User login
COVID-19 disease may actually cause preeclampsia, suggests study
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
FROM THE JOURNAL OF OBSTETRICS AND GYNECOLOGY
Nearly half of female surgeons surveyed lost a pregnancy
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does early delivery for FGR affect school outcomes?
Iatrogenic delivery for suspected fetal growth restriction (FGR) may be associated with an increased likelihood of poorer school outcomes among infants born severely small for gestational age, a study of children in Australia suggests.
researchers reported in JAMA.
“It raises the question: in our efforts to improve outcomes in babies that are small, are we potentially doing more harm than good?” said Robert M. Silver, MD, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, who was not involved in the study. “I think that is a very important question to ask.”
However, “we can’t make that conclusion based on this one study,” he said in an interview. It could be that, in cases where severely small infants were delivered early, there may have been testing that indicated acute risks, and these infants may have tended to be sicker overall. “It may have been that if those babies weren’t delivered, they would have suffered a stillbirth or major brain injury,” Dr. Silver said. “It is really important that we acknowledge that we shouldn’t change our clinical practice” based on this one study.”
At the same time, the study underscores questions and challenges that surround the definition, identification, and management of suspected FGR, Dr. Silver said.
The study authors described their research as exploratory. In a related editorial Dr. Silver and Nathan R. Blue, MD said the findings should be considered hypothesis generating.
For the study, Roshan John Selvaratnam, BMedSc(Hons), a researcher affiliated with Monash University, Melbourne, and colleagues analyzed data from 181,902 children with developmental outcomes and 425,717 children with educational outcomes in Australia. They included children born at 32 weeks’ or more gestation between 2003 and 2013.
Severely small infants delivered early for suspected FGR had an average gestation of 37.9 weeks, whereas those not suspected of having FGR had an average gestation of 39.4 weeks.
Among infants who were severely small for gestational age, those delivered early for suspected FGR were more likely to be in the bottom 10th percentile on at least two developmental domains when they started school, compared with those not suspected of having FGR (16.2% vs. 12.7%; adjusted odds ratio, 1.36). They also were more likely to have low test scores in subsequent years. In grade 7, for example, the adjusted odds ratio for scoring below the national minimum standard on at least two educational domains was 1.33 (13.4% vs. 10.5%).
The researchers defined severely small for gestational age as birth weight below the third percentile. Among infants with normal growth, defined as birth weight at the 10th percentile or greater, school outcomes did not significantly differ between those with early delivery for suspected FGR and those not suspected of having FGR. Approximately 8% of the infants with normal growth had poor developmental outcomes.
The study authors described the dilemma that clinicians face with suspected FGR: “Either intervene early to prevent a small risk of stillbirth but potentially cause immediate and lifelong harm to the child or accept the increasing risk of stillbirth associated with prolonging the pregnancy to avoid more common neonatal and longer-term morbidities.”
It could be that severely small infants with suspected FGR in the study were “more compromised than those not suspected of having FGR,” which might explain the outcomes, Mr. Selvaratnam and coauthors wrote.
Another more plausible explanation is that “iatrogenic prematurity was harmful,” they said.
The researchers were unable to adjust for many factors that may influence academic success, including smoking and alcohol use during pregnancy, maternal body mass index, and breastfeeding, they noted. They also lacked information about the etiology for FGR and whether children had genetic abnormalities.
The study also does not take into account neonatal, infant, and childhood complications, Dr. Silver and Dr. Blue wrote in their editorial. “Nonetheless, these data are a welcome contribution given the knowledge gaps with regard to the optimal obstetric management of FGR.”
The establishment of a diagnostic standard for FGR is needed to properly investigate ways to improve risk stratification, diagnosis, and management, Dr. Silver and Dr. Blue added.
“What we have to do is get better at predicting which babies are at very high risk for continuing the pregnancy and which babies are at low risk for continuing the pregnancy so that we can better decide which babies would benefit from slightly early delivery,” Dr. Silver said.
Improved detection and management of FGR may be on the horizon. “Our ability to image the placental function has gotten a lot better, and I think that is really going to help us,” Dr. Silver said. Studies that aim to further improve the ability to assess whether babies are getting adequate blood flow during pregnancy are ongoing, which could further help doctors evaluate risks.
The study investigators and Dr. Silver had no conflict of interest disclosures. Dr. Blue disclosed grants from Samsung Medison and personal fees from Elsevier. The study was supported by a grant from the Australian government’s National Health and Medical Research Council Program, and Mr. Selvaratnam is supported by scholarships from an Australian government research training program and the National Centre of Research Excellence in Stillbirth.
Iatrogenic delivery for suspected fetal growth restriction (FGR) may be associated with an increased likelihood of poorer school outcomes among infants born severely small for gestational age, a study of children in Australia suggests.
researchers reported in JAMA.
“It raises the question: in our efforts to improve outcomes in babies that are small, are we potentially doing more harm than good?” said Robert M. Silver, MD, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, who was not involved in the study. “I think that is a very important question to ask.”
However, “we can’t make that conclusion based on this one study,” he said in an interview. It could be that, in cases where severely small infants were delivered early, there may have been testing that indicated acute risks, and these infants may have tended to be sicker overall. “It may have been that if those babies weren’t delivered, they would have suffered a stillbirth or major brain injury,” Dr. Silver said. “It is really important that we acknowledge that we shouldn’t change our clinical practice” based on this one study.”
At the same time, the study underscores questions and challenges that surround the definition, identification, and management of suspected FGR, Dr. Silver said.
The study authors described their research as exploratory. In a related editorial Dr. Silver and Nathan R. Blue, MD said the findings should be considered hypothesis generating.
For the study, Roshan John Selvaratnam, BMedSc(Hons), a researcher affiliated with Monash University, Melbourne, and colleagues analyzed data from 181,902 children with developmental outcomes and 425,717 children with educational outcomes in Australia. They included children born at 32 weeks’ or more gestation between 2003 and 2013.
Severely small infants delivered early for suspected FGR had an average gestation of 37.9 weeks, whereas those not suspected of having FGR had an average gestation of 39.4 weeks.
Among infants who were severely small for gestational age, those delivered early for suspected FGR were more likely to be in the bottom 10th percentile on at least two developmental domains when they started school, compared with those not suspected of having FGR (16.2% vs. 12.7%; adjusted odds ratio, 1.36). They also were more likely to have low test scores in subsequent years. In grade 7, for example, the adjusted odds ratio for scoring below the national minimum standard on at least two educational domains was 1.33 (13.4% vs. 10.5%).
The researchers defined severely small for gestational age as birth weight below the third percentile. Among infants with normal growth, defined as birth weight at the 10th percentile or greater, school outcomes did not significantly differ between those with early delivery for suspected FGR and those not suspected of having FGR. Approximately 8% of the infants with normal growth had poor developmental outcomes.
The study authors described the dilemma that clinicians face with suspected FGR: “Either intervene early to prevent a small risk of stillbirth but potentially cause immediate and lifelong harm to the child or accept the increasing risk of stillbirth associated with prolonging the pregnancy to avoid more common neonatal and longer-term morbidities.”
It could be that severely small infants with suspected FGR in the study were “more compromised than those not suspected of having FGR,” which might explain the outcomes, Mr. Selvaratnam and coauthors wrote.
Another more plausible explanation is that “iatrogenic prematurity was harmful,” they said.
The researchers were unable to adjust for many factors that may influence academic success, including smoking and alcohol use during pregnancy, maternal body mass index, and breastfeeding, they noted. They also lacked information about the etiology for FGR and whether children had genetic abnormalities.
The study also does not take into account neonatal, infant, and childhood complications, Dr. Silver and Dr. Blue wrote in their editorial. “Nonetheless, these data are a welcome contribution given the knowledge gaps with regard to the optimal obstetric management of FGR.”
The establishment of a diagnostic standard for FGR is needed to properly investigate ways to improve risk stratification, diagnosis, and management, Dr. Silver and Dr. Blue added.
“What we have to do is get better at predicting which babies are at very high risk for continuing the pregnancy and which babies are at low risk for continuing the pregnancy so that we can better decide which babies would benefit from slightly early delivery,” Dr. Silver said.
Improved detection and management of FGR may be on the horizon. “Our ability to image the placental function has gotten a lot better, and I think that is really going to help us,” Dr. Silver said. Studies that aim to further improve the ability to assess whether babies are getting adequate blood flow during pregnancy are ongoing, which could further help doctors evaluate risks.
The study investigators and Dr. Silver had no conflict of interest disclosures. Dr. Blue disclosed grants from Samsung Medison and personal fees from Elsevier. The study was supported by a grant from the Australian government’s National Health and Medical Research Council Program, and Mr. Selvaratnam is supported by scholarships from an Australian government research training program and the National Centre of Research Excellence in Stillbirth.
Iatrogenic delivery for suspected fetal growth restriction (FGR) may be associated with an increased likelihood of poorer school outcomes among infants born severely small for gestational age, a study of children in Australia suggests.
researchers reported in JAMA.
“It raises the question: in our efforts to improve outcomes in babies that are small, are we potentially doing more harm than good?” said Robert M. Silver, MD, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, who was not involved in the study. “I think that is a very important question to ask.”
However, “we can’t make that conclusion based on this one study,” he said in an interview. It could be that, in cases where severely small infants were delivered early, there may have been testing that indicated acute risks, and these infants may have tended to be sicker overall. “It may have been that if those babies weren’t delivered, they would have suffered a stillbirth or major brain injury,” Dr. Silver said. “It is really important that we acknowledge that we shouldn’t change our clinical practice” based on this one study.”
At the same time, the study underscores questions and challenges that surround the definition, identification, and management of suspected FGR, Dr. Silver said.
The study authors described their research as exploratory. In a related editorial Dr. Silver and Nathan R. Blue, MD said the findings should be considered hypothesis generating.
For the study, Roshan John Selvaratnam, BMedSc(Hons), a researcher affiliated with Monash University, Melbourne, and colleagues analyzed data from 181,902 children with developmental outcomes and 425,717 children with educational outcomes in Australia. They included children born at 32 weeks’ or more gestation between 2003 and 2013.
Severely small infants delivered early for suspected FGR had an average gestation of 37.9 weeks, whereas those not suspected of having FGR had an average gestation of 39.4 weeks.
Among infants who were severely small for gestational age, those delivered early for suspected FGR were more likely to be in the bottom 10th percentile on at least two developmental domains when they started school, compared with those not suspected of having FGR (16.2% vs. 12.7%; adjusted odds ratio, 1.36). They also were more likely to have low test scores in subsequent years. In grade 7, for example, the adjusted odds ratio for scoring below the national minimum standard on at least two educational domains was 1.33 (13.4% vs. 10.5%).
The researchers defined severely small for gestational age as birth weight below the third percentile. Among infants with normal growth, defined as birth weight at the 10th percentile or greater, school outcomes did not significantly differ between those with early delivery for suspected FGR and those not suspected of having FGR. Approximately 8% of the infants with normal growth had poor developmental outcomes.
The study authors described the dilemma that clinicians face with suspected FGR: “Either intervene early to prevent a small risk of stillbirth but potentially cause immediate and lifelong harm to the child or accept the increasing risk of stillbirth associated with prolonging the pregnancy to avoid more common neonatal and longer-term morbidities.”
It could be that severely small infants with suspected FGR in the study were “more compromised than those not suspected of having FGR,” which might explain the outcomes, Mr. Selvaratnam and coauthors wrote.
Another more plausible explanation is that “iatrogenic prematurity was harmful,” they said.
The researchers were unable to adjust for many factors that may influence academic success, including smoking and alcohol use during pregnancy, maternal body mass index, and breastfeeding, they noted. They also lacked information about the etiology for FGR and whether children had genetic abnormalities.
The study also does not take into account neonatal, infant, and childhood complications, Dr. Silver and Dr. Blue wrote in their editorial. “Nonetheless, these data are a welcome contribution given the knowledge gaps with regard to the optimal obstetric management of FGR.”
The establishment of a diagnostic standard for FGR is needed to properly investigate ways to improve risk stratification, diagnosis, and management, Dr. Silver and Dr. Blue added.
“What we have to do is get better at predicting which babies are at very high risk for continuing the pregnancy and which babies are at low risk for continuing the pregnancy so that we can better decide which babies would benefit from slightly early delivery,” Dr. Silver said.
Improved detection and management of FGR may be on the horizon. “Our ability to image the placental function has gotten a lot better, and I think that is really going to help us,” Dr. Silver said. Studies that aim to further improve the ability to assess whether babies are getting adequate blood flow during pregnancy are ongoing, which could further help doctors evaluate risks.
The study investigators and Dr. Silver had no conflict of interest disclosures. Dr. Blue disclosed grants from Samsung Medison and personal fees from Elsevier. The study was supported by a grant from the Australian government’s National Health and Medical Research Council Program, and Mr. Selvaratnam is supported by scholarships from an Australian government research training program and the National Centre of Research Excellence in Stillbirth.
FROM JAMA
Placental allograft, cytology processor, cell-free RNA testing, and male infertility
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
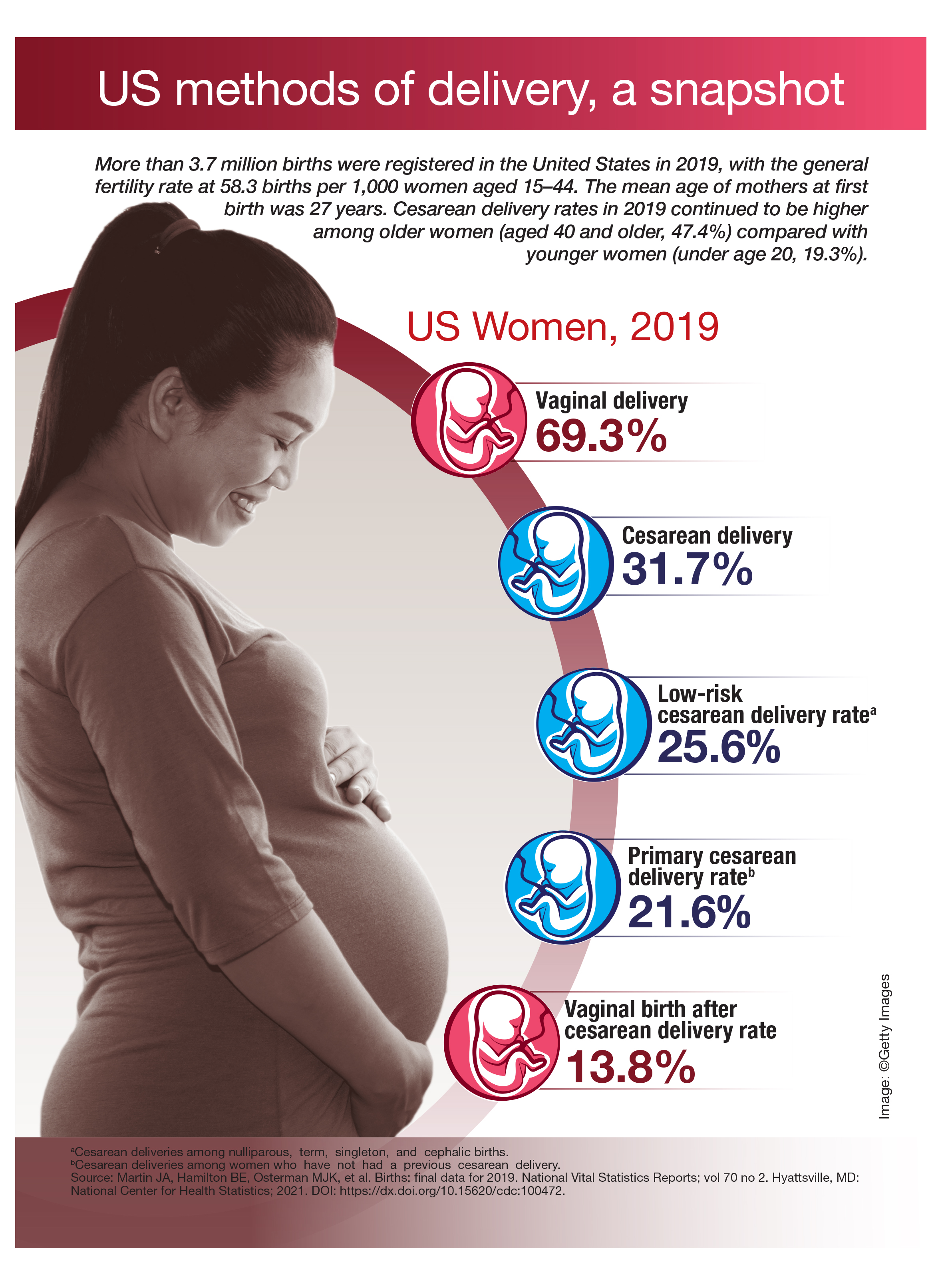
US methods of delivery, a snapshot


Neighborhood police complaints tied to Black preterm birth rates
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-dose aspirin did not reduce preterm birth rates but don’t rule it out yet
Women at risk of preterm birth who took daily low-dose aspirin did not have significantly lower rates of preterm birth than those who did not take aspirin, according to preliminary findings from a small randomized controlled trial. There was a trend toward lower rates, especially among those with the highest compliance, but the study was underpowered to detect a difference with statistical significance, said Anadeijda Landman, MD, of the Amsterdam University Medical Center. Dr. Landman presented the findings Jan. 28 at a meeting sponsored by the Society for Maternal-Fetal Medicine.
Preterm birth accounts for a third of all neonatal mortality, she told attendees. Among 15 million preterm births worldwide each year, 65% are spontaneous, indicating the need for effective preventive interventions. Dr. Landman reviewed several mechanisms by which aspirin may help reduce preterm birth via different pathways.
The researchers’ multicenter, placebo-controlled trial involved 8 tertiary care and 26 secondary care hospitals in the Netherlands between May 2016 and June 2019. Starting between 8 and 15 weeks’ gestation, women took either 80 mg of aspirin or a placebo daily until 36 weeks’ gestation or delivery. Women also received progesterone, cerclage, or pessary as indicated according to local protocols.
The study enrolled 406 women with singleton pregnancy and a history of preterm birth delivered between 22 and 37 weeks’ gestation. The final analysis, after exclusions for pregnancy termination, congenital anomalies, multiples pregnancy, or similar reasons, included 193 women in the intervention group and 194 in the placebo group. The women had similar baseline characteristics across both groups except a higher number of past mid-trimester fetal deaths in the aspirin group.
“It’s important to realize these women had multiple preterm births, as one of our inclusion criteria was previous spontaneous preterm birth later than 22 weeks’ gestation, so this particular group is very high risk for cervical insufficiency as a probable cause,” Dr. Landman told attendees.
Among women in the aspirin group, 21.2% delivered before 37 weeks, compared with 25.4% in the placebo group (P = .323). The rate of spontaneous birth was 20.1% in the aspirin group and 23.8% in the placebo group (P = .376). Though still not statistically significant, the difference between the groups was larger when the researchers limited their analysis to the 245 women with at least 80% compliance: 18.5% of women in the aspirin group had a preterm birth, compared with 24.8% of women in the placebo group (P = .238).
There were no significant differences between the groups in composite poor neonatal outcomes or in a range of prespecified newborn complications. The aspirin group did have two stillbirths, two mid-trimester fetal losses, and two extremely preterm newborns (at 24+2 weeks and 25+2 weeks). The placebo group had two mid-trimester fetal losses.
“These deaths are inherent to the study population, and it seems unlikely they are related to the use of aspirin,” Dr. Landman said. “Moreover other aspirin studies have not found an increased perinatal mortality rate, and some large studies indicated the neonatal mortality rate is even reduced.”
Although preterm birth only trended lower in the aspirin group, Dr. Landman said the researchers believe they cannot rule out an effect from aspirin.
“It’s also important to note that our study was underpowered as the recurrence risk of preterm birth in our study was lower than expected, so it’s possible a small treatment effect of aspirin could not be demonstrated in our study,” she said. “And, despite the proper randomization procedure, many more women in the aspirin group had a previous mid-trimester fetal loss. This indicates that the aspirin group might be more at risk for preterm birth than the placebo group, and this imbalance could also have diminished a small protective effect of aspirin.”
In response to an audience question, Dr. Landman acknowledged that more recent studies on aspirin have used 100- to 150-mg dosages, but that evidence was not as clear when their study began in 2015. She added that her research team does not advise changing clinical care currently and believes it is too soon to recommend aspirin to this population.
Tracy Manuck, MD, MS, an associate professor of ob.gyn. at the University of North Carolina in Chapel Hill, agreed that it is premature to begin prescribing aspirin for preterm birth prevention, but she noted that most of the patients she cares for clinically already meet criteria for aspirin based on their risk factors for preeclampsia.
“Additional research is needed in the form of a well-designed and large [randomized, controlled trial],” Dr Manuck, who moderated the session, said in an interview. “However, such a trial is becoming increasingly difficult to conduct because so many pregnant women qualify to receive aspirin for the prevention of preeclampsia due to their weight, medical comorbidities, or prior pregnancy history.”
She said she anticipates seeing patient-level data meta-analyses in the coming months as more data on aspirin for preterm birth prevention are published.
“Given that these data are supportive of the overall trends seen in prior publications, I do think that low-dose aspirin will eventually bear out as a helpful preventative measure to prevent recurrent preterm birth. Aspirin is low risk, readily available, and is inexpensive,” Dr. Manuck said. “I hope that meta-analysis data will provide additional information regarding the benefit of low-dose aspirin for prematurity prevention.”
The research was funded by the Dutch Organization for Health Care Research and the Dutch Consortium for Research in Women’s Health. Dr. Landman and Dr. Manuck had no disclosures.
Women at risk of preterm birth who took daily low-dose aspirin did not have significantly lower rates of preterm birth than those who did not take aspirin, according to preliminary findings from a small randomized controlled trial. There was a trend toward lower rates, especially among those with the highest compliance, but the study was underpowered to detect a difference with statistical significance, said Anadeijda Landman, MD, of the Amsterdam University Medical Center. Dr. Landman presented the findings Jan. 28 at a meeting sponsored by the Society for Maternal-Fetal Medicine.
Preterm birth accounts for a third of all neonatal mortality, she told attendees. Among 15 million preterm births worldwide each year, 65% are spontaneous, indicating the need for effective preventive interventions. Dr. Landman reviewed several mechanisms by which aspirin may help reduce preterm birth via different pathways.
The researchers’ multicenter, placebo-controlled trial involved 8 tertiary care and 26 secondary care hospitals in the Netherlands between May 2016 and June 2019. Starting between 8 and 15 weeks’ gestation, women took either 80 mg of aspirin or a placebo daily until 36 weeks’ gestation or delivery. Women also received progesterone, cerclage, or pessary as indicated according to local protocols.
The study enrolled 406 women with singleton pregnancy and a history of preterm birth delivered between 22 and 37 weeks’ gestation. The final analysis, after exclusions for pregnancy termination, congenital anomalies, multiples pregnancy, or similar reasons, included 193 women in the intervention group and 194 in the placebo group. The women had similar baseline characteristics across both groups except a higher number of past mid-trimester fetal deaths in the aspirin group.
“It’s important to realize these women had multiple preterm births, as one of our inclusion criteria was previous spontaneous preterm birth later than 22 weeks’ gestation, so this particular group is very high risk for cervical insufficiency as a probable cause,” Dr. Landman told attendees.
Among women in the aspirin group, 21.2% delivered before 37 weeks, compared with 25.4% in the placebo group (P = .323). The rate of spontaneous birth was 20.1% in the aspirin group and 23.8% in the placebo group (P = .376). Though still not statistically significant, the difference between the groups was larger when the researchers limited their analysis to the 245 women with at least 80% compliance: 18.5% of women in the aspirin group had a preterm birth, compared with 24.8% of women in the placebo group (P = .238).
There were no significant differences between the groups in composite poor neonatal outcomes or in a range of prespecified newborn complications. The aspirin group did have two stillbirths, two mid-trimester fetal losses, and two extremely preterm newborns (at 24+2 weeks and 25+2 weeks). The placebo group had two mid-trimester fetal losses.
“These deaths are inherent to the study population, and it seems unlikely they are related to the use of aspirin,” Dr. Landman said. “Moreover other aspirin studies have not found an increased perinatal mortality rate, and some large studies indicated the neonatal mortality rate is even reduced.”
Although preterm birth only trended lower in the aspirin group, Dr. Landman said the researchers believe they cannot rule out an effect from aspirin.
“It’s also important to note that our study was underpowered as the recurrence risk of preterm birth in our study was lower than expected, so it’s possible a small treatment effect of aspirin could not be demonstrated in our study,” she said. “And, despite the proper randomization procedure, many more women in the aspirin group had a previous mid-trimester fetal loss. This indicates that the aspirin group might be more at risk for preterm birth than the placebo group, and this imbalance could also have diminished a small protective effect of aspirin.”
In response to an audience question, Dr. Landman acknowledged that more recent studies on aspirin have used 100- to 150-mg dosages, but that evidence was not as clear when their study began in 2015. She added that her research team does not advise changing clinical care currently and believes it is too soon to recommend aspirin to this population.
Tracy Manuck, MD, MS, an associate professor of ob.gyn. at the University of North Carolina in Chapel Hill, agreed that it is premature to begin prescribing aspirin for preterm birth prevention, but she noted that most of the patients she cares for clinically already meet criteria for aspirin based on their risk factors for preeclampsia.
“Additional research is needed in the form of a well-designed and large [randomized, controlled trial],” Dr Manuck, who moderated the session, said in an interview. “However, such a trial is becoming increasingly difficult to conduct because so many pregnant women qualify to receive aspirin for the prevention of preeclampsia due to their weight, medical comorbidities, or prior pregnancy history.”
She said she anticipates seeing patient-level data meta-analyses in the coming months as more data on aspirin for preterm birth prevention are published.
“Given that these data are supportive of the overall trends seen in prior publications, I do think that low-dose aspirin will eventually bear out as a helpful preventative measure to prevent recurrent preterm birth. Aspirin is low risk, readily available, and is inexpensive,” Dr. Manuck said. “I hope that meta-analysis data will provide additional information regarding the benefit of low-dose aspirin for prematurity prevention.”
The research was funded by the Dutch Organization for Health Care Research and the Dutch Consortium for Research in Women’s Health. Dr. Landman and Dr. Manuck had no disclosures.
Women at risk of preterm birth who took daily low-dose aspirin did not have significantly lower rates of preterm birth than those who did not take aspirin, according to preliminary findings from a small randomized controlled trial. There was a trend toward lower rates, especially among those with the highest compliance, but the study was underpowered to detect a difference with statistical significance, said Anadeijda Landman, MD, of the Amsterdam University Medical Center. Dr. Landman presented the findings Jan. 28 at a meeting sponsored by the Society for Maternal-Fetal Medicine.
Preterm birth accounts for a third of all neonatal mortality, she told attendees. Among 15 million preterm births worldwide each year, 65% are spontaneous, indicating the need for effective preventive interventions. Dr. Landman reviewed several mechanisms by which aspirin may help reduce preterm birth via different pathways.
The researchers’ multicenter, placebo-controlled trial involved 8 tertiary care and 26 secondary care hospitals in the Netherlands between May 2016 and June 2019. Starting between 8 and 15 weeks’ gestation, women took either 80 mg of aspirin or a placebo daily until 36 weeks’ gestation or delivery. Women also received progesterone, cerclage, or pessary as indicated according to local protocols.
The study enrolled 406 women with singleton pregnancy and a history of preterm birth delivered between 22 and 37 weeks’ gestation. The final analysis, after exclusions for pregnancy termination, congenital anomalies, multiples pregnancy, or similar reasons, included 193 women in the intervention group and 194 in the placebo group. The women had similar baseline characteristics across both groups except a higher number of past mid-trimester fetal deaths in the aspirin group.
“It’s important to realize these women had multiple preterm births, as one of our inclusion criteria was previous spontaneous preterm birth later than 22 weeks’ gestation, so this particular group is very high risk for cervical insufficiency as a probable cause,” Dr. Landman told attendees.
Among women in the aspirin group, 21.2% delivered before 37 weeks, compared with 25.4% in the placebo group (P = .323). The rate of spontaneous birth was 20.1% in the aspirin group and 23.8% in the placebo group (P = .376). Though still not statistically significant, the difference between the groups was larger when the researchers limited their analysis to the 245 women with at least 80% compliance: 18.5% of women in the aspirin group had a preterm birth, compared with 24.8% of women in the placebo group (P = .238).
There were no significant differences between the groups in composite poor neonatal outcomes or in a range of prespecified newborn complications. The aspirin group did have two stillbirths, two mid-trimester fetal losses, and two extremely preterm newborns (at 24+2 weeks and 25+2 weeks). The placebo group had two mid-trimester fetal losses.
“These deaths are inherent to the study population, and it seems unlikely they are related to the use of aspirin,” Dr. Landman said. “Moreover other aspirin studies have not found an increased perinatal mortality rate, and some large studies indicated the neonatal mortality rate is even reduced.”
Although preterm birth only trended lower in the aspirin group, Dr. Landman said the researchers believe they cannot rule out an effect from aspirin.
“It’s also important to note that our study was underpowered as the recurrence risk of preterm birth in our study was lower than expected, so it’s possible a small treatment effect of aspirin could not be demonstrated in our study,” she said. “And, despite the proper randomization procedure, many more women in the aspirin group had a previous mid-trimester fetal loss. This indicates that the aspirin group might be more at risk for preterm birth than the placebo group, and this imbalance could also have diminished a small protective effect of aspirin.”
In response to an audience question, Dr. Landman acknowledged that more recent studies on aspirin have used 100- to 150-mg dosages, but that evidence was not as clear when their study began in 2015. She added that her research team does not advise changing clinical care currently and believes it is too soon to recommend aspirin to this population.
Tracy Manuck, MD, MS, an associate professor of ob.gyn. at the University of North Carolina in Chapel Hill, agreed that it is premature to begin prescribing aspirin for preterm birth prevention, but she noted that most of the patients she cares for clinically already meet criteria for aspirin based on their risk factors for preeclampsia.
“Additional research is needed in the form of a well-designed and large [randomized, controlled trial],” Dr Manuck, who moderated the session, said in an interview. “However, such a trial is becoming increasingly difficult to conduct because so many pregnant women qualify to receive aspirin for the prevention of preeclampsia due to their weight, medical comorbidities, or prior pregnancy history.”
She said she anticipates seeing patient-level data meta-analyses in the coming months as more data on aspirin for preterm birth prevention are published.
“Given that these data are supportive of the overall trends seen in prior publications, I do think that low-dose aspirin will eventually bear out as a helpful preventative measure to prevent recurrent preterm birth. Aspirin is low risk, readily available, and is inexpensive,” Dr. Manuck said. “I hope that meta-analysis data will provide additional information regarding the benefit of low-dose aspirin for prematurity prevention.”
The research was funded by the Dutch Organization for Health Care Research and the Dutch Consortium for Research in Women’s Health. Dr. Landman and Dr. Manuck had no disclosures.
FROM THE PREGNANCY MEETING
COVID-19 in pregnancy raises risk of preterm birth and severe disease
based on data from two studies published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
In a study of birth and infant outcomes, rates of preterm birth (less than 37 weeks’ gestational age) were higher among women with confirmed SARS-CoV-2 infections compared with the national average (12.9% vs. 10.2%) wrote Kate R. Woodworth, MD, and colleagues of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team.
The researchers collected information on pregnancy and infant outcomes from 16 jurisdictions through the Surveillance for Emerging Threats to Mothers and Babies Network (SET-NET). The study included 5,252 women with laboratory-confirmed SARS-CoV-2 infection reported during March 29–Oct. 14, 2020.
Overall, 12.9% of the 3,912 live births with known gestational age were preterm. A total of 610 infants were tested for SARS-CoV-2, and 2.6% were positive. Most of these perinatal infections (85%) occurred among infants born to women with SARS-CoV-2 infection within 1 week of delivery.
Half of the infants with positive test results were preterm, possibly reflecting higher screening rates in the ICU, the researchers said. “These findings also support the growing evidence that although severe COVID-19 does occur in neonates the majority of term neonates experience asymptomatic infection or mild disease; however, information on long term outcomes among exposed infants is unknown.”
Address disparities that amplify risk
The study findings were limited by several factors including inconsistent symptom reporting, overrepresentation of Hispanic women, and incomplete information on pregnancy loss, Dr. Woodworth and associates noted. However, the results add to the knowledge about the impact of COVID-19 disease on pregnancy by providing a large, population-based cohort with completed pregnancy outcomes as well as infant testing.
“SET-NET will continue to follow pregnancies affected by SARS-CoV-2 through completion of pregnancy and infants until age 6 months to guide clinical and public health practice,” the researchers noted. “Longer-term investigation into solutions to alleviate underlying inequities in social determinants of health associated with disparities in maternal morbidity, mortality, and adverse pregnancy outcomes, and effectively addressing these inequities, could reduce the prevalence of conditions and experiences that might amplify risks from COVID-19,” they added.
Severe disease and death increased in pregnant women
In a second study published in the MMWR, Laura D. Zambrano, PhD, and colleagues, also of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team, compared data on 23,434 reportedly pregnant and 386,028 nonpregnant women of reproductive age (15-44 years) with confirmed and symptomatic SARS-CoV-2 infections reported to the CDC between Jan. 22, 2020, and Oct. 3, 2020.
After adjustment for age, race, and underlying medical conditions, pregnant women with COVID-19 disease were significantly more likely than were nonpregnant women to be admitted to intensive care (10.5 per 1,000 cases vs. 3.9 per 1,000 cases), to receive invasive ventilation (2.9 vs. 1.1), receive extracorporeal membrane oxygenation (0.7 vs. 0.3) and to die (1.5 vs. 1.2).
“Irrespective of pregnancy status, ICU admissions, receipt of invasive ventilation, and death occurred more often among women aged 35-44 years than among those aged 15-24 years,” Dr. Zambrano and associates noted. In addition, non-Hispanic Black and Black women comprised 14.1% of the study population but accounted for 36.6% of deaths overall (9 in pregnant women and 167 in nonpregnant women).
The findings in the study of characteristics were limited by several factors including the voluntary reporting of COVID-19 cases, potential reporting bias, and inadequate time to assess severe cases, the researchers noted. However, “data from previous influenza pandemics, including 2009 H1N1, have shown that pregnant women are at increased risk for severe outcomes including death and the absolute risks for severe outcomes were higher than in this study of COVID-19 during pregnancy.”
“Pregnant women should be informed of their risk for severe COVID-19–associated illness and the warning signs of severe COVID-19,” Dr. Zambrano and associates said. “Providers who care for pregnant women should be familiar with guidelines for medical management of COVID-19, including considerations for management of COVID-19 in pregnancy.”
More data needed for informed counseling
“It is important to conduct research trials involving pregnant women so that we have reliable data regarding outcomes with which to counsel women,” Angela Bianco, MD, a maternal fetal medicine specialist at Mount Sinai Hospital in New York, said in an interview.
“Often pregnant women are excluded from research trials, but the impact of the current public health crisis affects all persons regardless of pregnancy status,” she said.
Dr. Bianco said that she was not surprised by the findings of either study. “In fact, our own research produced similar results.”
“These recent publications found that age-matched pregnant versus nonpregnant women had more severe manifestations of COVID-19, and specifically that pregnant women had a higher risk of requiring ventilation and intensive care admission, as well as higher risk of death,” she said. “Previous studies examining the effect of other SARS viruses have demonstrated that pregnancy is associated with worse outcomes; these findings are likely attributable to the relative state of immunosuppression in pregnancy.” Also, “one of these trials found a greater risk of premature birth in women with COVID-19; this may largely be attributable to iatrogenic delivery due to maternal illness as opposed to spontaneous preterm birth,” Dr. Bianco explained.
“Data are emerging regarding the impact of SARS-CoV-2 on pregnancy outcomes, however information remains limited,” Dr. Bianco noted. “Clinicians need to make patients aware that SARS-CoV-2 infection during pregnancy is associated with a greater risk of severe illness requiring intensive care and/or ventilatory support and even death; however, the precise rates remain unknown. “COVID-19 during pregnancy may result in a preterm birth, but at this time the rate of fetal infection remains unknown,” she said. “Clinicians need to reinforce the importance of physical distancing, mask use, and proper hand hygiene, particularly in this vulnerable population.”
Dr. Bianco emphasized: “Longitudinal studies assessing the impact of SARS-CoV-2 infection at various gestational age periods are needed, as at this time most of the available data includes women with SARS-CoV-2 infection around the time of delivery. Long-term infant outcomes are needed, as well as studies assessing the risk of fetal infection.”
The studies were supported by the Centers for Disease Control and Prevention. The researchers had no financial conflicts to disclose. Dr. Bianco had no relevant financial disclosures.
SOURCE: Woodworth KR et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e2; Zambrano LD et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e3.
based on data from two studies published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
In a study of birth and infant outcomes, rates of preterm birth (less than 37 weeks’ gestational age) were higher among women with confirmed SARS-CoV-2 infections compared with the national average (12.9% vs. 10.2%) wrote Kate R. Woodworth, MD, and colleagues of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team.
The researchers collected information on pregnancy and infant outcomes from 16 jurisdictions through the Surveillance for Emerging Threats to Mothers and Babies Network (SET-NET). The study included 5,252 women with laboratory-confirmed SARS-CoV-2 infection reported during March 29–Oct. 14, 2020.
Overall, 12.9% of the 3,912 live births with known gestational age were preterm. A total of 610 infants were tested for SARS-CoV-2, and 2.6% were positive. Most of these perinatal infections (85%) occurred among infants born to women with SARS-CoV-2 infection within 1 week of delivery.
Half of the infants with positive test results were preterm, possibly reflecting higher screening rates in the ICU, the researchers said. “These findings also support the growing evidence that although severe COVID-19 does occur in neonates the majority of term neonates experience asymptomatic infection or mild disease; however, information on long term outcomes among exposed infants is unknown.”
Address disparities that amplify risk
The study findings were limited by several factors including inconsistent symptom reporting, overrepresentation of Hispanic women, and incomplete information on pregnancy loss, Dr. Woodworth and associates noted. However, the results add to the knowledge about the impact of COVID-19 disease on pregnancy by providing a large, population-based cohort with completed pregnancy outcomes as well as infant testing.
“SET-NET will continue to follow pregnancies affected by SARS-CoV-2 through completion of pregnancy and infants until age 6 months to guide clinical and public health practice,” the researchers noted. “Longer-term investigation into solutions to alleviate underlying inequities in social determinants of health associated with disparities in maternal morbidity, mortality, and adverse pregnancy outcomes, and effectively addressing these inequities, could reduce the prevalence of conditions and experiences that might amplify risks from COVID-19,” they added.
Severe disease and death increased in pregnant women
In a second study published in the MMWR, Laura D. Zambrano, PhD, and colleagues, also of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team, compared data on 23,434 reportedly pregnant and 386,028 nonpregnant women of reproductive age (15-44 years) with confirmed and symptomatic SARS-CoV-2 infections reported to the CDC between Jan. 22, 2020, and Oct. 3, 2020.
After adjustment for age, race, and underlying medical conditions, pregnant women with COVID-19 disease were significantly more likely than were nonpregnant women to be admitted to intensive care (10.5 per 1,000 cases vs. 3.9 per 1,000 cases), to receive invasive ventilation (2.9 vs. 1.1), receive extracorporeal membrane oxygenation (0.7 vs. 0.3) and to die (1.5 vs. 1.2).
“Irrespective of pregnancy status, ICU admissions, receipt of invasive ventilation, and death occurred more often among women aged 35-44 years than among those aged 15-24 years,” Dr. Zambrano and associates noted. In addition, non-Hispanic Black and Black women comprised 14.1% of the study population but accounted for 36.6% of deaths overall (9 in pregnant women and 167 in nonpregnant women).
The findings in the study of characteristics were limited by several factors including the voluntary reporting of COVID-19 cases, potential reporting bias, and inadequate time to assess severe cases, the researchers noted. However, “data from previous influenza pandemics, including 2009 H1N1, have shown that pregnant women are at increased risk for severe outcomes including death and the absolute risks for severe outcomes were higher than in this study of COVID-19 during pregnancy.”
“Pregnant women should be informed of their risk for severe COVID-19–associated illness and the warning signs of severe COVID-19,” Dr. Zambrano and associates said. “Providers who care for pregnant women should be familiar with guidelines for medical management of COVID-19, including considerations for management of COVID-19 in pregnancy.”
More data needed for informed counseling
“It is important to conduct research trials involving pregnant women so that we have reliable data regarding outcomes with which to counsel women,” Angela Bianco, MD, a maternal fetal medicine specialist at Mount Sinai Hospital in New York, said in an interview.
“Often pregnant women are excluded from research trials, but the impact of the current public health crisis affects all persons regardless of pregnancy status,” she said.
Dr. Bianco said that she was not surprised by the findings of either study. “In fact, our own research produced similar results.”
“These recent publications found that age-matched pregnant versus nonpregnant women had more severe manifestations of COVID-19, and specifically that pregnant women had a higher risk of requiring ventilation and intensive care admission, as well as higher risk of death,” she said. “Previous studies examining the effect of other SARS viruses have demonstrated that pregnancy is associated with worse outcomes; these findings are likely attributable to the relative state of immunosuppression in pregnancy.” Also, “one of these trials found a greater risk of premature birth in women with COVID-19; this may largely be attributable to iatrogenic delivery due to maternal illness as opposed to spontaneous preterm birth,” Dr. Bianco explained.
“Data are emerging regarding the impact of SARS-CoV-2 on pregnancy outcomes, however information remains limited,” Dr. Bianco noted. “Clinicians need to make patients aware that SARS-CoV-2 infection during pregnancy is associated with a greater risk of severe illness requiring intensive care and/or ventilatory support and even death; however, the precise rates remain unknown. “COVID-19 during pregnancy may result in a preterm birth, but at this time the rate of fetal infection remains unknown,” she said. “Clinicians need to reinforce the importance of physical distancing, mask use, and proper hand hygiene, particularly in this vulnerable population.”
Dr. Bianco emphasized: “Longitudinal studies assessing the impact of SARS-CoV-2 infection at various gestational age periods are needed, as at this time most of the available data includes women with SARS-CoV-2 infection around the time of delivery. Long-term infant outcomes are needed, as well as studies assessing the risk of fetal infection.”
The studies were supported by the Centers for Disease Control and Prevention. The researchers had no financial conflicts to disclose. Dr. Bianco had no relevant financial disclosures.
SOURCE: Woodworth KR et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e2; Zambrano LD et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e3.
based on data from two studies published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.
In a study of birth and infant outcomes, rates of preterm birth (less than 37 weeks’ gestational age) were higher among women with confirmed SARS-CoV-2 infections compared with the national average (12.9% vs. 10.2%) wrote Kate R. Woodworth, MD, and colleagues of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team.
The researchers collected information on pregnancy and infant outcomes from 16 jurisdictions through the Surveillance for Emerging Threats to Mothers and Babies Network (SET-NET). The study included 5,252 women with laboratory-confirmed SARS-CoV-2 infection reported during March 29–Oct. 14, 2020.
Overall, 12.9% of the 3,912 live births with known gestational age were preterm. A total of 610 infants were tested for SARS-CoV-2, and 2.6% were positive. Most of these perinatal infections (85%) occurred among infants born to women with SARS-CoV-2 infection within 1 week of delivery.
Half of the infants with positive test results were preterm, possibly reflecting higher screening rates in the ICU, the researchers said. “These findings also support the growing evidence that although severe COVID-19 does occur in neonates the majority of term neonates experience asymptomatic infection or mild disease; however, information on long term outcomes among exposed infants is unknown.”
Address disparities that amplify risk
The study findings were limited by several factors including inconsistent symptom reporting, overrepresentation of Hispanic women, and incomplete information on pregnancy loss, Dr. Woodworth and associates noted. However, the results add to the knowledge about the impact of COVID-19 disease on pregnancy by providing a large, population-based cohort with completed pregnancy outcomes as well as infant testing.
“SET-NET will continue to follow pregnancies affected by SARS-CoV-2 through completion of pregnancy and infants until age 6 months to guide clinical and public health practice,” the researchers noted. “Longer-term investigation into solutions to alleviate underlying inequities in social determinants of health associated with disparities in maternal morbidity, mortality, and adverse pregnancy outcomes, and effectively addressing these inequities, could reduce the prevalence of conditions and experiences that might amplify risks from COVID-19,” they added.
Severe disease and death increased in pregnant women
In a second study published in the MMWR, Laura D. Zambrano, PhD, and colleagues, also of the CDC COVID-19 Response Pregnancy and Linked Outcomes Team, compared data on 23,434 reportedly pregnant and 386,028 nonpregnant women of reproductive age (15-44 years) with confirmed and symptomatic SARS-CoV-2 infections reported to the CDC between Jan. 22, 2020, and Oct. 3, 2020.
After adjustment for age, race, and underlying medical conditions, pregnant women with COVID-19 disease were significantly more likely than were nonpregnant women to be admitted to intensive care (10.5 per 1,000 cases vs. 3.9 per 1,000 cases), to receive invasive ventilation (2.9 vs. 1.1), receive extracorporeal membrane oxygenation (0.7 vs. 0.3) and to die (1.5 vs. 1.2).
“Irrespective of pregnancy status, ICU admissions, receipt of invasive ventilation, and death occurred more often among women aged 35-44 years than among those aged 15-24 years,” Dr. Zambrano and associates noted. In addition, non-Hispanic Black and Black women comprised 14.1% of the study population but accounted for 36.6% of deaths overall (9 in pregnant women and 167 in nonpregnant women).
The findings in the study of characteristics were limited by several factors including the voluntary reporting of COVID-19 cases, potential reporting bias, and inadequate time to assess severe cases, the researchers noted. However, “data from previous influenza pandemics, including 2009 H1N1, have shown that pregnant women are at increased risk for severe outcomes including death and the absolute risks for severe outcomes were higher than in this study of COVID-19 during pregnancy.”
“Pregnant women should be informed of their risk for severe COVID-19–associated illness and the warning signs of severe COVID-19,” Dr. Zambrano and associates said. “Providers who care for pregnant women should be familiar with guidelines for medical management of COVID-19, including considerations for management of COVID-19 in pregnancy.”
More data needed for informed counseling
“It is important to conduct research trials involving pregnant women so that we have reliable data regarding outcomes with which to counsel women,” Angela Bianco, MD, a maternal fetal medicine specialist at Mount Sinai Hospital in New York, said in an interview.
“Often pregnant women are excluded from research trials, but the impact of the current public health crisis affects all persons regardless of pregnancy status,” she said.
Dr. Bianco said that she was not surprised by the findings of either study. “In fact, our own research produced similar results.”
“These recent publications found that age-matched pregnant versus nonpregnant women had more severe manifestations of COVID-19, and specifically that pregnant women had a higher risk of requiring ventilation and intensive care admission, as well as higher risk of death,” she said. “Previous studies examining the effect of other SARS viruses have demonstrated that pregnancy is associated with worse outcomes; these findings are likely attributable to the relative state of immunosuppression in pregnancy.” Also, “one of these trials found a greater risk of premature birth in women with COVID-19; this may largely be attributable to iatrogenic delivery due to maternal illness as opposed to spontaneous preterm birth,” Dr. Bianco explained.
“Data are emerging regarding the impact of SARS-CoV-2 on pregnancy outcomes, however information remains limited,” Dr. Bianco noted. “Clinicians need to make patients aware that SARS-CoV-2 infection during pregnancy is associated with a greater risk of severe illness requiring intensive care and/or ventilatory support and even death; however, the precise rates remain unknown. “COVID-19 during pregnancy may result in a preterm birth, but at this time the rate of fetal infection remains unknown,” she said. “Clinicians need to reinforce the importance of physical distancing, mask use, and proper hand hygiene, particularly in this vulnerable population.”
Dr. Bianco emphasized: “Longitudinal studies assessing the impact of SARS-CoV-2 infection at various gestational age periods are needed, as at this time most of the available data includes women with SARS-CoV-2 infection around the time of delivery. Long-term infant outcomes are needed, as well as studies assessing the risk of fetal infection.”
The studies were supported by the Centers for Disease Control and Prevention. The researchers had no financial conflicts to disclose. Dr. Bianco had no relevant financial disclosures.
SOURCE: Woodworth KR et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e2; Zambrano LD et al. MMWR. 2020 Nov 2. doi: 10.15585/mmwr.mm6944e3.
FROM MMWR
Proposed withdrawal of approval of preterm drug: Two opposing views
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FDA proposes withdrawing Makena’s approval
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.