User login
Aaron Beck: An appreciation
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
City or country life? Genetic risk for mental illness may decide
Individuals with a genetic predisposition to schizophrenia, bipolar disorder (BD), autism spectrum disorder (ASD), or anorexia nervosa (AN) are significantly more likely to move from a rural to an urban setting, whereas those at high genetic risk for attention-deficit/hyperactivity disorder were more likely to do the opposite.
The findings held even in those at high genetic risk who had never been diagnosed with a psychiatric disorder, highlighting a genetic factor that previous research linking urban living to mental illness has not explored.
“It’s not as simple as saying that urban environment is responsible for schizophrenia and everyone should move out of urban environments and they will be safe,” study investigator Evangelos Vassos, MD, PhD, senior clinical research fellow at King’s College London, and a consulting psychiatrist, said in an interview. “If you are genetically predisposed to schizophrenia, you will still be predisposed to schizophrenia even if you move.”
The study was published online in JAMA Psychiatry.
Genetic influence
The study results don’t rule out environmental influence, but offer evidence that the migration pattern researchers have tracked for years may have a multifactorial explanation.
“Our research shows that, at some level, an individual’s genes select their environment and that the relationship between environmental and genetic influences on mental health is interrelated,” Jessye Maxwell, MSc, lead author and a PhD candidate in psychiatry at King’s College, said in a statement. “This overlap needs to be considered when developing models to predict the risk of people developing mental health conditions in the future.”
For the study, the investigators calculated polygenic risk scores (PRS) of different psychiatric illnesses for 385,793 U.K. Biobank participants aged 37-73. PRS analyzes genetic information across a person’s entire genome, rather than by individual genes.
They used address history and U.K. census records from 1931 to 2011 to map population density over time.
PRS analyses showed significant associations with higher population density throughout adulthood, reaching highest significance between age 45 and 55 years for schizophrenia (88 people/km2; 95% confidence interval, 65-98 people/km2), BD (44 people/km2; 95%CI, 34-54 people/km2), AN (36 people/km2; 95%CI, 22-50 people/km2), and ASD (35 people/km2; 95%CI, 25-45 people/km2).
When they compared those who were born and stayed in rural or suburban areas to their counterparts who moved from those areas to cities, they found the odds of moving to urban areas ranged from 5% among people at high genetic risk for schizophrenia to 13% of those with a high risk for BD. Only people at high risk for ADHD were more likely to move to rural areas.
However, the study is not without its limitations. Only people of European descent were included, family medical history was unavailable for some participants, and only about 50,000 people had a lifetime diagnosis of mental illness, which is not representative of the general population.
‘Convincing evidence’
Still, the research adds another piece of the puzzle scientists seek to solve about where people live and mental illness risk, said Jordan DeVylder, PhD, associate professor of social work at Fordham University, New York, who commented on the study for this news organization.
Dr. DeVylder, who has also published research on the topic but was not part of the current study, noted that urban living has long been thought to be among the most consistent environmental risk factors for psychosis. However, he noted, “this association can also be explained by genetic selection, in which the same genes that predispose one to schizophrenia also predispose one to choose urban living.”
“This study presents the most convincing evidence to date that genetics have a major role in this association, at least in the countries where this association between urban living and psychosis exists,” he said.
The study was funded by National Institute for Health Research, Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. The authors and Dr. DeVylder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a genetic predisposition to schizophrenia, bipolar disorder (BD), autism spectrum disorder (ASD), or anorexia nervosa (AN) are significantly more likely to move from a rural to an urban setting, whereas those at high genetic risk for attention-deficit/hyperactivity disorder were more likely to do the opposite.
The findings held even in those at high genetic risk who had never been diagnosed with a psychiatric disorder, highlighting a genetic factor that previous research linking urban living to mental illness has not explored.
“It’s not as simple as saying that urban environment is responsible for schizophrenia and everyone should move out of urban environments and they will be safe,” study investigator Evangelos Vassos, MD, PhD, senior clinical research fellow at King’s College London, and a consulting psychiatrist, said in an interview. “If you are genetically predisposed to schizophrenia, you will still be predisposed to schizophrenia even if you move.”
The study was published online in JAMA Psychiatry.
Genetic influence
The study results don’t rule out environmental influence, but offer evidence that the migration pattern researchers have tracked for years may have a multifactorial explanation.
“Our research shows that, at some level, an individual’s genes select their environment and that the relationship between environmental and genetic influences on mental health is interrelated,” Jessye Maxwell, MSc, lead author and a PhD candidate in psychiatry at King’s College, said in a statement. “This overlap needs to be considered when developing models to predict the risk of people developing mental health conditions in the future.”
For the study, the investigators calculated polygenic risk scores (PRS) of different psychiatric illnesses for 385,793 U.K. Biobank participants aged 37-73. PRS analyzes genetic information across a person’s entire genome, rather than by individual genes.
They used address history and U.K. census records from 1931 to 2011 to map population density over time.
PRS analyses showed significant associations with higher population density throughout adulthood, reaching highest significance between age 45 and 55 years for schizophrenia (88 people/km2; 95% confidence interval, 65-98 people/km2), BD (44 people/km2; 95%CI, 34-54 people/km2), AN (36 people/km2; 95%CI, 22-50 people/km2), and ASD (35 people/km2; 95%CI, 25-45 people/km2).
When they compared those who were born and stayed in rural or suburban areas to their counterparts who moved from those areas to cities, they found the odds of moving to urban areas ranged from 5% among people at high genetic risk for schizophrenia to 13% of those with a high risk for BD. Only people at high risk for ADHD were more likely to move to rural areas.
However, the study is not without its limitations. Only people of European descent were included, family medical history was unavailable for some participants, and only about 50,000 people had a lifetime diagnosis of mental illness, which is not representative of the general population.
‘Convincing evidence’
Still, the research adds another piece of the puzzle scientists seek to solve about where people live and mental illness risk, said Jordan DeVylder, PhD, associate professor of social work at Fordham University, New York, who commented on the study for this news organization.
Dr. DeVylder, who has also published research on the topic but was not part of the current study, noted that urban living has long been thought to be among the most consistent environmental risk factors for psychosis. However, he noted, “this association can also be explained by genetic selection, in which the same genes that predispose one to schizophrenia also predispose one to choose urban living.”
“This study presents the most convincing evidence to date that genetics have a major role in this association, at least in the countries where this association between urban living and psychosis exists,” he said.
The study was funded by National Institute for Health Research, Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. The authors and Dr. DeVylder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a genetic predisposition to schizophrenia, bipolar disorder (BD), autism spectrum disorder (ASD), or anorexia nervosa (AN) are significantly more likely to move from a rural to an urban setting, whereas those at high genetic risk for attention-deficit/hyperactivity disorder were more likely to do the opposite.
The findings held even in those at high genetic risk who had never been diagnosed with a psychiatric disorder, highlighting a genetic factor that previous research linking urban living to mental illness has not explored.
“It’s not as simple as saying that urban environment is responsible for schizophrenia and everyone should move out of urban environments and they will be safe,” study investigator Evangelos Vassos, MD, PhD, senior clinical research fellow at King’s College London, and a consulting psychiatrist, said in an interview. “If you are genetically predisposed to schizophrenia, you will still be predisposed to schizophrenia even if you move.”
The study was published online in JAMA Psychiatry.
Genetic influence
The study results don’t rule out environmental influence, but offer evidence that the migration pattern researchers have tracked for years may have a multifactorial explanation.
“Our research shows that, at some level, an individual’s genes select their environment and that the relationship between environmental and genetic influences on mental health is interrelated,” Jessye Maxwell, MSc, lead author and a PhD candidate in psychiatry at King’s College, said in a statement. “This overlap needs to be considered when developing models to predict the risk of people developing mental health conditions in the future.”
For the study, the investigators calculated polygenic risk scores (PRS) of different psychiatric illnesses for 385,793 U.K. Biobank participants aged 37-73. PRS analyzes genetic information across a person’s entire genome, rather than by individual genes.
They used address history and U.K. census records from 1931 to 2011 to map population density over time.
PRS analyses showed significant associations with higher population density throughout adulthood, reaching highest significance between age 45 and 55 years for schizophrenia (88 people/km2; 95% confidence interval, 65-98 people/km2), BD (44 people/km2; 95%CI, 34-54 people/km2), AN (36 people/km2; 95%CI, 22-50 people/km2), and ASD (35 people/km2; 95%CI, 25-45 people/km2).
When they compared those who were born and stayed in rural or suburban areas to their counterparts who moved from those areas to cities, they found the odds of moving to urban areas ranged from 5% among people at high genetic risk for schizophrenia to 13% of those with a high risk for BD. Only people at high risk for ADHD were more likely to move to rural areas.
However, the study is not without its limitations. Only people of European descent were included, family medical history was unavailable for some participants, and only about 50,000 people had a lifetime diagnosis of mental illness, which is not representative of the general population.
‘Convincing evidence’
Still, the research adds another piece of the puzzle scientists seek to solve about where people live and mental illness risk, said Jordan DeVylder, PhD, associate professor of social work at Fordham University, New York, who commented on the study for this news organization.
Dr. DeVylder, who has also published research on the topic but was not part of the current study, noted that urban living has long been thought to be among the most consistent environmental risk factors for psychosis. However, he noted, “this association can also be explained by genetic selection, in which the same genes that predispose one to schizophrenia also predispose one to choose urban living.”
“This study presents the most convincing evidence to date that genetics have a major role in this association, at least in the countries where this association between urban living and psychosis exists,” he said.
The study was funded by National Institute for Health Research, Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. The authors and Dr. DeVylder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
FDA not recognizing efficacy of psychopharmacologic therapies
Many years ago, drug development in psychiatry turned to control of specific symptoms across disorders rather than within disorders, but regulatory agencies are still not yet on board, according to an expert psychopharmacologist outlining the ongoing evolution at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
If this reorientation is going to lead to the broad indications the newer drugs likely deserve, which is control of specific types of symptoms regardless of the diagnosis, “we have to move the [Food and Drug Administration] along,” said Stephen M. Stahl, MD, PhD, chairman of the Neuroscience Institute and an adjunct professor of psychiatry at the University of California, San Diego.
On the side of drug development and clinical practice, the reorientation has already taken place. Dr. Stahl described numerous brain circuits known to produce symptoms when function is altered that are now treatment targets. This includes the ventral medial prefrontal cortex where deficient information processing leads to depression and the orbital frontal cortex where altered function leads to impulsivity.
“It is not like each part of the brain does a little bit of everything. Rather, each part of the brain has an assignment and duty and function,” Dr. Stahl explained. By addressing the disturbed signaling in brain circuits that lead to depression, impulsivity, agitation, or other symptoms, there is an opportunity for control, regardless of the psychiatric diagnosis with which the symptom is associated.
For example, Dr. Stahl predicted that pimavanserin, a highly selective 5-HT2A inverse agonist that is already approved for psychosis in Parkinson’s disease, is now likely to be approved for psychosis associated with other conditions on the basis of recent positive clinical studies in these other disorders.
Brexpiprazole, a serotonin-dopamine activity modulator already known to be useful for control of the agitation characteristic of schizophrenia, is now showing the same type of activity against agitation when it is associated with Alzheimer’s disease. Again, Dr. Stahl thinks this drug is on course for an indication across diseases once studies are conducted in each disease individually.
Another drug being evaluated for agitation, the N-methyl-D-aspartate receptor antagonist dextromethorphan bupropion, is also being tested for treatment of symptoms across multiple disorders, he reported.
However, the FDA has so far taken the position that each drug must be tested separately for a given symptom in each disorder for which it is being considered despite the underlying premise that it is the symptom, not the disease, that is important.
Unlike physiological diseases where symptoms, like a fever or abdominal cramps, are the product of a disease, psychiatric symptoms are the disease and a fundamental target – regardless of the DSM-based diagnosis.
To some degree, the symptoms of psychiatric disorders have always been the focus of treatment, but a pivot toward developing therapies that will control a symptom regardless of the underlying diagnosis is an important conceptual change. It is being made possible by advances in the detail with which the neuropathology of these symptoms is understood .
“By my count, 79 symptoms are described in DSM-5, but they are spread across hundreds of syndromes because they are grouped together in different ways,” Dr. Stahl observed.
He noted that clinicians make a diagnosis on the basis symptom groupings, but their interventions are selected to address the manifestations of the disease, not the disease itself.
“If you are a real psychopharmacologist treating real patients, you are treating the specific symptoms of the specific patient,” according to Dr. Stahl.
So far, the FDA has not made this leap, insisting on trials in these categorical disorders rather than permitting trial designs that allow benefit to be demonstrated against a symptom regardless of the syndrome with which it is associated.
Of egregious examples, Dr. Stahl recounted a recent trial of a 5-HT2 antagonist that looked so promising against psychosis in Alzheimer’s disease that the trialists enrolled patients with psychosis regardless of type of dementia, such as vascular dementia and Lewy body disease. The efficacy was impressive.
“It worked so well that they stopped the trial, but the FDA declined to approve it,” Dr. Stahl recounted. Despite clear evidence of benefit, the regulators insisted that the investigators needed to show a significant benefit in each condition individually.
While the trial investigators acknowledged that there was not enough power in the trial to show a statistically significant benefit in each category, they argued that the overall benefit and the consistent response across categories required them to stop the trial for ethical reasons.
“That’s your problem, the FDA said to the investigators,” according to Dr. Stahl.
The failure of the FDA to recognize the efficacy of psychopharmacologic therapies across symptoms regardless of the associated disease is a failure to stay current with an important evolution in medicine, Dr. Stahl indicated.
“What we have come to understand is the neurobiology of any given symptom is likely to be the same across disorders,” he said.
Agency’s arbitrary decisions cited
“I completely agree with Dr. Stahl,” said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience, University of Cincinnati.
In addition to the fact that symptoms are present across multiple categories, many patients manifest multiple symptoms at one time, Dr. Nasrallah pointed out. For neurodegenerative disorders associated with psychosis, depression, anxiety, aggression, and other symptoms, it is already well known that the heterogeneous symptoms “cannot be treated with a single drug,” he said. Rather different drugs targeting each symptom individually is essential for effective management.
Dr. Nasrallah, who chaired the Psychopharmacology Update meeting, has made this point many times in the past, including in his role as the editor of Current Psychiatry. In one editorial 10 years ago, he wrote that “it makes little sense for the FDA to mandate that a drug must work for a DSM diagnosis instead of specific symptoms.”
“The FDA must update its old policy, which has led to the widespread off-label use of psychiatric drugs, an artificial concept, simply because the FDA arbitrarily decided a long time ago that new drugs must be approved for a specific DSM diagnosis,” Dr. Nasrallah said.
Dr. Stahl reported financial relationships with more than 20 pharmaceutical companies, including those that are involved in the development of drugs included in his talk. Medscape Live and this news organization are owned by the same parent company.
Many years ago, drug development in psychiatry turned to control of specific symptoms across disorders rather than within disorders, but regulatory agencies are still not yet on board, according to an expert psychopharmacologist outlining the ongoing evolution at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
If this reorientation is going to lead to the broad indications the newer drugs likely deserve, which is control of specific types of symptoms regardless of the diagnosis, “we have to move the [Food and Drug Administration] along,” said Stephen M. Stahl, MD, PhD, chairman of the Neuroscience Institute and an adjunct professor of psychiatry at the University of California, San Diego.
On the side of drug development and clinical practice, the reorientation has already taken place. Dr. Stahl described numerous brain circuits known to produce symptoms when function is altered that are now treatment targets. This includes the ventral medial prefrontal cortex where deficient information processing leads to depression and the orbital frontal cortex where altered function leads to impulsivity.
“It is not like each part of the brain does a little bit of everything. Rather, each part of the brain has an assignment and duty and function,” Dr. Stahl explained. By addressing the disturbed signaling in brain circuits that lead to depression, impulsivity, agitation, or other symptoms, there is an opportunity for control, regardless of the psychiatric diagnosis with which the symptom is associated.
For example, Dr. Stahl predicted that pimavanserin, a highly selective 5-HT2A inverse agonist that is already approved for psychosis in Parkinson’s disease, is now likely to be approved for psychosis associated with other conditions on the basis of recent positive clinical studies in these other disorders.
Brexpiprazole, a serotonin-dopamine activity modulator already known to be useful for control of the agitation characteristic of schizophrenia, is now showing the same type of activity against agitation when it is associated with Alzheimer’s disease. Again, Dr. Stahl thinks this drug is on course for an indication across diseases once studies are conducted in each disease individually.
Another drug being evaluated for agitation, the N-methyl-D-aspartate receptor antagonist dextromethorphan bupropion, is also being tested for treatment of symptoms across multiple disorders, he reported.
However, the FDA has so far taken the position that each drug must be tested separately for a given symptom in each disorder for which it is being considered despite the underlying premise that it is the symptom, not the disease, that is important.
Unlike physiological diseases where symptoms, like a fever or abdominal cramps, are the product of a disease, psychiatric symptoms are the disease and a fundamental target – regardless of the DSM-based diagnosis.
To some degree, the symptoms of psychiatric disorders have always been the focus of treatment, but a pivot toward developing therapies that will control a symptom regardless of the underlying diagnosis is an important conceptual change. It is being made possible by advances in the detail with which the neuropathology of these symptoms is understood .
“By my count, 79 symptoms are described in DSM-5, but they are spread across hundreds of syndromes because they are grouped together in different ways,” Dr. Stahl observed.
He noted that clinicians make a diagnosis on the basis symptom groupings, but their interventions are selected to address the manifestations of the disease, not the disease itself.
“If you are a real psychopharmacologist treating real patients, you are treating the specific symptoms of the specific patient,” according to Dr. Stahl.
So far, the FDA has not made this leap, insisting on trials in these categorical disorders rather than permitting trial designs that allow benefit to be demonstrated against a symptom regardless of the syndrome with which it is associated.
Of egregious examples, Dr. Stahl recounted a recent trial of a 5-HT2 antagonist that looked so promising against psychosis in Alzheimer’s disease that the trialists enrolled patients with psychosis regardless of type of dementia, such as vascular dementia and Lewy body disease. The efficacy was impressive.
“It worked so well that they stopped the trial, but the FDA declined to approve it,” Dr. Stahl recounted. Despite clear evidence of benefit, the regulators insisted that the investigators needed to show a significant benefit in each condition individually.
While the trial investigators acknowledged that there was not enough power in the trial to show a statistically significant benefit in each category, they argued that the overall benefit and the consistent response across categories required them to stop the trial for ethical reasons.
“That’s your problem, the FDA said to the investigators,” according to Dr. Stahl.
The failure of the FDA to recognize the efficacy of psychopharmacologic therapies across symptoms regardless of the associated disease is a failure to stay current with an important evolution in medicine, Dr. Stahl indicated.
“What we have come to understand is the neurobiology of any given symptom is likely to be the same across disorders,” he said.
Agency’s arbitrary decisions cited
“I completely agree with Dr. Stahl,” said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience, University of Cincinnati.
In addition to the fact that symptoms are present across multiple categories, many patients manifest multiple symptoms at one time, Dr. Nasrallah pointed out. For neurodegenerative disorders associated with psychosis, depression, anxiety, aggression, and other symptoms, it is already well known that the heterogeneous symptoms “cannot be treated with a single drug,” he said. Rather different drugs targeting each symptom individually is essential for effective management.
Dr. Nasrallah, who chaired the Psychopharmacology Update meeting, has made this point many times in the past, including in his role as the editor of Current Psychiatry. In one editorial 10 years ago, he wrote that “it makes little sense for the FDA to mandate that a drug must work for a DSM diagnosis instead of specific symptoms.”
“The FDA must update its old policy, which has led to the widespread off-label use of psychiatric drugs, an artificial concept, simply because the FDA arbitrarily decided a long time ago that new drugs must be approved for a specific DSM diagnosis,” Dr. Nasrallah said.
Dr. Stahl reported financial relationships with more than 20 pharmaceutical companies, including those that are involved in the development of drugs included in his talk. Medscape Live and this news organization are owned by the same parent company.
Many years ago, drug development in psychiatry turned to control of specific symptoms across disorders rather than within disorders, but regulatory agencies are still not yet on board, according to an expert psychopharmacologist outlining the ongoing evolution at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
If this reorientation is going to lead to the broad indications the newer drugs likely deserve, which is control of specific types of symptoms regardless of the diagnosis, “we have to move the [Food and Drug Administration] along,” said Stephen M. Stahl, MD, PhD, chairman of the Neuroscience Institute and an adjunct professor of psychiatry at the University of California, San Diego.
On the side of drug development and clinical practice, the reorientation has already taken place. Dr. Stahl described numerous brain circuits known to produce symptoms when function is altered that are now treatment targets. This includes the ventral medial prefrontal cortex where deficient information processing leads to depression and the orbital frontal cortex where altered function leads to impulsivity.
“It is not like each part of the brain does a little bit of everything. Rather, each part of the brain has an assignment and duty and function,” Dr. Stahl explained. By addressing the disturbed signaling in brain circuits that lead to depression, impulsivity, agitation, or other symptoms, there is an opportunity for control, regardless of the psychiatric diagnosis with which the symptom is associated.
For example, Dr. Stahl predicted that pimavanserin, a highly selective 5-HT2A inverse agonist that is already approved for psychosis in Parkinson’s disease, is now likely to be approved for psychosis associated with other conditions on the basis of recent positive clinical studies in these other disorders.
Brexpiprazole, a serotonin-dopamine activity modulator already known to be useful for control of the agitation characteristic of schizophrenia, is now showing the same type of activity against agitation when it is associated with Alzheimer’s disease. Again, Dr. Stahl thinks this drug is on course for an indication across diseases once studies are conducted in each disease individually.
Another drug being evaluated for agitation, the N-methyl-D-aspartate receptor antagonist dextromethorphan bupropion, is also being tested for treatment of symptoms across multiple disorders, he reported.
However, the FDA has so far taken the position that each drug must be tested separately for a given symptom in each disorder for which it is being considered despite the underlying premise that it is the symptom, not the disease, that is important.
Unlike physiological diseases where symptoms, like a fever or abdominal cramps, are the product of a disease, psychiatric symptoms are the disease and a fundamental target – regardless of the DSM-based diagnosis.
To some degree, the symptoms of psychiatric disorders have always been the focus of treatment, but a pivot toward developing therapies that will control a symptom regardless of the underlying diagnosis is an important conceptual change. It is being made possible by advances in the detail with which the neuropathology of these symptoms is understood .
“By my count, 79 symptoms are described in DSM-5, but they are spread across hundreds of syndromes because they are grouped together in different ways,” Dr. Stahl observed.
He noted that clinicians make a diagnosis on the basis symptom groupings, but their interventions are selected to address the manifestations of the disease, not the disease itself.
“If you are a real psychopharmacologist treating real patients, you are treating the specific symptoms of the specific patient,” according to Dr. Stahl.
So far, the FDA has not made this leap, insisting on trials in these categorical disorders rather than permitting trial designs that allow benefit to be demonstrated against a symptom regardless of the syndrome with which it is associated.
Of egregious examples, Dr. Stahl recounted a recent trial of a 5-HT2 antagonist that looked so promising against psychosis in Alzheimer’s disease that the trialists enrolled patients with psychosis regardless of type of dementia, such as vascular dementia and Lewy body disease. The efficacy was impressive.
“It worked so well that they stopped the trial, but the FDA declined to approve it,” Dr. Stahl recounted. Despite clear evidence of benefit, the regulators insisted that the investigators needed to show a significant benefit in each condition individually.
While the trial investigators acknowledged that there was not enough power in the trial to show a statistically significant benefit in each category, they argued that the overall benefit and the consistent response across categories required them to stop the trial for ethical reasons.
“That’s your problem, the FDA said to the investigators,” according to Dr. Stahl.
The failure of the FDA to recognize the efficacy of psychopharmacologic therapies across symptoms regardless of the associated disease is a failure to stay current with an important evolution in medicine, Dr. Stahl indicated.
“What we have come to understand is the neurobiology of any given symptom is likely to be the same across disorders,” he said.
Agency’s arbitrary decisions cited
“I completely agree with Dr. Stahl,” said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience, University of Cincinnati.
In addition to the fact that symptoms are present across multiple categories, many patients manifest multiple symptoms at one time, Dr. Nasrallah pointed out. For neurodegenerative disorders associated with psychosis, depression, anxiety, aggression, and other symptoms, it is already well known that the heterogeneous symptoms “cannot be treated with a single drug,” he said. Rather different drugs targeting each symptom individually is essential for effective management.
Dr. Nasrallah, who chaired the Psychopharmacology Update meeting, has made this point many times in the past, including in his role as the editor of Current Psychiatry. In one editorial 10 years ago, he wrote that “it makes little sense for the FDA to mandate that a drug must work for a DSM diagnosis instead of specific symptoms.”
“The FDA must update its old policy, which has led to the widespread off-label use of psychiatric drugs, an artificial concept, simply because the FDA arbitrarily decided a long time ago that new drugs must be approved for a specific DSM diagnosis,” Dr. Nasrallah said.
Dr. Stahl reported financial relationships with more than 20 pharmaceutical companies, including those that are involved in the development of drugs included in his talk. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Open notes: Big benefits, few harms in psychiatry, experts say
There are multiple benefits and few harms from sharing clinical notes in patients with mental illness, results of a poll of international experts show.
As of April 5, 2021, new federal rules in the United States mandate that all patients are offered online access to their electronic health record.
“Given that sharing notes in psychiatry is likely to be more complicated than in some other specialties, we were unsure whether experts would consider the practice more harmful than beneficial,” Charlotte Blease, PhD, of Beth Israel Deaconess Medical Center in Boston, told this news organization.
“However, the results of our poll suggest clinicians’ anxieties about sharing mental health notes with patients may be misplaced. We found clear consensus among experts that the benefits of online access to clinical notes could outweigh the risks,” Dr. Blease said in a news release.
The study was published online in PLOS ONE.
Empowering patients
Investigators used an online Delphi poll, an established methodology used to investigate emerging health care policy – including in psychiatry – to solicit the views of an international panel of experts on the mental health effects of sharing clinical notes.
The panel included clinicians, chief medical information officers, patient advocates, and informatics experts with extensive experience and research knowledge about patient access to mental health notes.
There was consensus among the panel that offering online access to mental health notes could enhance patients’ understanding about their diagnosis, care plan, and rationale for treatments.
There was also consensus that access to clinical notes could enhance patient recall about what was communicated and improve mental health patients’ sense of control over their health care.
The panel also agreed that blocking mental health notes could lead to greater harms including increased feelings of stigmatization.
Confirmatory findings
The poll results support an earlier study by Dr. Blease and colleagues that evaluated the experiences of patients in accessing their online clinical notes.
Among these patients with major depressive disorder, schizophrenia, schizoaffective disorder, or bipolar-related disorder, “access helped to clarify why medications had been prescribed, improved understanding about side effects, and 20% of patients reported doing a better job taking their meds as prescribed,” said Dr. Blease.
However, the expert panel in the Delphi poll predicted that with “open notes” some patients might demand changes to their clinical notes, and that mental health clinicians might be less detailed/accurate in documenting negative aspects of the patient relationship, details about patients’ personalities, or symptoms of paranoia in patients.
“If some patients feel more judged or offended by what they read, this may undermine the therapeutic relationship. ,” she added.
“In some clinical cases where there is more focus on emergency care than in forming a therapeutic relationship, for example emergency department visits, we know almost nothing about the risks and benefits associated with OpenNotes,” senior author John Torous, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, said in an interview.
“One thing is clear,” Dr. Blease said. “Patient access to their online medical records is now mainstream, and we need more clinician education on how to write notes that patients will read, and more guidance among patients on the benefits and risks of accessing their notes.”
Support for this research was provided by a J. F. Keane Scholar Award and a Swedish Research Council on Health, Working Life, and Welfare grant. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are multiple benefits and few harms from sharing clinical notes in patients with mental illness, results of a poll of international experts show.
As of April 5, 2021, new federal rules in the United States mandate that all patients are offered online access to their electronic health record.
“Given that sharing notes in psychiatry is likely to be more complicated than in some other specialties, we were unsure whether experts would consider the practice more harmful than beneficial,” Charlotte Blease, PhD, of Beth Israel Deaconess Medical Center in Boston, told this news organization.
“However, the results of our poll suggest clinicians’ anxieties about sharing mental health notes with patients may be misplaced. We found clear consensus among experts that the benefits of online access to clinical notes could outweigh the risks,” Dr. Blease said in a news release.
The study was published online in PLOS ONE.
Empowering patients
Investigators used an online Delphi poll, an established methodology used to investigate emerging health care policy – including in psychiatry – to solicit the views of an international panel of experts on the mental health effects of sharing clinical notes.
The panel included clinicians, chief medical information officers, patient advocates, and informatics experts with extensive experience and research knowledge about patient access to mental health notes.
There was consensus among the panel that offering online access to mental health notes could enhance patients’ understanding about their diagnosis, care plan, and rationale for treatments.
There was also consensus that access to clinical notes could enhance patient recall about what was communicated and improve mental health patients’ sense of control over their health care.
The panel also agreed that blocking mental health notes could lead to greater harms including increased feelings of stigmatization.
Confirmatory findings
The poll results support an earlier study by Dr. Blease and colleagues that evaluated the experiences of patients in accessing their online clinical notes.
Among these patients with major depressive disorder, schizophrenia, schizoaffective disorder, or bipolar-related disorder, “access helped to clarify why medications had been prescribed, improved understanding about side effects, and 20% of patients reported doing a better job taking their meds as prescribed,” said Dr. Blease.
However, the expert panel in the Delphi poll predicted that with “open notes” some patients might demand changes to their clinical notes, and that mental health clinicians might be less detailed/accurate in documenting negative aspects of the patient relationship, details about patients’ personalities, or symptoms of paranoia in patients.
“If some patients feel more judged or offended by what they read, this may undermine the therapeutic relationship. ,” she added.
“In some clinical cases where there is more focus on emergency care than in forming a therapeutic relationship, for example emergency department visits, we know almost nothing about the risks and benefits associated with OpenNotes,” senior author John Torous, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, said in an interview.
“One thing is clear,” Dr. Blease said. “Patient access to their online medical records is now mainstream, and we need more clinician education on how to write notes that patients will read, and more guidance among patients on the benefits and risks of accessing their notes.”
Support for this research was provided by a J. F. Keane Scholar Award and a Swedish Research Council on Health, Working Life, and Welfare grant. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are multiple benefits and few harms from sharing clinical notes in patients with mental illness, results of a poll of international experts show.
As of April 5, 2021, new federal rules in the United States mandate that all patients are offered online access to their electronic health record.
“Given that sharing notes in psychiatry is likely to be more complicated than in some other specialties, we were unsure whether experts would consider the practice more harmful than beneficial,” Charlotte Blease, PhD, of Beth Israel Deaconess Medical Center in Boston, told this news organization.
“However, the results of our poll suggest clinicians’ anxieties about sharing mental health notes with patients may be misplaced. We found clear consensus among experts that the benefits of online access to clinical notes could outweigh the risks,” Dr. Blease said in a news release.
The study was published online in PLOS ONE.
Empowering patients
Investigators used an online Delphi poll, an established methodology used to investigate emerging health care policy – including in psychiatry – to solicit the views of an international panel of experts on the mental health effects of sharing clinical notes.
The panel included clinicians, chief medical information officers, patient advocates, and informatics experts with extensive experience and research knowledge about patient access to mental health notes.
There was consensus among the panel that offering online access to mental health notes could enhance patients’ understanding about their diagnosis, care plan, and rationale for treatments.
There was also consensus that access to clinical notes could enhance patient recall about what was communicated and improve mental health patients’ sense of control over their health care.
The panel also agreed that blocking mental health notes could lead to greater harms including increased feelings of stigmatization.
Confirmatory findings
The poll results support an earlier study by Dr. Blease and colleagues that evaluated the experiences of patients in accessing their online clinical notes.
Among these patients with major depressive disorder, schizophrenia, schizoaffective disorder, or bipolar-related disorder, “access helped to clarify why medications had been prescribed, improved understanding about side effects, and 20% of patients reported doing a better job taking their meds as prescribed,” said Dr. Blease.
However, the expert panel in the Delphi poll predicted that with “open notes” some patients might demand changes to their clinical notes, and that mental health clinicians might be less detailed/accurate in documenting negative aspects of the patient relationship, details about patients’ personalities, or symptoms of paranoia in patients.
“If some patients feel more judged or offended by what they read, this may undermine the therapeutic relationship. ,” she added.
“In some clinical cases where there is more focus on emergency care than in forming a therapeutic relationship, for example emergency department visits, we know almost nothing about the risks and benefits associated with OpenNotes,” senior author John Torous, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, said in an interview.
“One thing is clear,” Dr. Blease said. “Patient access to their online medical records is now mainstream, and we need more clinician education on how to write notes that patients will read, and more guidance among patients on the benefits and risks of accessing their notes.”
Support for this research was provided by a J. F. Keane Scholar Award and a Swedish Research Council on Health, Working Life, and Welfare grant. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
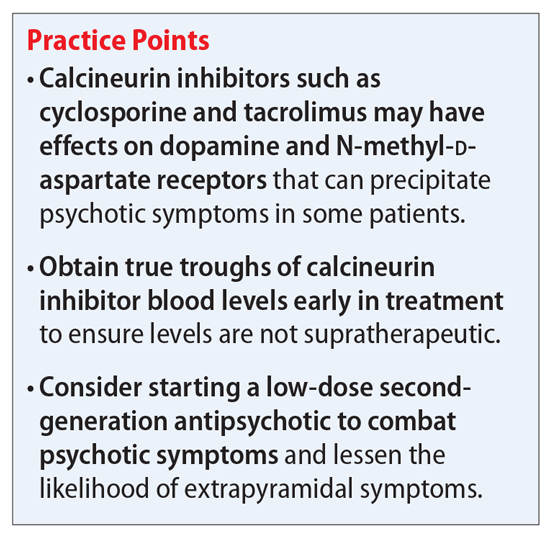
Calcineurin inhibitor–induced psychosis
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Sleep problems in mental illness highly pervasive
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
Substance use or substance use disorder: A question of judgment
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.
Alleged on-the-job violence, racism, prompts psych workers to head to D.C.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
Should clinicians recommend vitamin D for psychiatric patients during COVID-19?
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Early interventions for psychosis
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php