User login
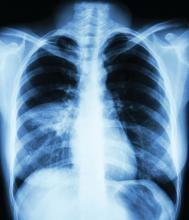
Mystery pneumonia in China has health officials on alert
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Despite PCV, pediatric asthma patients face pneumococcal risks
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
FROM PEDIATRICS
ART treatment at birth found to benefit neonates with HIV
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Antiretroviral treatment initiation immediately after birth reduced HIV-1 viral reservoir size and alters innate immune responses in neonates.
Major finding: Very-early ART intervention in neonates infected with HIV limited the number of virally infected cells and improves immune response.
Study details: A cohort study of 10 infants infected with HIV who were born in Botswana, Africa.
Disclosures: The Early Infant Treatment study is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
Source: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
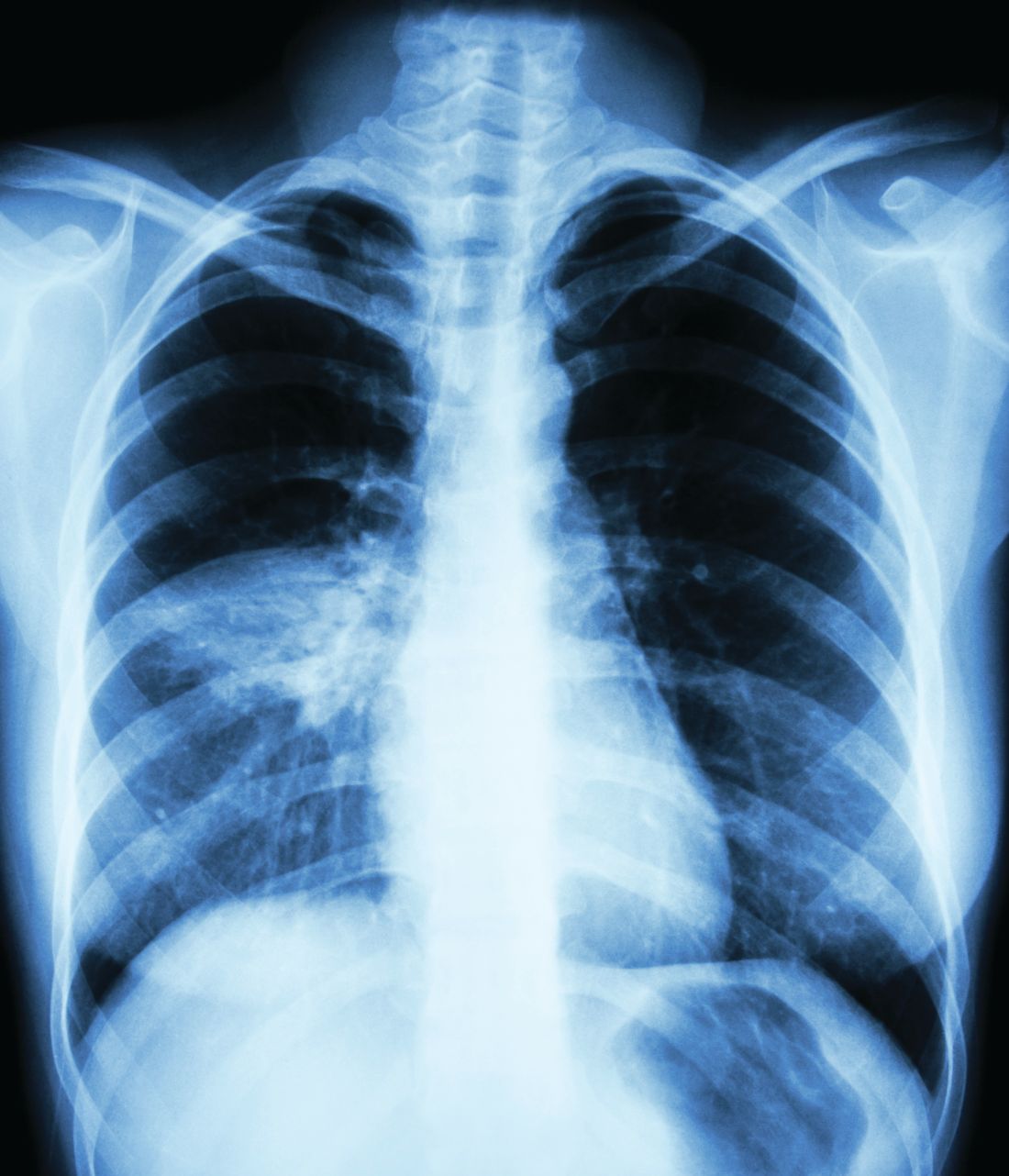
Guideline: Diagnosis and treatment of adults with community-acquired pneumonia
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
In-hospital flu shot reduced readmissions in pneumonia patients
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
REPORTING FROM CHEST 2019
IPD in children may be a signal of immunodeficiency
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
FROM JAMA PEDIATRICS
Lefamulin found noninferior to moxifloxacin for bacterial pneumonia
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
FROM JAMA
FDA approves Xenleta for community-acquired bacterial pneumonia treatment
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
Patients with COPD at heightened risk for community-acquired pneumonia requiring hospitalization
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
FROM CLINICAL MICROBIOLOGY AND INFECTION
ACIP favors shared decision on pneumococcal vaccine for older adults
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING