data were collected through a cross-sectional study coordinated by the Antibacterial Resistance Leadership Group, which is funded and supported by the National Institute of Allergy and Infectious Diseases.
The FDA granted marketing clearance to Hologic and Cepheid for the Aptima Combo 2 Assay and the Xpert CT/NG, respectively.
The new tests, the Aptima Combo 2 Assay and Xpert CT/NG, use samples from the throat and rectum to test for chlamydia and gonorrhea, according to a statement from the FDA.
“It is best for patients if both [chlamydia and gonorrhea] are caught and treated right away, as significant complications can occur if left untreated,” noted Tim Stenzel, MD, in the statement.
“Today’s clearances provide a mechanism for more easily diagnosing these infections,” said Dr. Stenzel, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
The two tests were reviewed through the premarket notification – or 510(k) – pathway, which seeks to demonstrate to the FDA that the device to be marketed is equivalent or better in safety and effectiveness to the legally marketed device.
In the FDA’s evaluation of the tests, it reviewed clinical data from a multisite study of more than 2,500 patients. This study evaluated the diagnostic accuracy of multiple commercially available nucleic acid amplification tests for detection of Neisseria gonorrhoeae and Chlamydia trachomatis from throat and rectal sites. The results of this study and other information reviewed by the FDA demonstrated that the two tests “are safe and effective for extragenital testing for chlamydia and gonorrhea,” according to the statement.
The data were collected through a cross-sectional study coordinated by the Antibacterial Resistance Leadership Group, which is funded and supported by the National Institute of Allergy and Infectious Diseases.
The FDA granted marketing clearance to Hologic and Cepheid for the Aptima Combo 2 Assay and the Xpert CT/NG, respectively.
The new tests, the Aptima Combo 2 Assay and Xpert CT/NG, use samples from the throat and rectum to test for chlamydia and gonorrhea, according to a statement from the FDA.
“It is best for patients if both [chlamydia and gonorrhea] are caught and treated right away, as significant complications can occur if left untreated,” noted Tim Stenzel, MD, in the statement.
“Today’s clearances provide a mechanism for more easily diagnosing these infections,” said Dr. Stenzel, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
The two tests were reviewed through the premarket notification – or 510(k) – pathway, which seeks to demonstrate to the FDA that the device to be marketed is equivalent or better in safety and effectiveness to the legally marketed device.
In the FDA’s evaluation of the tests, it reviewed clinical data from a multisite study of more than 2,500 patients. This study evaluated the diagnostic accuracy of multiple commercially available nucleic acid amplification tests for detection of Neisseria gonorrhoeae and Chlamydia trachomatis from throat and rectal sites. The results of this study and other information reviewed by the FDA demonstrated that the two tests “are safe and effective for extragenital testing for chlamydia and gonorrhea,” according to the statement.
The data were collected through a cross-sectional study coordinated by the Antibacterial Resistance Leadership Group, which is funded and supported by the National Institute of Allergy and Infectious Diseases.
The FDA granted marketing clearance to Hologic and Cepheid for the Aptima Combo 2 Assay and the Xpert CT/NG, respectively.
Flu vaccine visits reveal missed opportunities for HPV vaccination
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Understanding the terminology of gender identity
Use vocabulary to reduce barriers
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.
Use vocabulary to reduce barriers
Use vocabulary to reduce barriers
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.
Female Veterans’ Experiences With VHA Treatment for Military Sexual Trauma
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
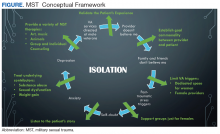
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
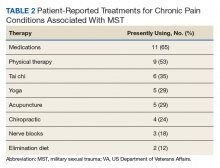
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
Proposed triple I criteria may overlook febrile women at risk post partum
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The sensitivity and specificity of the suspected triple I criteria to predict an adverse clinical infectious outcome were 68% for the suspected triple I group and 38% for the isolated maternal fever group.
Study details: A retrospective cohort study of 339 women with intrapartum fever from June 2015 to September 2017.
Disclosures: The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
Source: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
Rate of STIs is rising, and many U.S. teens are sexually active
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
EXPERT ANALYSIS FROM AAP 18
Single-dose zoliflodacin successfully treats uncomplicated urogenital gonorrhea
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Cure rates of 96% and 100% were reported for urethral/cervical and rectal infections, respectively, and ranged from 50% to 82% in pharyngeal infections.
Study details: A randomized, open-label, phase 2 study including 181 patients with uncomplicated urogenital gonorrhea who received zoliflodacin or ceftriaxone.
Disclosures: The study authors reported disclosures related to AstraZeneca (parent company of Entasis Therapeutics, which is developing zoliflodacin) and other pharmaceutical companies.
Source: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
FDA expands approval of 9-valent HPV vaccine
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
HPV testing detects cervical precancers earlier than cytology
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
FROM JAMA
Key clinical point: Compared with a Pap smear, HPV testing detected CIN3+ more often and earlier.
Major finding: Women who had HPV testing were 58% less likely to be CIN3+ 48 months later.
Study details: The prospective randomized trial comprised 19,009 women.
Disclosures: The Canadian Institute of Health Research funded the study, Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
Source: Ogilvie GS et al. JAMA. 2018;320:43-52.
Make the Diagnosis - May 2018
and through close skin contact, as well as contaminated clothes and bedding. Adult lice can live up to 36 hours away from its host. Pubic areas most commonly are affected, although other hair-bearing parts of the body often are affected, including eyelashes.
Pruritus can be severe. Secondary bacterial infections may occur as maculae ceruleae, or blue-colored macules, on the skin. The lice are visible to the naked eye and are approximately 1 mm in length. They have a crablike appearance, six legs, and a wide body. Nits may be present on the hair shaft. Unlike hair casts, which can be moved up and down along the hair shaft, nits firmly adhere to the hair. Diagnosis should prompt a workup for other sexually transmitted diseases, including HIV.
Treatment for patients and their sexual partners include permethrin topically; and laundering of clothing and bedding. Lice on the eyelashes can be treated with 8 days of twice-daily applications of petrolatum. Ivermectin can be used when topical therapy fails, although this is an off-label treatment (not approved by the Food and Drug Administration).
Pediculosis corporis – body lice or clothing lice – is also known as “vagabond’s disease” and is caused by Pediculus humanus var corporis. Body lice lay their eggs in clothing seams and can live in clothing for up to 1 month without feeding on human blood. Often homeless individuals and those living in overcrowded areas can be affected. The louse and nits also are visible to the naked eye. They have a longer, narrower body than Phthirus pubis and are more similar in appearance to head lice. They rarely are found on the skin.
Body lice may carry disease such as epidemic typhus, relapsing fever, and trench fever or endocarditis. Permethrin is the most widely used treatment to kill both lice and ova. Other treatments include Malathion, Lindane, and Crotamiton. Clothing and bedding should be laundered.
Scabies is a mite infestation caused by Sarcoptes scabiei. Unlike lice, scabies often affects the hands and feet. Characteristic linear burrows may be seen in the finger web spaces. The circle of Hebra describes the areas commonly infected by mites: axillae, antecubital fossa, wrists, hands, and the groin. Pruritus may be severe and worse at night. Patients may be afflicted with both lice and scabies at the same time. Mites are not visible to the naked eye but can be seen microscopically. Topical permethrin cream is used most often for treatment. All household contacts should be treated at the same time. As in louse infestations, clothing and bedding should be laundered. Ivermectin can be used for crusted scabies, although this is an off-label treatment.
This case and photo were submitted by Maria Hicks, MD, Advanced Dermatology and Cosmetic Surgery, Tampa, and Dr. Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
and through close skin contact, as well as contaminated clothes and bedding. Adult lice can live up to 36 hours away from its host. Pubic areas most commonly are affected, although other hair-bearing parts of the body often are affected, including eyelashes.
Pruritus can be severe. Secondary bacterial infections may occur as maculae ceruleae, or blue-colored macules, on the skin. The lice are visible to the naked eye and are approximately 1 mm in length. They have a crablike appearance, six legs, and a wide body. Nits may be present on the hair shaft. Unlike hair casts, which can be moved up and down along the hair shaft, nits firmly adhere to the hair. Diagnosis should prompt a workup for other sexually transmitted diseases, including HIV.
Treatment for patients and their sexual partners include permethrin topically; and laundering of clothing and bedding. Lice on the eyelashes can be treated with 8 days of twice-daily applications of petrolatum. Ivermectin can be used when topical therapy fails, although this is an off-label treatment (not approved by the Food and Drug Administration).
Pediculosis corporis – body lice or clothing lice – is also known as “vagabond’s disease” and is caused by Pediculus humanus var corporis. Body lice lay their eggs in clothing seams and can live in clothing for up to 1 month without feeding on human blood. Often homeless individuals and those living in overcrowded areas can be affected. The louse and nits also are visible to the naked eye. They have a longer, narrower body than Phthirus pubis and are more similar in appearance to head lice. They rarely are found on the skin.
Body lice may carry disease such as epidemic typhus, relapsing fever, and trench fever or endocarditis. Permethrin is the most widely used treatment to kill both lice and ova. Other treatments include Malathion, Lindane, and Crotamiton. Clothing and bedding should be laundered.
Scabies is a mite infestation caused by Sarcoptes scabiei. Unlike lice, scabies often affects the hands and feet. Characteristic linear burrows may be seen in the finger web spaces. The circle of Hebra describes the areas commonly infected by mites: axillae, antecubital fossa, wrists, hands, and the groin. Pruritus may be severe and worse at night. Patients may be afflicted with both lice and scabies at the same time. Mites are not visible to the naked eye but can be seen microscopically. Topical permethrin cream is used most often for treatment. All household contacts should be treated at the same time. As in louse infestations, clothing and bedding should be laundered. Ivermectin can be used for crusted scabies, although this is an off-label treatment.
This case and photo were submitted by Maria Hicks, MD, Advanced Dermatology and Cosmetic Surgery, Tampa, and Dr. Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
and through close skin contact, as well as contaminated clothes and bedding. Adult lice can live up to 36 hours away from its host. Pubic areas most commonly are affected, although other hair-bearing parts of the body often are affected, including eyelashes.
Pruritus can be severe. Secondary bacterial infections may occur as maculae ceruleae, or blue-colored macules, on the skin. The lice are visible to the naked eye and are approximately 1 mm in length. They have a crablike appearance, six legs, and a wide body. Nits may be present on the hair shaft. Unlike hair casts, which can be moved up and down along the hair shaft, nits firmly adhere to the hair. Diagnosis should prompt a workup for other sexually transmitted diseases, including HIV.
Treatment for patients and their sexual partners include permethrin topically; and laundering of clothing and bedding. Lice on the eyelashes can be treated with 8 days of twice-daily applications of petrolatum. Ivermectin can be used when topical therapy fails, although this is an off-label treatment (not approved by the Food and Drug Administration).
Pediculosis corporis – body lice or clothing lice – is also known as “vagabond’s disease” and is caused by Pediculus humanus var corporis. Body lice lay their eggs in clothing seams and can live in clothing for up to 1 month without feeding on human blood. Often homeless individuals and those living in overcrowded areas can be affected. The louse and nits also are visible to the naked eye. They have a longer, narrower body than Phthirus pubis and are more similar in appearance to head lice. They rarely are found on the skin.
Body lice may carry disease such as epidemic typhus, relapsing fever, and trench fever or endocarditis. Permethrin is the most widely used treatment to kill both lice and ova. Other treatments include Malathion, Lindane, and Crotamiton. Clothing and bedding should be laundered.
Scabies is a mite infestation caused by Sarcoptes scabiei. Unlike lice, scabies often affects the hands and feet. Characteristic linear burrows may be seen in the finger web spaces. The circle of Hebra describes the areas commonly infected by mites: axillae, antecubital fossa, wrists, hands, and the groin. Pruritus may be severe and worse at night. Patients may be afflicted with both lice and scabies at the same time. Mites are not visible to the naked eye but can be seen microscopically. Topical permethrin cream is used most often for treatment. All household contacts should be treated at the same time. As in louse infestations, clothing and bedding should be laundered. Ivermectin can be used for crusted scabies, although this is an off-label treatment.
This case and photo were submitted by Maria Hicks, MD, Advanced Dermatology and Cosmetic Surgery, Tampa, and Dr. Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
A 40-year-old HIV-positive male presented with a 1-month history of severely pruritic papules on his chest. The patient reported that he "removes bugs" from his skin. Microscopic examination of a hair clipping was performed.
Make the Diagnosis: