User login
Medicare may best Medicare Advantage at reducing readmissions
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
FROM ANNALS OF INTERNAL MEDICINE
Most measles cases in 25 years prompts government pleas to vaccinate
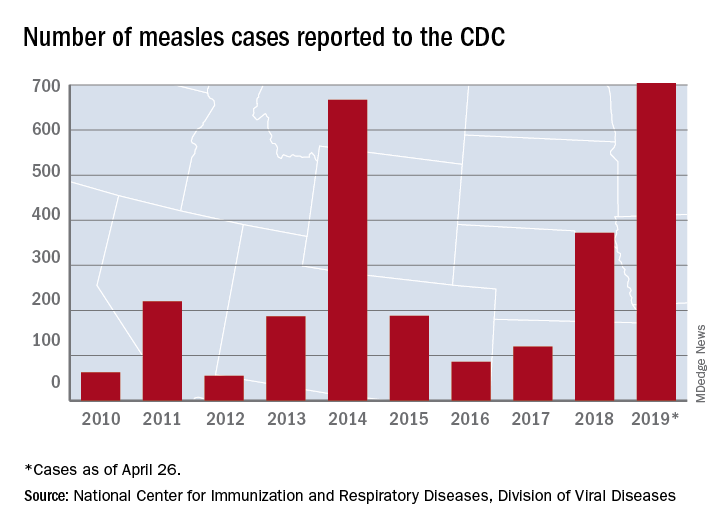
The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”
FROM A CDC TELEBRIEFING
Ibrexafungerp effective against C. auris in two early case reports
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
FROM ECCMID 2019
Diagnostic test helps clinicians identify IPF with nonsurgical biopsy
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
FROM THE LANCET RESPIRATORY MEDICINE
No increase in severe community-acquired pneumonia after PCV13
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Pediatric community-acquired pneumonia cases dropped from 6.3 to 3.5 cases per 1,000 visits from 2010 to 2014 and increased to 3.8 cases per 1,000 visits in May 2017.
Study details: The findings are based on a prospective time series analysis of 12,587 pediatric pneumonia cases (under 15 years old) in eight French emergency departments from June 2009 to May 2017.
Disclosures: Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research, and a Pfizer Investigator Initiated Research grant. Dr. Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received nonfinancial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer, and/or Sanofi Pasteur.
Source: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Prescribed opioids increase pneumonia risk in patients with, without HIV
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Prescribed opioids, especially those with immunosuppressive properties, are associated with increased community-acquired pneumonia risk.
Major finding: For currently prescribed immunosuppressive opioids, the adjusted odds ratio for community-acquired pneumonia was 3.18 (95% confidence interval, 2.44-4.14).
Study details: A nested case-control study of 25,392 patients in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Disclosures: Funding was provided by a variety of government organizations and Yale University, New Haven, Conn. The authors reported that they had no conflicts.
Source: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Children who are coughing: Is it flu or bacterial pneumonia?
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
Hospital Readmissions Reduction Program may be doing more harm than good
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
FROM JAMA
Key clinical point:
Major finding: Heart failure patients saw mortality increase 0.52% after HRRP launched.
Study details: A retrospective cohort study across 10 years, including time before and after the implementation of the HRRP.
Disclosures: The Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology funded the study. No relevant conflicts of interest were disclosed.
Source: Wadhera R et al. JAMA 2018 Dec 25. doi: 10.1001/jama.2018.19232.
Guideline-concordant treatment still unlikely in nonchildren’s hospitals for pediatric CAP
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
FROM JAMA PEDIATRICS
ICU-acquired pneumonia mortality risk may be underestimated
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
FROM CRITICAL CARE MEDICINE
Key clinical point: Hospital-acquired pneumonia poses a greater risk of death in the ICU than ventilator-associated pneumonia.
Major finding: After prognostic adjustment, the mortality hazard ratios were 1.82 and 1.38 for HAP and VAP, respectively.
Study details: Observational cohort study.
Disclosures: The researchers had no disclosures.
Source: Saied WI et al. Crit Care Med. 2018 Nov 7; doi: 10.1097/CCM.0000000000003553.