User login
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
RSV resurgence likely in wake of COVID-19
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
FROM JAMA NETWORK OPEN
Axilla swelling after COVID booster puts focus on mammogram timing
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
Poorly controlled asthma predicts COVID-19 hospitalization in children
Children and adolescents with poorly controlled asthma were three to six times more likely to be hospitalized with COVID-19 infections, based on data from a national study of more than 750,000 children in Scotland.
Although the majority of COVID-19 cases in children have been mild, some children require hospitalization, wrote Ting Shi, PhD, of the University of Edinburgh (Scotland) and colleagues.
Vaccination policies to potentially reduce infection and hospitalization of children remain inconsistent, the researchers said. Identifying which school-age children would derive the greatest benefit from vaccination “could help to reduce the risk of infection and consequently the need for children to have time off school; and might also reduce the risk of spread of SARS-CoV-2 within schools and households,” but the potential benefits of vaccination for children with asthma in particular have not been well studied, they wrote.
The United Kingdom’s Joint Commission on Vaccination and Immunisation commissioned research on the rates of hospitalization among children with poorly controlled asthma. In a national incidence cohort study published in The Lancet Respiratory Medicine, the researchers reviewed data from all children aged 5-17 years in Scotland who were enrolled in the linked dataset of Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II). The total number of children in the dataset was 752,867, and 63,463 (8.4%) of these had diagnosed asthma. Among the children with asthma, 4,339 (6.8%) had confirmed COVID-19 infections between March 1, 2020, and July 27, 2021. A total of 67 infected children were hospitalized. Of the 689,404 children without asthma, 40,231 (5.8%) had confirmed COVID-19 infections, and 382 (0.9%) of these children were hospitalized.
Overall, hospital admission rates for COVID-19 were significantly higher among children with asthma, compared to those without asthma (adjusted hazard ratio, 1.49), and the rates increased among children with poorly controlled asthma.
The researchers used previous hospital admission for asthma as a measure of uncontrolled asthma, and found that hospitalization was at least six times as likely for children with poorly controlled asthma, compared with those with no asthma (aHR, 6.40), although children with well-controlled asthma also had an increased risk of hospitalization, compared with those with no asthma (aHR, 1.36).
When the researchers used oral corticosteroid prescriptions as an indicator of uncontrolled asthma, the adjusted hazard ratios were 3.38, 3.53, 1.52, and 1.34 for children with prescribed corticosteroid courses of three or more, two, one, and none, respectively, compared with children with no asthma.
These hazard ratios remained significant after controlling for factors including age, sex, socioeconomic status, comorbidity, and previous hospital admission, the researchers wrote.
In an age-based analysis, results were similar for children aged 12-17 years, but in children aged 5-11 years, the hospitalization risk decreased for those with one course of corticosteroids and reached the highest rate for those with three or more courses, rather than two courses.
The study findings were limited by several factors including the relatively small numbers of COVID-19 hospitalizations, ICU admissions, and deaths in children with asthma, the researchers noted. Other limitations include potential changes in asthma control over the study period, and lack of data on certain confounders such as tobacco use, unsuitable housing, and ethnicity, they noted. However, the results were strengthened by the use of a large, national dataset, and access to electronic health records, they said.
The findings reflect data from previous studies suggesting increased risk of hospitalization for patients with respiratory illness who develop COVID-19 infections, the researchers wrote.
The results emphasize the importance of good asthma control to protect children from severe COVID-19, and careful monitoring of children with poorly controlled asthma who do become infected, they added.
“The findings from this linkage of multiple data sources have helped inform the prioritisation of school-aged children with poorly controlled asthma for vaccines,” they concluded.
Findings support value of vaccination for children with asthma
“Pediatricians see many children who suffer from asthma, and although one could assume that these children would have more serious consequences from contracting COVID-19, the current study examines a large database in a way not possible in the United States to address the severity question,” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The authors used prior hospitalization rate or two prescriptions for oral corticosteroids as markers of asthma severity prior to the onset of COVID-19 in Scotland, and they collected retrospective data for 16 months of the pandemic through July of 2021, showing a significant increase in hospitalization for those children,” she said. Dr. Boulter said she was not surprised by this finding, given the impact of COVID-19 on the respiratory system.
“Pediatricians have found significant challenges from some groups of parents when discussing the indications and need for vaccination in their patients,” said Dr. Boulter. “Having this data on the increased risk of morbidity and mortality in children with asthma might help parents who are uncertain about the risk/benefit ratio of the vaccine make their decision,” she said.
Dr. Boulter said she hoped that additional studies will yield ongoing information about hospitalization rates for COVID-19 not only about asthma, but also other diagnoses affecting children in the United States and worldwide.
“It would also be important to see a breakdown of ethnic factors and adverse childhood experiences and how they relate to hospitalization and death from COVID-19,” Dr. Boulter said.
“The results of this study are not surprising, as we have known for a long time that children with severe asthma are more susceptible to severe respiratory viruses,” Francis E. Rushton, MD, a pediatrician in Beaufort, S.C., said in an interview. “But the study is still important, as it helps us determine which children are most urgently in need of protection from COVID-19 in any of its forms,” he emphasized. In particular, the current study underlines the importance of vaccinating children with unstable asthma, Dr. Rushton said.
Going forward, “it would be interesting to do additional studies looking at other markers for poor asthma control that could guide our vaccine efforts so that they are focused on those most at risk,” he added.
The study was supported by the UK Research and Innovation (Medical Research Council), Research and Innovation Industrial Strategy Challenge Fund, Health Data Research UK, and the Scottish Government. Lead author Dr. Shi had no financial conflicts to disclose. Dr. Rushton and Dr. Boulter had no financial conflicts to disclose, but each serves on the Editorial Advisory Board of Pediatric News.
Children and adolescents with poorly controlled asthma were three to six times more likely to be hospitalized with COVID-19 infections, based on data from a national study of more than 750,000 children in Scotland.
Although the majority of COVID-19 cases in children have been mild, some children require hospitalization, wrote Ting Shi, PhD, of the University of Edinburgh (Scotland) and colleagues.
Vaccination policies to potentially reduce infection and hospitalization of children remain inconsistent, the researchers said. Identifying which school-age children would derive the greatest benefit from vaccination “could help to reduce the risk of infection and consequently the need for children to have time off school; and might also reduce the risk of spread of SARS-CoV-2 within schools and households,” but the potential benefits of vaccination for children with asthma in particular have not been well studied, they wrote.
The United Kingdom’s Joint Commission on Vaccination and Immunisation commissioned research on the rates of hospitalization among children with poorly controlled asthma. In a national incidence cohort study published in The Lancet Respiratory Medicine, the researchers reviewed data from all children aged 5-17 years in Scotland who were enrolled in the linked dataset of Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II). The total number of children in the dataset was 752,867, and 63,463 (8.4%) of these had diagnosed asthma. Among the children with asthma, 4,339 (6.8%) had confirmed COVID-19 infections between March 1, 2020, and July 27, 2021. A total of 67 infected children were hospitalized. Of the 689,404 children without asthma, 40,231 (5.8%) had confirmed COVID-19 infections, and 382 (0.9%) of these children were hospitalized.
Overall, hospital admission rates for COVID-19 were significantly higher among children with asthma, compared to those without asthma (adjusted hazard ratio, 1.49), and the rates increased among children with poorly controlled asthma.
The researchers used previous hospital admission for asthma as a measure of uncontrolled asthma, and found that hospitalization was at least six times as likely for children with poorly controlled asthma, compared with those with no asthma (aHR, 6.40), although children with well-controlled asthma also had an increased risk of hospitalization, compared with those with no asthma (aHR, 1.36).
When the researchers used oral corticosteroid prescriptions as an indicator of uncontrolled asthma, the adjusted hazard ratios were 3.38, 3.53, 1.52, and 1.34 for children with prescribed corticosteroid courses of three or more, two, one, and none, respectively, compared with children with no asthma.
These hazard ratios remained significant after controlling for factors including age, sex, socioeconomic status, comorbidity, and previous hospital admission, the researchers wrote.
In an age-based analysis, results were similar for children aged 12-17 years, but in children aged 5-11 years, the hospitalization risk decreased for those with one course of corticosteroids and reached the highest rate for those with three or more courses, rather than two courses.
The study findings were limited by several factors including the relatively small numbers of COVID-19 hospitalizations, ICU admissions, and deaths in children with asthma, the researchers noted. Other limitations include potential changes in asthma control over the study period, and lack of data on certain confounders such as tobacco use, unsuitable housing, and ethnicity, they noted. However, the results were strengthened by the use of a large, national dataset, and access to electronic health records, they said.
The findings reflect data from previous studies suggesting increased risk of hospitalization for patients with respiratory illness who develop COVID-19 infections, the researchers wrote.
The results emphasize the importance of good asthma control to protect children from severe COVID-19, and careful monitoring of children with poorly controlled asthma who do become infected, they added.
“The findings from this linkage of multiple data sources have helped inform the prioritisation of school-aged children with poorly controlled asthma for vaccines,” they concluded.
Findings support value of vaccination for children with asthma
“Pediatricians see many children who suffer from asthma, and although one could assume that these children would have more serious consequences from contracting COVID-19, the current study examines a large database in a way not possible in the United States to address the severity question,” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The authors used prior hospitalization rate or two prescriptions for oral corticosteroids as markers of asthma severity prior to the onset of COVID-19 in Scotland, and they collected retrospective data for 16 months of the pandemic through July of 2021, showing a significant increase in hospitalization for those children,” she said. Dr. Boulter said she was not surprised by this finding, given the impact of COVID-19 on the respiratory system.
“Pediatricians have found significant challenges from some groups of parents when discussing the indications and need for vaccination in their patients,” said Dr. Boulter. “Having this data on the increased risk of morbidity and mortality in children with asthma might help parents who are uncertain about the risk/benefit ratio of the vaccine make their decision,” she said.
Dr. Boulter said she hoped that additional studies will yield ongoing information about hospitalization rates for COVID-19 not only about asthma, but also other diagnoses affecting children in the United States and worldwide.
“It would also be important to see a breakdown of ethnic factors and adverse childhood experiences and how they relate to hospitalization and death from COVID-19,” Dr. Boulter said.
“The results of this study are not surprising, as we have known for a long time that children with severe asthma are more susceptible to severe respiratory viruses,” Francis E. Rushton, MD, a pediatrician in Beaufort, S.C., said in an interview. “But the study is still important, as it helps us determine which children are most urgently in need of protection from COVID-19 in any of its forms,” he emphasized. In particular, the current study underlines the importance of vaccinating children with unstable asthma, Dr. Rushton said.
Going forward, “it would be interesting to do additional studies looking at other markers for poor asthma control that could guide our vaccine efforts so that they are focused on those most at risk,” he added.
The study was supported by the UK Research and Innovation (Medical Research Council), Research and Innovation Industrial Strategy Challenge Fund, Health Data Research UK, and the Scottish Government. Lead author Dr. Shi had no financial conflicts to disclose. Dr. Rushton and Dr. Boulter had no financial conflicts to disclose, but each serves on the Editorial Advisory Board of Pediatric News.
Children and adolescents with poorly controlled asthma were three to six times more likely to be hospitalized with COVID-19 infections, based on data from a national study of more than 750,000 children in Scotland.
Although the majority of COVID-19 cases in children have been mild, some children require hospitalization, wrote Ting Shi, PhD, of the University of Edinburgh (Scotland) and colleagues.
Vaccination policies to potentially reduce infection and hospitalization of children remain inconsistent, the researchers said. Identifying which school-age children would derive the greatest benefit from vaccination “could help to reduce the risk of infection and consequently the need for children to have time off school; and might also reduce the risk of spread of SARS-CoV-2 within schools and households,” but the potential benefits of vaccination for children with asthma in particular have not been well studied, they wrote.
The United Kingdom’s Joint Commission on Vaccination and Immunisation commissioned research on the rates of hospitalization among children with poorly controlled asthma. In a national incidence cohort study published in The Lancet Respiratory Medicine, the researchers reviewed data from all children aged 5-17 years in Scotland who were enrolled in the linked dataset of Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II). The total number of children in the dataset was 752,867, and 63,463 (8.4%) of these had diagnosed asthma. Among the children with asthma, 4,339 (6.8%) had confirmed COVID-19 infections between March 1, 2020, and July 27, 2021. A total of 67 infected children were hospitalized. Of the 689,404 children without asthma, 40,231 (5.8%) had confirmed COVID-19 infections, and 382 (0.9%) of these children were hospitalized.
Overall, hospital admission rates for COVID-19 were significantly higher among children with asthma, compared to those without asthma (adjusted hazard ratio, 1.49), and the rates increased among children with poorly controlled asthma.
The researchers used previous hospital admission for asthma as a measure of uncontrolled asthma, and found that hospitalization was at least six times as likely for children with poorly controlled asthma, compared with those with no asthma (aHR, 6.40), although children with well-controlled asthma also had an increased risk of hospitalization, compared with those with no asthma (aHR, 1.36).
When the researchers used oral corticosteroid prescriptions as an indicator of uncontrolled asthma, the adjusted hazard ratios were 3.38, 3.53, 1.52, and 1.34 for children with prescribed corticosteroid courses of three or more, two, one, and none, respectively, compared with children with no asthma.
These hazard ratios remained significant after controlling for factors including age, sex, socioeconomic status, comorbidity, and previous hospital admission, the researchers wrote.
In an age-based analysis, results were similar for children aged 12-17 years, but in children aged 5-11 years, the hospitalization risk decreased for those with one course of corticosteroids and reached the highest rate for those with three or more courses, rather than two courses.
The study findings were limited by several factors including the relatively small numbers of COVID-19 hospitalizations, ICU admissions, and deaths in children with asthma, the researchers noted. Other limitations include potential changes in asthma control over the study period, and lack of data on certain confounders such as tobacco use, unsuitable housing, and ethnicity, they noted. However, the results were strengthened by the use of a large, national dataset, and access to electronic health records, they said.
The findings reflect data from previous studies suggesting increased risk of hospitalization for patients with respiratory illness who develop COVID-19 infections, the researchers wrote.
The results emphasize the importance of good asthma control to protect children from severe COVID-19, and careful monitoring of children with poorly controlled asthma who do become infected, they added.
“The findings from this linkage of multiple data sources have helped inform the prioritisation of school-aged children with poorly controlled asthma for vaccines,” they concluded.
Findings support value of vaccination for children with asthma
“Pediatricians see many children who suffer from asthma, and although one could assume that these children would have more serious consequences from contracting COVID-19, the current study examines a large database in a way not possible in the United States to address the severity question,” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “The authors used prior hospitalization rate or two prescriptions for oral corticosteroids as markers of asthma severity prior to the onset of COVID-19 in Scotland, and they collected retrospective data for 16 months of the pandemic through July of 2021, showing a significant increase in hospitalization for those children,” she said. Dr. Boulter said she was not surprised by this finding, given the impact of COVID-19 on the respiratory system.
“Pediatricians have found significant challenges from some groups of parents when discussing the indications and need for vaccination in their patients,” said Dr. Boulter. “Having this data on the increased risk of morbidity and mortality in children with asthma might help parents who are uncertain about the risk/benefit ratio of the vaccine make their decision,” she said.
Dr. Boulter said she hoped that additional studies will yield ongoing information about hospitalization rates for COVID-19 not only about asthma, but also other diagnoses affecting children in the United States and worldwide.
“It would also be important to see a breakdown of ethnic factors and adverse childhood experiences and how they relate to hospitalization and death from COVID-19,” Dr. Boulter said.
“The results of this study are not surprising, as we have known for a long time that children with severe asthma are more susceptible to severe respiratory viruses,” Francis E. Rushton, MD, a pediatrician in Beaufort, S.C., said in an interview. “But the study is still important, as it helps us determine which children are most urgently in need of protection from COVID-19 in any of its forms,” he emphasized. In particular, the current study underlines the importance of vaccinating children with unstable asthma, Dr. Rushton said.
Going forward, “it would be interesting to do additional studies looking at other markers for poor asthma control that could guide our vaccine efforts so that they are focused on those most at risk,” he added.
The study was supported by the UK Research and Innovation (Medical Research Council), Research and Innovation Industrial Strategy Challenge Fund, Health Data Research UK, and the Scottish Government. Lead author Dr. Shi had no financial conflicts to disclose. Dr. Rushton and Dr. Boulter had no financial conflicts to disclose, but each serves on the Editorial Advisory Board of Pediatric News.
FROM THE LANCET
For older adults, smelling the roses may be more difficult
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Does zinc really help treat colds?
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Influenza tied to long-term increased risk for Parkinson’s disease
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Fluoroquinolones linked to sudden death risk for those on hemodialysis
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even one vaccinated member can cut family’s COVID risk
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
Effect of COVID-19 pandemic on respiratory infectious diseases in primary care practice
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.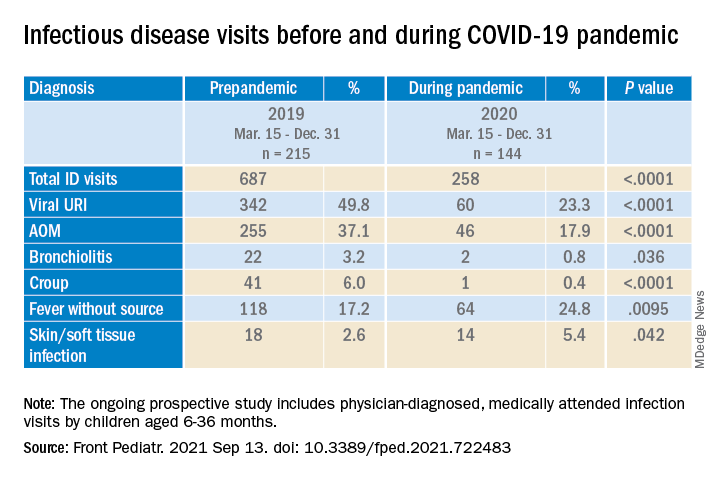
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.



