User login
Calcium Protective Against Hyperparathyroidism
Increased calcium intake is associated with a reduced risk for primary hyperparathyroidism in women, according to an analysis of data from the Nurses’ Health Study published on Oct. 18.
In what the researchers called the first prospective study to assess the link between hyperparathyroidism and calcium intake, Dr. Julie M. Paik of Brigham and Women’s Hospital in Boston and her colleagues looked at 58,354 women from the Nurses’ Health Study I, an ongoing, prospective cohort study that began in 1976. Participants in the study complete questionnaires every 2 years.
Dietary intake was assessed using the food frequency questionnaire, which asked about participants’ average intake of more than 130 individual food items and 22 beverages during the previous year.
The survey also asked about calcium supplements, vitamin D supplements, and multivitamins, with calcium intake determined by the brand, type, and frequency of use.
Only cases of primary hyperparathyroidism diagnosed between the date on which the 1986 questionnaire was returned and May 31, 2008, were included in the study.
Overall, during 1,475,978 person-years of follow-up, there were 277 cases of incident primary hyperparathyroidism.
After the data were adjusted for age, women in the highest quintile of dietary intake had a relative risk for developing hyperparathyroidism of 0.61, compared with women in the lowest quintile (95% confidence interval, 0.42-0.90; P = .03), the investigators reported (BMJ 2012;345:e6390).
Further adjustments for body mass index, race, smoking, calcium supplement use, intake of vitamin D, dietary intake of vitamin A and protein, alcohol intake, and diuretic use only strengthened the effect: The relative risk among the highest quintile of intake was 0.56, by this calculation, compared with patients in the lowest intake category (95% CI, 0.37-0.86; P = .009).
The authors also looked at supplemental calcium intake, although "because of an insufficient number of cases of primary hyperparathyroidism among the different categories of supplemental calcium use in the earlier time periods, our analysis on the relation between supplemental calcium intake and primary hyperparathyroidism began in 1994 with follow-up until 2008," wrote Dr. Paik and her coinvestigators.
In this analysis, which included 985,628 person-years of follow-up, there were a total of 257 cases of incident primary hyperparathyroidism.
When the findings were adjusted for age, "the relative risk for women taking more than 500 mg/day of calcium supplements compared with those not taking calcium supplements was 0.69 (95% CI, 0.50-0.94; P less than .001)," wrote the authors.
As with dietary intake, the protective effect was only strengthened in multivariable analysis, including adjustment for dietary calcium: The higher-intake group’s risk was 0.41, compared with the lower-intake group (95% CI, 0.29-0.60; P less than .001).
The authors conceded that the study population was exclusively female and almost entirely white, making the findings not necessarily generalizable to men or to women of other races.
Additionally, "although the food frequency questionnaires have been well validated, calcium intake was not perfectly assessed in this study," they wrote.
"However, because of the prospective design, any misclassification would be random with respect to case status, and therefore would probably underestimate the magnitude of the inverse association between calcium intake and risk of primary hyperparathyroidism."
The study investigators stated that they had no relevant financial disclosures. The study was supported by grants from the National Institutes of Health.
"Most clinicians do advise patients to take adequate calcium and have done [so] for decades, given its known benefits on bone health," wrote Dr. James Norman.
In recent years, however, this advice has come into question, "as evidence has emerged that taking supplemental calcium may be associated with a higher incidence of myocardial infarction."
Indeed, "some patients now question whether they should take supplemental calcium even when they have overt osteoporosis," he added.
In the current study, "Paik and colleagues found that even a dose as low as 500 mg/day was associated with a decreased incidence of primary hyperparathyroidism. This is important because such a modest dose is less likely to be controversial."
He added that the study "provides evidence to support physicians in confidently encouraging female patients to take calcium supplements."
Dr. Norman is chief of surgery at the Norman parathyroid center at Tampa General Hospital. These remarks were adapted from his editorial accompanying the article (BMJ 2012;345:e6646). He disclosed having no financial conflicts associated with this editorial.
"Most clinicians do advise patients to take adequate calcium and have done [so] for decades, given its known benefits on bone health," wrote Dr. James Norman.
In recent years, however, this advice has come into question, "as evidence has emerged that taking supplemental calcium may be associated with a higher incidence of myocardial infarction."
Indeed, "some patients now question whether they should take supplemental calcium even when they have overt osteoporosis," he added.
In the current study, "Paik and colleagues found that even a dose as low as 500 mg/day was associated with a decreased incidence of primary hyperparathyroidism. This is important because such a modest dose is less likely to be controversial."
He added that the study "provides evidence to support physicians in confidently encouraging female patients to take calcium supplements."
Dr. Norman is chief of surgery at the Norman parathyroid center at Tampa General Hospital. These remarks were adapted from his editorial accompanying the article (BMJ 2012;345:e6646). He disclosed having no financial conflicts associated with this editorial.
"Most clinicians do advise patients to take adequate calcium and have done [so] for decades, given its known benefits on bone health," wrote Dr. James Norman.
In recent years, however, this advice has come into question, "as evidence has emerged that taking supplemental calcium may be associated with a higher incidence of myocardial infarction."
Indeed, "some patients now question whether they should take supplemental calcium even when they have overt osteoporosis," he added.
In the current study, "Paik and colleagues found that even a dose as low as 500 mg/day was associated with a decreased incidence of primary hyperparathyroidism. This is important because such a modest dose is less likely to be controversial."
He added that the study "provides evidence to support physicians in confidently encouraging female patients to take calcium supplements."
Dr. Norman is chief of surgery at the Norman parathyroid center at Tampa General Hospital. These remarks were adapted from his editorial accompanying the article (BMJ 2012;345:e6646). He disclosed having no financial conflicts associated with this editorial.
Increased calcium intake is associated with a reduced risk for primary hyperparathyroidism in women, according to an analysis of data from the Nurses’ Health Study published on Oct. 18.
In what the researchers called the first prospective study to assess the link between hyperparathyroidism and calcium intake, Dr. Julie M. Paik of Brigham and Women’s Hospital in Boston and her colleagues looked at 58,354 women from the Nurses’ Health Study I, an ongoing, prospective cohort study that began in 1976. Participants in the study complete questionnaires every 2 years.
Dietary intake was assessed using the food frequency questionnaire, which asked about participants’ average intake of more than 130 individual food items and 22 beverages during the previous year.
The survey also asked about calcium supplements, vitamin D supplements, and multivitamins, with calcium intake determined by the brand, type, and frequency of use.
Only cases of primary hyperparathyroidism diagnosed between the date on which the 1986 questionnaire was returned and May 31, 2008, were included in the study.
Overall, during 1,475,978 person-years of follow-up, there were 277 cases of incident primary hyperparathyroidism.
After the data were adjusted for age, women in the highest quintile of dietary intake had a relative risk for developing hyperparathyroidism of 0.61, compared with women in the lowest quintile (95% confidence interval, 0.42-0.90; P = .03), the investigators reported (BMJ 2012;345:e6390).
Further adjustments for body mass index, race, smoking, calcium supplement use, intake of vitamin D, dietary intake of vitamin A and protein, alcohol intake, and diuretic use only strengthened the effect: The relative risk among the highest quintile of intake was 0.56, by this calculation, compared with patients in the lowest intake category (95% CI, 0.37-0.86; P = .009).
The authors also looked at supplemental calcium intake, although "because of an insufficient number of cases of primary hyperparathyroidism among the different categories of supplemental calcium use in the earlier time periods, our analysis on the relation between supplemental calcium intake and primary hyperparathyroidism began in 1994 with follow-up until 2008," wrote Dr. Paik and her coinvestigators.
In this analysis, which included 985,628 person-years of follow-up, there were a total of 257 cases of incident primary hyperparathyroidism.
When the findings were adjusted for age, "the relative risk for women taking more than 500 mg/day of calcium supplements compared with those not taking calcium supplements was 0.69 (95% CI, 0.50-0.94; P less than .001)," wrote the authors.
As with dietary intake, the protective effect was only strengthened in multivariable analysis, including adjustment for dietary calcium: The higher-intake group’s risk was 0.41, compared with the lower-intake group (95% CI, 0.29-0.60; P less than .001).
The authors conceded that the study population was exclusively female and almost entirely white, making the findings not necessarily generalizable to men or to women of other races.
Additionally, "although the food frequency questionnaires have been well validated, calcium intake was not perfectly assessed in this study," they wrote.
"However, because of the prospective design, any misclassification would be random with respect to case status, and therefore would probably underestimate the magnitude of the inverse association between calcium intake and risk of primary hyperparathyroidism."
The study investigators stated that they had no relevant financial disclosures. The study was supported by grants from the National Institutes of Health.
Increased calcium intake is associated with a reduced risk for primary hyperparathyroidism in women, according to an analysis of data from the Nurses’ Health Study published on Oct. 18.
In what the researchers called the first prospective study to assess the link between hyperparathyroidism and calcium intake, Dr. Julie M. Paik of Brigham and Women’s Hospital in Boston and her colleagues looked at 58,354 women from the Nurses’ Health Study I, an ongoing, prospective cohort study that began in 1976. Participants in the study complete questionnaires every 2 years.
Dietary intake was assessed using the food frequency questionnaire, which asked about participants’ average intake of more than 130 individual food items and 22 beverages during the previous year.
The survey also asked about calcium supplements, vitamin D supplements, and multivitamins, with calcium intake determined by the brand, type, and frequency of use.
Only cases of primary hyperparathyroidism diagnosed between the date on which the 1986 questionnaire was returned and May 31, 2008, were included in the study.
Overall, during 1,475,978 person-years of follow-up, there were 277 cases of incident primary hyperparathyroidism.
After the data were adjusted for age, women in the highest quintile of dietary intake had a relative risk for developing hyperparathyroidism of 0.61, compared with women in the lowest quintile (95% confidence interval, 0.42-0.90; P = .03), the investigators reported (BMJ 2012;345:e6390).
Further adjustments for body mass index, race, smoking, calcium supplement use, intake of vitamin D, dietary intake of vitamin A and protein, alcohol intake, and diuretic use only strengthened the effect: The relative risk among the highest quintile of intake was 0.56, by this calculation, compared with patients in the lowest intake category (95% CI, 0.37-0.86; P = .009).
The authors also looked at supplemental calcium intake, although "because of an insufficient number of cases of primary hyperparathyroidism among the different categories of supplemental calcium use in the earlier time periods, our analysis on the relation between supplemental calcium intake and primary hyperparathyroidism began in 1994 with follow-up until 2008," wrote Dr. Paik and her coinvestigators.
In this analysis, which included 985,628 person-years of follow-up, there were a total of 257 cases of incident primary hyperparathyroidism.
When the findings were adjusted for age, "the relative risk for women taking more than 500 mg/day of calcium supplements compared with those not taking calcium supplements was 0.69 (95% CI, 0.50-0.94; P less than .001)," wrote the authors.
As with dietary intake, the protective effect was only strengthened in multivariable analysis, including adjustment for dietary calcium: The higher-intake group’s risk was 0.41, compared with the lower-intake group (95% CI, 0.29-0.60; P less than .001).
The authors conceded that the study population was exclusively female and almost entirely white, making the findings not necessarily generalizable to men or to women of other races.
Additionally, "although the food frequency questionnaires have been well validated, calcium intake was not perfectly assessed in this study," they wrote.
"However, because of the prospective design, any misclassification would be random with respect to case status, and therefore would probably underestimate the magnitude of the inverse association between calcium intake and risk of primary hyperparathyroidism."
The study investigators stated that they had no relevant financial disclosures. The study was supported by grants from the National Institutes of Health.
FROM BMJ
Major Finding: Women in the highest quintile of dietary calcium intake had a relative risk for developing hyperparathyroidism of 0.61, compared with women in the lowest quintile.
Data Source: Data are from a subcohort of more than 58,000 participants from the Nurses’ Health Study I.
Disclosures: The researchers stated that they had no relevant financial conflicts. The study was supported by grants from the National Institutes of Health.
Resistant Hypothyroidism? Consider Adding Liothyronine
ESTES PARK, COLO. – Adding liothyronine is a reasonable treatment strategy when symptoms of hypothyroidism persist on optimal levothyroxine alone, according to Dr. Michael T. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at the University of Colorado Hospital, Aurora.
Combination levothyroxine/liothyronine (LT4/LT3) therapy is supported by a biologically plausible mechanism of benefit in symptomatic patients who are biochemically euthyroid on LT4. But it’s a step that belongs near the bottom of the management plan for the difficult hypothyroid patient. It should be considered only after other actions have been taken, including a search for coexisting autoimmune conditions or other medical illnesses, Dr. McDermott said at an update on internal medicine sponsored by the University of Colorado.
Every physician who treats hypothyroidism has patients who experience lingering fatigue, memory problems, and other symptoms even though their on-treatment TSH is in the target range of 0.5-2.0 mU/L. Five published studies underscore just how common this situation is. Dr. McDermott cited as an example a study of 397 hypothyroid patients with a TSH in the goal range; 34% scored in the abnormal range on the short-form General Health Questionnaire and 49% had elevated scores on a thyroid symptom-specific questionnaire (Clin. Endocrinol. [Oxf.] 2002;57:577-85).
These are patients who require further general medical evaluation. Their symptoms may be due to a coexistent autoimmune disease. After all, autoimmune diseases tend to cluster. Indeed, when British investigators did a systematic work-up of 495 patients with Hashimoto’s thyroiditis, they found a 14% prevalence of another autoimmune disease. Leading the way was rheumatoid arthritis, present in 4% of subjects. The Hashimoto’s thyroiditis group also had greater than 10-fold increased relative risks of celiac disease, vitiligo, SLE, and Addison’s disease (Am. J. Med. 2010;183:e1-9).
Alternatively, the patient’s lingering symptoms may be due in part to Hashimoto’s thyroiditis itself rather than to hypothyroidism per se. A study of 426 euthyroid women with goiter undergoing thyroidectomy showed that those with antithyroperoxidase antibodies had higher levels of fatigue, nervousness, and irritability, and lower quality of life (Thyroid 2011;21:161-7).
Dr. McDermott routinely obtains a serum 25 vitamin D measurement in his difficult cases of hypothyroidism, since low vitamin D levels are a common cause of fatigue. He also encourages patients with lingering symptoms to eat a well-balanced diet and get regular exercise and sleep, and he refers them for treatment of depression when indicated.
Only after doing all that, does he consider adding LT3. Combined LT4/LT3 therapy is controversial. The first-ever randomized controlled clinical trial was positive (N. Engl. J. Med. 1999;340:424-9), but it was followed by a spate of negative studies. Some physicians closed the book on this treatment strategy after a meta-analysis of all 11 randomized trials showed combination therapy was without benefit (J. Clin. Endocrinol. Metab. 2006; 91: 2,592-9).
However, more recent work has shown that a relatively common polymorphism of the deiodinase 2 gene known as Thr92Ala may predict less responsiveness of psychological symptoms of hypothyroidism to optimal LT4 monotherapy. Deiodinase 2 is responsible for maintaining brain T3 levels, and there is evidence to indicate that the Thr92Ala polymorphism subtly impairs T4 to T3 conversion in the brain.
In a secondary analysis of a study involving 552 hypothyroid patients randomized to LT4 or LT4/LT3, the prevalence of Thr92Ala homozygosity was 16%, and psychological well being in patients with the deiodinase 2 polymorphism improved significantly more on combination therapy than with LT4 alone (J. Clin. Endocrinol. Metab. 2009;94:1623-9).
The fact that the Thr92Ala polymorphism is present in only 16% of individuals on thyroid hormone therapy might explain why so many randomized trials of LT4 versus combination therapy were negative: With study populations of only 20-141 patients, the trials would have been underpowered to detect a significant difference in treatment effect. Unfortunately, genetic testing for deiodinase polymorphisms is not commercially available, the endocrinologist observed.
When he does resort to combination therapy, Dr. McDermott prescribes it in an LT4:LT3 ratio of 10-14:1 to mimic normal thyroid secretion. He generally has patients take LT3 twice daily, with the second dose no later than about 6 p.m. so it doesn’t interfere with sleep. Once-daily slow-release formulations of LT3 are available in Europe and work very well. Several companies are interested in developing a slow-release LT3 for the United States, which would be a welcome development, according to Dr. McDermott.
Another option, once all else has been tried and failed, is to switch to another brand of LT4, he continued. Some patients may have adverse reactions to the various dyes and fillers contained in LT4 pills. When this is a potential concern, levothyroxine sodium (Tirosint), approved by the Food and Drug Administration a couple of years ago, is an attractive option. The LT4 in Tirosint is contained in oil in a liquid gelcap with no dyes or fillers, differentiating it from all other brand name and generic products, Dr. McDermott noted.
He emphasized the importance of avoiding overtreatment with LT4 in an attempt to improve quality of life in patients with residual symptoms despite a TSH of 0.5-2.0 mU/L. Subclinical hyperthyroidism as defined by a TSH below 0.1 mU/L has been shown to significantly increase the risk of hip and spine fractures, atrial fibrillation, and cardiovascular mortality.
Dr. McDermott reported having no financial conflicts.
ESTES PARK, COLO. – Adding liothyronine is a reasonable treatment strategy when symptoms of hypothyroidism persist on optimal levothyroxine alone, according to Dr. Michael T. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at the University of Colorado Hospital, Aurora.
Combination levothyroxine/liothyronine (LT4/LT3) therapy is supported by a biologically plausible mechanism of benefit in symptomatic patients who are biochemically euthyroid on LT4. But it’s a step that belongs near the bottom of the management plan for the difficult hypothyroid patient. It should be considered only after other actions have been taken, including a search for coexisting autoimmune conditions or other medical illnesses, Dr. McDermott said at an update on internal medicine sponsored by the University of Colorado.
Every physician who treats hypothyroidism has patients who experience lingering fatigue, memory problems, and other symptoms even though their on-treatment TSH is in the target range of 0.5-2.0 mU/L. Five published studies underscore just how common this situation is. Dr. McDermott cited as an example a study of 397 hypothyroid patients with a TSH in the goal range; 34% scored in the abnormal range on the short-form General Health Questionnaire and 49% had elevated scores on a thyroid symptom-specific questionnaire (Clin. Endocrinol. [Oxf.] 2002;57:577-85).
These are patients who require further general medical evaluation. Their symptoms may be due to a coexistent autoimmune disease. After all, autoimmune diseases tend to cluster. Indeed, when British investigators did a systematic work-up of 495 patients with Hashimoto’s thyroiditis, they found a 14% prevalence of another autoimmune disease. Leading the way was rheumatoid arthritis, present in 4% of subjects. The Hashimoto’s thyroiditis group also had greater than 10-fold increased relative risks of celiac disease, vitiligo, SLE, and Addison’s disease (Am. J. Med. 2010;183:e1-9).
Alternatively, the patient’s lingering symptoms may be due in part to Hashimoto’s thyroiditis itself rather than to hypothyroidism per se. A study of 426 euthyroid women with goiter undergoing thyroidectomy showed that those with antithyroperoxidase antibodies had higher levels of fatigue, nervousness, and irritability, and lower quality of life (Thyroid 2011;21:161-7).
Dr. McDermott routinely obtains a serum 25 vitamin D measurement in his difficult cases of hypothyroidism, since low vitamin D levels are a common cause of fatigue. He also encourages patients with lingering symptoms to eat a well-balanced diet and get regular exercise and sleep, and he refers them for treatment of depression when indicated.
Only after doing all that, does he consider adding LT3. Combined LT4/LT3 therapy is controversial. The first-ever randomized controlled clinical trial was positive (N. Engl. J. Med. 1999;340:424-9), but it was followed by a spate of negative studies. Some physicians closed the book on this treatment strategy after a meta-analysis of all 11 randomized trials showed combination therapy was without benefit (J. Clin. Endocrinol. Metab. 2006; 91: 2,592-9).
However, more recent work has shown that a relatively common polymorphism of the deiodinase 2 gene known as Thr92Ala may predict less responsiveness of psychological symptoms of hypothyroidism to optimal LT4 monotherapy. Deiodinase 2 is responsible for maintaining brain T3 levels, and there is evidence to indicate that the Thr92Ala polymorphism subtly impairs T4 to T3 conversion in the brain.
In a secondary analysis of a study involving 552 hypothyroid patients randomized to LT4 or LT4/LT3, the prevalence of Thr92Ala homozygosity was 16%, and psychological well being in patients with the deiodinase 2 polymorphism improved significantly more on combination therapy than with LT4 alone (J. Clin. Endocrinol. Metab. 2009;94:1623-9).
The fact that the Thr92Ala polymorphism is present in only 16% of individuals on thyroid hormone therapy might explain why so many randomized trials of LT4 versus combination therapy were negative: With study populations of only 20-141 patients, the trials would have been underpowered to detect a significant difference in treatment effect. Unfortunately, genetic testing for deiodinase polymorphisms is not commercially available, the endocrinologist observed.
When he does resort to combination therapy, Dr. McDermott prescribes it in an LT4:LT3 ratio of 10-14:1 to mimic normal thyroid secretion. He generally has patients take LT3 twice daily, with the second dose no later than about 6 p.m. so it doesn’t interfere with sleep. Once-daily slow-release formulations of LT3 are available in Europe and work very well. Several companies are interested in developing a slow-release LT3 for the United States, which would be a welcome development, according to Dr. McDermott.
Another option, once all else has been tried and failed, is to switch to another brand of LT4, he continued. Some patients may have adverse reactions to the various dyes and fillers contained in LT4 pills. When this is a potential concern, levothyroxine sodium (Tirosint), approved by the Food and Drug Administration a couple of years ago, is an attractive option. The LT4 in Tirosint is contained in oil in a liquid gelcap with no dyes or fillers, differentiating it from all other brand name and generic products, Dr. McDermott noted.
He emphasized the importance of avoiding overtreatment with LT4 in an attempt to improve quality of life in patients with residual symptoms despite a TSH of 0.5-2.0 mU/L. Subclinical hyperthyroidism as defined by a TSH below 0.1 mU/L has been shown to significantly increase the risk of hip and spine fractures, atrial fibrillation, and cardiovascular mortality.
Dr. McDermott reported having no financial conflicts.
ESTES PARK, COLO. – Adding liothyronine is a reasonable treatment strategy when symptoms of hypothyroidism persist on optimal levothyroxine alone, according to Dr. Michael T. McDermott, professor of medicine and clinical pharmacology and director of endocrinology and diabetes practice at the University of Colorado Hospital, Aurora.
Combination levothyroxine/liothyronine (LT4/LT3) therapy is supported by a biologically plausible mechanism of benefit in symptomatic patients who are biochemically euthyroid on LT4. But it’s a step that belongs near the bottom of the management plan for the difficult hypothyroid patient. It should be considered only after other actions have been taken, including a search for coexisting autoimmune conditions or other medical illnesses, Dr. McDermott said at an update on internal medicine sponsored by the University of Colorado.
Every physician who treats hypothyroidism has patients who experience lingering fatigue, memory problems, and other symptoms even though their on-treatment TSH is in the target range of 0.5-2.0 mU/L. Five published studies underscore just how common this situation is. Dr. McDermott cited as an example a study of 397 hypothyroid patients with a TSH in the goal range; 34% scored in the abnormal range on the short-form General Health Questionnaire and 49% had elevated scores on a thyroid symptom-specific questionnaire (Clin. Endocrinol. [Oxf.] 2002;57:577-85).
These are patients who require further general medical evaluation. Their symptoms may be due to a coexistent autoimmune disease. After all, autoimmune diseases tend to cluster. Indeed, when British investigators did a systematic work-up of 495 patients with Hashimoto’s thyroiditis, they found a 14% prevalence of another autoimmune disease. Leading the way was rheumatoid arthritis, present in 4% of subjects. The Hashimoto’s thyroiditis group also had greater than 10-fold increased relative risks of celiac disease, vitiligo, SLE, and Addison’s disease (Am. J. Med. 2010;183:e1-9).
Alternatively, the patient’s lingering symptoms may be due in part to Hashimoto’s thyroiditis itself rather than to hypothyroidism per se. A study of 426 euthyroid women with goiter undergoing thyroidectomy showed that those with antithyroperoxidase antibodies had higher levels of fatigue, nervousness, and irritability, and lower quality of life (Thyroid 2011;21:161-7).
Dr. McDermott routinely obtains a serum 25 vitamin D measurement in his difficult cases of hypothyroidism, since low vitamin D levels are a common cause of fatigue. He also encourages patients with lingering symptoms to eat a well-balanced diet and get regular exercise and sleep, and he refers them for treatment of depression when indicated.
Only after doing all that, does he consider adding LT3. Combined LT4/LT3 therapy is controversial. The first-ever randomized controlled clinical trial was positive (N. Engl. J. Med. 1999;340:424-9), but it was followed by a spate of negative studies. Some physicians closed the book on this treatment strategy after a meta-analysis of all 11 randomized trials showed combination therapy was without benefit (J. Clin. Endocrinol. Metab. 2006; 91: 2,592-9).
However, more recent work has shown that a relatively common polymorphism of the deiodinase 2 gene known as Thr92Ala may predict less responsiveness of psychological symptoms of hypothyroidism to optimal LT4 monotherapy. Deiodinase 2 is responsible for maintaining brain T3 levels, and there is evidence to indicate that the Thr92Ala polymorphism subtly impairs T4 to T3 conversion in the brain.
In a secondary analysis of a study involving 552 hypothyroid patients randomized to LT4 or LT4/LT3, the prevalence of Thr92Ala homozygosity was 16%, and psychological well being in patients with the deiodinase 2 polymorphism improved significantly more on combination therapy than with LT4 alone (J. Clin. Endocrinol. Metab. 2009;94:1623-9).
The fact that the Thr92Ala polymorphism is present in only 16% of individuals on thyroid hormone therapy might explain why so many randomized trials of LT4 versus combination therapy were negative: With study populations of only 20-141 patients, the trials would have been underpowered to detect a significant difference in treatment effect. Unfortunately, genetic testing for deiodinase polymorphisms is not commercially available, the endocrinologist observed.
When he does resort to combination therapy, Dr. McDermott prescribes it in an LT4:LT3 ratio of 10-14:1 to mimic normal thyroid secretion. He generally has patients take LT3 twice daily, with the second dose no later than about 6 p.m. so it doesn’t interfere with sleep. Once-daily slow-release formulations of LT3 are available in Europe and work very well. Several companies are interested in developing a slow-release LT3 for the United States, which would be a welcome development, according to Dr. McDermott.
Another option, once all else has been tried and failed, is to switch to another brand of LT4, he continued. Some patients may have adverse reactions to the various dyes and fillers contained in LT4 pills. When this is a potential concern, levothyroxine sodium (Tirosint), approved by the Food and Drug Administration a couple of years ago, is an attractive option. The LT4 in Tirosint is contained in oil in a liquid gelcap with no dyes or fillers, differentiating it from all other brand name and generic products, Dr. McDermott noted.
He emphasized the importance of avoiding overtreatment with LT4 in an attempt to improve quality of life in patients with residual symptoms despite a TSH of 0.5-2.0 mU/L. Subclinical hyperthyroidism as defined by a TSH below 0.1 mU/L has been shown to significantly increase the risk of hip and spine fractures, atrial fibrillation, and cardiovascular mortality.
Dr. McDermott reported having no financial conflicts.
EXPERT OPINION FROM AN UPDATE ON INTERNAL MEDICINE SPONSORED BY THE UNIVERSITY OF COLORADO
Traumatic Brain Injury Linked to GH Deficiency
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Twenty-five percent of a group of military veterans with mild traumatic brain injury had growth hormone deficiency, which was associated with specific neuropsychologic abnormalities.
Data Source: This was a pilot study of 20 men with combat-induced mild traumatic brain injury.
Disclosures: The study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
Oral Relaxin Improves Poststroke Recovery
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Treatment with oral porcine relaxin at 40 mg/day plus rehabilitation therapy resulted in greater psychoneurologic and functional recovery than rehabilitation therapy alone in a group of poststroke patients.
Data Source: A prospective, randomized, controlled trial of 40 poststroke patients admitted to a rehabilitation center.
Disclosures: The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
Experimental Drug Improves Muscle Strength in Cancer
HOUSTON – An experimental drug significantly improved physical function in men with cancer-related muscle wasting, compared with placebo – and more so in the men with low testosterone levels – in a secondary analysis of a randomized, double-blind, phase II clinical trial.
In all, 60% of the 93 men for whom baseline testosterone levels were available had hypogonadism when the patients were randomized to treatment with daily placebo or 1 mg or 3 mg of enobosarm for 16 weeks. Before treatment, the eugonadal males showed significantly better physical function, as measured by stair-climb power, compared with the hypogonadal patients (174 W vs. 147 W). Lower testosterone levels correlated with lower physical function.
Enobosarm significantly improved physical function (a secondary end point in the trial) with an average 17% increase in stair-climb power in hypogonadal men, and a 12% increase in power in eugonadal men, compared with baseline, Dr. Adrian Dobs and her associates reported at the annual meeting of the Endocrine Society.
Among men on placebo, stair-climb power increased by about 10% in hypogonadal men and by roughly 1% in eugonadal men, compared with baseline, but the changes were not statistically significant, said Dr. Dobs, professor of medicine and oncology at Johns Hopkins University, Baltimore.
Adverse events included fatigue in 21% of both treatment groups, anemia in 19% of those on enobosarm and 15% in those on placebo, nausea in 18% on enobosarm and 14% on placebo, diarrhea in 16% on enobosarm and 14% on placebo, vomiting in 12% of each treatment group, weight decrease in 12% on enobosarm and 10% on placebo, and constipation in 14% on enobosarm and 4% on placebo.
The investigators defined hypogonadism as a testosterone level below 300 ng/dL.
Enobosarm is a nonsteroidal SARM (selective androgen receptor modulator) that produces anabolic effects in bone and muscle without causing the prostate effects in men or hair growth in women that is seen with steroids.
The analysis used data from a larger trial in 159 people with cancer (65% of whom were men) who had lost at least 2% (and an average of 8%) of their weight in the previous 6 months. The men were older than 45 years of age, the women were postmenopausal, and each had a body mass index less than 35 mg/kg2.
The multicenter study as a whole met its primary end point of increasing lean body mass (muscle) and its secondary end point of improving physical function, Dr. Dobs said in an interview. Those results were reported on the website of the company that is developing the drug, GTx Inc., and at previous medical meetings, according to an interview in the Los Angeles Times.
Dr. Dobs did not report results for lean body mass in the current analysis based on gonadal status.
Cancer diagnoses included colorectal cancer, non–small cell lung cancer, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and breast cancer. Patients participated in the study prior to or between courses of chemotherapy. Rates of hypogonadism were similar for each type of cancer. Patients with hypogonadism were less likely to complete the study than were eugonadal men.
Patients with cancer develop cachexia (muscle wasting) because of reduced anabolic activity, increased catabolic activity, or both, accounting for a progressive catabolic state. Up to 50% of patients with lung cancer show severe cachexia at the time of diagnosis, and the muscle wasting increases throughout the course of the malignancy, Dr. Dobs said.
Two phase III clinical trials are underway to study effects of 3 mg/day of enobosarm for the prevention and treatment of muscle wasting in patients with non–small cell lung cancer.
GTx Inc., the company that is developing enobosarm, funded the current study. Dr. Dobs reported having no other financial disclosures.
HOUSTON – An experimental drug significantly improved physical function in men with cancer-related muscle wasting, compared with placebo – and more so in the men with low testosterone levels – in a secondary analysis of a randomized, double-blind, phase II clinical trial.
In all, 60% of the 93 men for whom baseline testosterone levels were available had hypogonadism when the patients were randomized to treatment with daily placebo or 1 mg or 3 mg of enobosarm for 16 weeks. Before treatment, the eugonadal males showed significantly better physical function, as measured by stair-climb power, compared with the hypogonadal patients (174 W vs. 147 W). Lower testosterone levels correlated with lower physical function.
Enobosarm significantly improved physical function (a secondary end point in the trial) with an average 17% increase in stair-climb power in hypogonadal men, and a 12% increase in power in eugonadal men, compared with baseline, Dr. Adrian Dobs and her associates reported at the annual meeting of the Endocrine Society.
Among men on placebo, stair-climb power increased by about 10% in hypogonadal men and by roughly 1% in eugonadal men, compared with baseline, but the changes were not statistically significant, said Dr. Dobs, professor of medicine and oncology at Johns Hopkins University, Baltimore.
Adverse events included fatigue in 21% of both treatment groups, anemia in 19% of those on enobosarm and 15% in those on placebo, nausea in 18% on enobosarm and 14% on placebo, diarrhea in 16% on enobosarm and 14% on placebo, vomiting in 12% of each treatment group, weight decrease in 12% on enobosarm and 10% on placebo, and constipation in 14% on enobosarm and 4% on placebo.
The investigators defined hypogonadism as a testosterone level below 300 ng/dL.
Enobosarm is a nonsteroidal SARM (selective androgen receptor modulator) that produces anabolic effects in bone and muscle without causing the prostate effects in men or hair growth in women that is seen with steroids.
The analysis used data from a larger trial in 159 people with cancer (65% of whom were men) who had lost at least 2% (and an average of 8%) of their weight in the previous 6 months. The men were older than 45 years of age, the women were postmenopausal, and each had a body mass index less than 35 mg/kg2.
The multicenter study as a whole met its primary end point of increasing lean body mass (muscle) and its secondary end point of improving physical function, Dr. Dobs said in an interview. Those results were reported on the website of the company that is developing the drug, GTx Inc., and at previous medical meetings, according to an interview in the Los Angeles Times.
Dr. Dobs did not report results for lean body mass in the current analysis based on gonadal status.
Cancer diagnoses included colorectal cancer, non–small cell lung cancer, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and breast cancer. Patients participated in the study prior to or between courses of chemotherapy. Rates of hypogonadism were similar for each type of cancer. Patients with hypogonadism were less likely to complete the study than were eugonadal men.
Patients with cancer develop cachexia (muscle wasting) because of reduced anabolic activity, increased catabolic activity, or both, accounting for a progressive catabolic state. Up to 50% of patients with lung cancer show severe cachexia at the time of diagnosis, and the muscle wasting increases throughout the course of the malignancy, Dr. Dobs said.
Two phase III clinical trials are underway to study effects of 3 mg/day of enobosarm for the prevention and treatment of muscle wasting in patients with non–small cell lung cancer.
GTx Inc., the company that is developing enobosarm, funded the current study. Dr. Dobs reported having no other financial disclosures.
HOUSTON – An experimental drug significantly improved physical function in men with cancer-related muscle wasting, compared with placebo – and more so in the men with low testosterone levels – in a secondary analysis of a randomized, double-blind, phase II clinical trial.
In all, 60% of the 93 men for whom baseline testosterone levels were available had hypogonadism when the patients were randomized to treatment with daily placebo or 1 mg or 3 mg of enobosarm for 16 weeks. Before treatment, the eugonadal males showed significantly better physical function, as measured by stair-climb power, compared with the hypogonadal patients (174 W vs. 147 W). Lower testosterone levels correlated with lower physical function.
Enobosarm significantly improved physical function (a secondary end point in the trial) with an average 17% increase in stair-climb power in hypogonadal men, and a 12% increase in power in eugonadal men, compared with baseline, Dr. Adrian Dobs and her associates reported at the annual meeting of the Endocrine Society.
Among men on placebo, stair-climb power increased by about 10% in hypogonadal men and by roughly 1% in eugonadal men, compared with baseline, but the changes were not statistically significant, said Dr. Dobs, professor of medicine and oncology at Johns Hopkins University, Baltimore.
Adverse events included fatigue in 21% of both treatment groups, anemia in 19% of those on enobosarm and 15% in those on placebo, nausea in 18% on enobosarm and 14% on placebo, diarrhea in 16% on enobosarm and 14% on placebo, vomiting in 12% of each treatment group, weight decrease in 12% on enobosarm and 10% on placebo, and constipation in 14% on enobosarm and 4% on placebo.
The investigators defined hypogonadism as a testosterone level below 300 ng/dL.
Enobosarm is a nonsteroidal SARM (selective androgen receptor modulator) that produces anabolic effects in bone and muscle without causing the prostate effects in men or hair growth in women that is seen with steroids.
The analysis used data from a larger trial in 159 people with cancer (65% of whom were men) who had lost at least 2% (and an average of 8%) of their weight in the previous 6 months. The men were older than 45 years of age, the women were postmenopausal, and each had a body mass index less than 35 mg/kg2.
The multicenter study as a whole met its primary end point of increasing lean body mass (muscle) and its secondary end point of improving physical function, Dr. Dobs said in an interview. Those results were reported on the website of the company that is developing the drug, GTx Inc., and at previous medical meetings, according to an interview in the Los Angeles Times.
Dr. Dobs did not report results for lean body mass in the current analysis based on gonadal status.
Cancer diagnoses included colorectal cancer, non–small cell lung cancer, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and breast cancer. Patients participated in the study prior to or between courses of chemotherapy. Rates of hypogonadism were similar for each type of cancer. Patients with hypogonadism were less likely to complete the study than were eugonadal men.
Patients with cancer develop cachexia (muscle wasting) because of reduced anabolic activity, increased catabolic activity, or both, accounting for a progressive catabolic state. Up to 50% of patients with lung cancer show severe cachexia at the time of diagnosis, and the muscle wasting increases throughout the course of the malignancy, Dr. Dobs said.
Two phase III clinical trials are underway to study effects of 3 mg/day of enobosarm for the prevention and treatment of muscle wasting in patients with non–small cell lung cancer.
GTx Inc., the company that is developing enobosarm, funded the current study. Dr. Dobs reported having no other financial disclosures.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: The experimental drug enobosarm significantly improved physical function on a stair-climb test by 17% in hypogonadal men with cancer and by 12% in eugonadal men with cancer, compared with nonsignificant improvements in men on placebo.
Data Source: Data are from an analysis of data on a secondary end point (physical function) in 93 men from a randomized, double-blind, multicenter trial in 159 cancer patients.
Disclosures: GTx, the company that is developing enobosarm, funded the study. Dr. Dobs reported having no other financial disclosures.
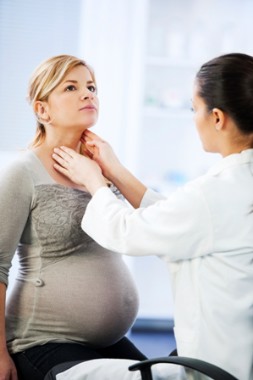
Treatment Guidelines for Thyroid Dysfunction in Pregnancy Updated
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
GnRH Antagonist Improves Rheumatoid Arthritis
HOUSTON – Use of a gonadotropin-releasing hormone antagonist showed early promise as a novel therapy for rheumatoid arthritis in a brief, proof-of-concept study that was presented at the annual meeting of the Endocrine Society.
Five days of subcutaneous injections of cetrorelix (Cetrotide) that were aimed at rapidly reducing levels of luteinizing hormone and follicle-stimulating hormone safely improved signs and symptoms of RA and reduced tumor necrosis factor–alpha levels in a 99-patient, placebo-controlled, randomized trial. Larger and longer-term clinical trials of GnRH antagonists as a potential treatment for RA are planned, according to Dr. Anita Kass of Betanien Hospital in Skien, Norway.
All study participants had active RA and were on stable doses of disease-modifying antirheumatic drugs. They were randomized to placebo or subcutaneous cetrorelix dosed at 5 mg on days 1-3 and 3 mg on days 4-5.
Outcomes were assessed on day 5, when LH and FSH levels in the cetrorelix arm were at their nadir. At that time, an ACR 20 (American College of Rheumatology scale, based on a 20% improvement in specified criteria) response was documented in 40% of patients on cetrorelix, compared with 18% of controls. Remission according to the DAS28 (Disease Activity Score, including a 28-joint count) was achieved in 13% of patients on the GnRH antagonist, compared with none on placebo. The erythrocyte sedimentation rate had declined by a mean of 1.06 mm/hour, compared with baseline, in the active treatment group, compared with a 5.04-mm/hour increase in controls.
In addition, the cetrorelix group had a mean 0.58 log pg/mL decrease in TNF-alpha compared with baseline, whereas controls experienced a 0.02 log pg/mL reduction. All of these differences between the cetrorelix and control groups were statistically significant.
The reduction in DAS28 score by day 5 was 0.82 in the cetrorelix group and 0.57 in controls, a trend that didn’t reach significance.
Adverse events in the two study arms did not differ.
Dr. Kass credited the impetus for this line of clinical investigation to earlier work by other investigators at the Weizmann Institute of Science in Rehovot, Israel. They showed that GnRH and GnRH-II are produced by T cells, and that the hormones have roles that extend beyond the reproductive system to include immune system regulation. The hormones trigger T-cell chemotaxis and homing to specific organs (Nat. Med. 2002;8:1421-6).
This proof-of-concept study was supported by the Norwegian Health and Rehabilitation Organization. The presenter reported having no financial conflicts.
HOUSTON – Use of a gonadotropin-releasing hormone antagonist showed early promise as a novel therapy for rheumatoid arthritis in a brief, proof-of-concept study that was presented at the annual meeting of the Endocrine Society.
Five days of subcutaneous injections of cetrorelix (Cetrotide) that were aimed at rapidly reducing levels of luteinizing hormone and follicle-stimulating hormone safely improved signs and symptoms of RA and reduced tumor necrosis factor–alpha levels in a 99-patient, placebo-controlled, randomized trial. Larger and longer-term clinical trials of GnRH antagonists as a potential treatment for RA are planned, according to Dr. Anita Kass of Betanien Hospital in Skien, Norway.
All study participants had active RA and were on stable doses of disease-modifying antirheumatic drugs. They were randomized to placebo or subcutaneous cetrorelix dosed at 5 mg on days 1-3 and 3 mg on days 4-5.
Outcomes were assessed on day 5, when LH and FSH levels in the cetrorelix arm were at their nadir. At that time, an ACR 20 (American College of Rheumatology scale, based on a 20% improvement in specified criteria) response was documented in 40% of patients on cetrorelix, compared with 18% of controls. Remission according to the DAS28 (Disease Activity Score, including a 28-joint count) was achieved in 13% of patients on the GnRH antagonist, compared with none on placebo. The erythrocyte sedimentation rate had declined by a mean of 1.06 mm/hour, compared with baseline, in the active treatment group, compared with a 5.04-mm/hour increase in controls.
In addition, the cetrorelix group had a mean 0.58 log pg/mL decrease in TNF-alpha compared with baseline, whereas controls experienced a 0.02 log pg/mL reduction. All of these differences between the cetrorelix and control groups were statistically significant.
The reduction in DAS28 score by day 5 was 0.82 in the cetrorelix group and 0.57 in controls, a trend that didn’t reach significance.
Adverse events in the two study arms did not differ.
Dr. Kass credited the impetus for this line of clinical investigation to earlier work by other investigators at the Weizmann Institute of Science in Rehovot, Israel. They showed that GnRH and GnRH-II are produced by T cells, and that the hormones have roles that extend beyond the reproductive system to include immune system regulation. The hormones trigger T-cell chemotaxis and homing to specific organs (Nat. Med. 2002;8:1421-6).
This proof-of-concept study was supported by the Norwegian Health and Rehabilitation Organization. The presenter reported having no financial conflicts.
HOUSTON – Use of a gonadotropin-releasing hormone antagonist showed early promise as a novel therapy for rheumatoid arthritis in a brief, proof-of-concept study that was presented at the annual meeting of the Endocrine Society.
Five days of subcutaneous injections of cetrorelix (Cetrotide) that were aimed at rapidly reducing levels of luteinizing hormone and follicle-stimulating hormone safely improved signs and symptoms of RA and reduced tumor necrosis factor–alpha levels in a 99-patient, placebo-controlled, randomized trial. Larger and longer-term clinical trials of GnRH antagonists as a potential treatment for RA are planned, according to Dr. Anita Kass of Betanien Hospital in Skien, Norway.
All study participants had active RA and were on stable doses of disease-modifying antirheumatic drugs. They were randomized to placebo or subcutaneous cetrorelix dosed at 5 mg on days 1-3 and 3 mg on days 4-5.
Outcomes were assessed on day 5, when LH and FSH levels in the cetrorelix arm were at their nadir. At that time, an ACR 20 (American College of Rheumatology scale, based on a 20% improvement in specified criteria) response was documented in 40% of patients on cetrorelix, compared with 18% of controls. Remission according to the DAS28 (Disease Activity Score, including a 28-joint count) was achieved in 13% of patients on the GnRH antagonist, compared with none on placebo. The erythrocyte sedimentation rate had declined by a mean of 1.06 mm/hour, compared with baseline, in the active treatment group, compared with a 5.04-mm/hour increase in controls.
In addition, the cetrorelix group had a mean 0.58 log pg/mL decrease in TNF-alpha compared with baseline, whereas controls experienced a 0.02 log pg/mL reduction. All of these differences between the cetrorelix and control groups were statistically significant.
The reduction in DAS28 score by day 5 was 0.82 in the cetrorelix group and 0.57 in controls, a trend that didn’t reach significance.
Adverse events in the two study arms did not differ.
Dr. Kass credited the impetus for this line of clinical investigation to earlier work by other investigators at the Weizmann Institute of Science in Rehovot, Israel. They showed that GnRH and GnRH-II are produced by T cells, and that the hormones have roles that extend beyond the reproductive system to include immune system regulation. The hormones trigger T-cell chemotaxis and homing to specific organs (Nat. Med. 2002;8:1421-6).
This proof-of-concept study was supported by the Norwegian Health and Rehabilitation Organization. The presenter reported having no financial conflicts.
AT THE ANNUAL MEETING OF ENDOCRINE SOCIETY
Major Finding: Inhibition of GnRH improved the signs and symptoms of RA and reduced TNF-alpha levels.
Data Source: This was an exploratory pilot study involving 99 patients with active RA who were randomized to 5 days of the GnRH antagonist cetrorelix or placebo.
Disclosures: This proof-of-concept study was supported by the Norwegian Health and Rehabilitation Organization. The presenter reported having no financial conflicts.
Elevated Cardiovascular Risk Persists Long After Thyroidectomy
HOUSTON – Patients with surgically treated hyperthyroidism have a lingering elevation in cardiovascular risk that persists for at least 2 decades post thyroidectomy, according to a Finnish national study.
“This is the first study to show increased risk of hospitalization for cardiovascular disease after thyroid surgery. The elevated risk is sustained 20 years after surgery. This is in line with previous studies done in patients treated with radioactive iodine. Thus, it’s probably the disease rather than the treatment that affects the patient’s heart permanently,” said Dr. Saara Metso of Tampere (Finland) University Hospital.
At the annual meeting of the Endocrine Society, she presented a population-based cohort study involving 4,334 hyperthyroid patients treated with thyroidectomy at any hospital in Finland during 1986-2007, along with 12,991 controls drawn from the general population.
The hospitalization rate for all cardiovascular causes during a median 10.5 years of follow-up was 240.5 per 10,000 person-years in the thyroidectomy group, compared with 206.2 per 10,000 person-years among controls.
These rates translated into a highly significant 17% increased risk of cardiovascular hospitalization in patients after they have undergone thyroidectomy. The risk, however, was not elevated across the board for all forms of cardiovascular disease. For example, there was no significant difference between patients and controls in hospitalization rates for coronary artery disease, cerebrovascular disease, or heart failure. On the other hand, patients with surgically treated hyperthyroidism had a 27% greater rate of hospitalization for hypertension; a 51% increase in admissions for atrial fibrillation; and a 54% greater hospitalization rate for nonbacterial endo-, peri- and myocardial diseases, valvular diseases, and cardiomyopathy, she continued.
Dr. Metso noted that a recent meta-analysis of six studies featuring long-term follow-up of patients treated for hyperthyroidism showed that the subjects had a 19% increase in cardiovascular mortality relative to age- and sex-matched controls (Eur. J. Endocrinol. 2011;165:491-7). However, most of these patients had been treated with radioiodine. This is what prompted Dr. Metso and her coinvestigators to take a close look at surgically treated patients. The most common causes of their surgically treated hyperthyroidism were Graves disease in 48% of patients, multinodular goiter in 33%, and toxic adenoma in 6%.
Several audience members, while quick to praise the Finnish study as an important advance in the field, expressed a wish that Dr. Metso and her coworkers would have included radioiodine-treated hyperthyroid patients as a control group. Audience members also would have welcomed information on postthyroidectomy thyroid replacement hormone dosing to assess whether that could be a potential contributor to the observed increase in cardiovascular risk in thyroidectomized patients.
“It’s hard to understand how the adverse cardiovascular effects could last 2 decades after the end of hyperthyroidism,” one physician commented.
The Finnish national study was funded by the Pirkanmaa Hospital Research Fund. Dr. Metso reported having no financial conflicts.
HOUSTON – Patients with surgically treated hyperthyroidism have a lingering elevation in cardiovascular risk that persists for at least 2 decades post thyroidectomy, according to a Finnish national study.
“This is the first study to show increased risk of hospitalization for cardiovascular disease after thyroid surgery. The elevated risk is sustained 20 years after surgery. This is in line with previous studies done in patients treated with radioactive iodine. Thus, it’s probably the disease rather than the treatment that affects the patient’s heart permanently,” said Dr. Saara Metso of Tampere (Finland) University Hospital.
At the annual meeting of the Endocrine Society, she presented a population-based cohort study involving 4,334 hyperthyroid patients treated with thyroidectomy at any hospital in Finland during 1986-2007, along with 12,991 controls drawn from the general population.
The hospitalization rate for all cardiovascular causes during a median 10.5 years of follow-up was 240.5 per 10,000 person-years in the thyroidectomy group, compared with 206.2 per 10,000 person-years among controls.
These rates translated into a highly significant 17% increased risk of cardiovascular hospitalization in patients after they have undergone thyroidectomy. The risk, however, was not elevated across the board for all forms of cardiovascular disease. For example, there was no significant difference between patients and controls in hospitalization rates for coronary artery disease, cerebrovascular disease, or heart failure. On the other hand, patients with surgically treated hyperthyroidism had a 27% greater rate of hospitalization for hypertension; a 51% increase in admissions for atrial fibrillation; and a 54% greater hospitalization rate for nonbacterial endo-, peri- and myocardial diseases, valvular diseases, and cardiomyopathy, she continued.
Dr. Metso noted that a recent meta-analysis of six studies featuring long-term follow-up of patients treated for hyperthyroidism showed that the subjects had a 19% increase in cardiovascular mortality relative to age- and sex-matched controls (Eur. J. Endocrinol. 2011;165:491-7). However, most of these patients had been treated with radioiodine. This is what prompted Dr. Metso and her coinvestigators to take a close look at surgically treated patients. The most common causes of their surgically treated hyperthyroidism were Graves disease in 48% of patients, multinodular goiter in 33%, and toxic adenoma in 6%.
Several audience members, while quick to praise the Finnish study as an important advance in the field, expressed a wish that Dr. Metso and her coworkers would have included radioiodine-treated hyperthyroid patients as a control group. Audience members also would have welcomed information on postthyroidectomy thyroid replacement hormone dosing to assess whether that could be a potential contributor to the observed increase in cardiovascular risk in thyroidectomized patients.
“It’s hard to understand how the adverse cardiovascular effects could last 2 decades after the end of hyperthyroidism,” one physician commented.
The Finnish national study was funded by the Pirkanmaa Hospital Research Fund. Dr. Metso reported having no financial conflicts.
HOUSTON – Patients with surgically treated hyperthyroidism have a lingering elevation in cardiovascular risk that persists for at least 2 decades post thyroidectomy, according to a Finnish national study.
“This is the first study to show increased risk of hospitalization for cardiovascular disease after thyroid surgery. The elevated risk is sustained 20 years after surgery. This is in line with previous studies done in patients treated with radioactive iodine. Thus, it’s probably the disease rather than the treatment that affects the patient’s heart permanently,” said Dr. Saara Metso of Tampere (Finland) University Hospital.
At the annual meeting of the Endocrine Society, she presented a population-based cohort study involving 4,334 hyperthyroid patients treated with thyroidectomy at any hospital in Finland during 1986-2007, along with 12,991 controls drawn from the general population.
The hospitalization rate for all cardiovascular causes during a median 10.5 years of follow-up was 240.5 per 10,000 person-years in the thyroidectomy group, compared with 206.2 per 10,000 person-years among controls.
These rates translated into a highly significant 17% increased risk of cardiovascular hospitalization in patients after they have undergone thyroidectomy. The risk, however, was not elevated across the board for all forms of cardiovascular disease. For example, there was no significant difference between patients and controls in hospitalization rates for coronary artery disease, cerebrovascular disease, or heart failure. On the other hand, patients with surgically treated hyperthyroidism had a 27% greater rate of hospitalization for hypertension; a 51% increase in admissions for atrial fibrillation; and a 54% greater hospitalization rate for nonbacterial endo-, peri- and myocardial diseases, valvular diseases, and cardiomyopathy, she continued.
Dr. Metso noted that a recent meta-analysis of six studies featuring long-term follow-up of patients treated for hyperthyroidism showed that the subjects had a 19% increase in cardiovascular mortality relative to age- and sex-matched controls (Eur. J. Endocrinol. 2011;165:491-7). However, most of these patients had been treated with radioiodine. This is what prompted Dr. Metso and her coinvestigators to take a close look at surgically treated patients. The most common causes of their surgically treated hyperthyroidism were Graves disease in 48% of patients, multinodular goiter in 33%, and toxic adenoma in 6%.
Several audience members, while quick to praise the Finnish study as an important advance in the field, expressed a wish that Dr. Metso and her coworkers would have included radioiodine-treated hyperthyroid patients as a control group. Audience members also would have welcomed information on postthyroidectomy thyroid replacement hormone dosing to assess whether that could be a potential contributor to the observed increase in cardiovascular risk in thyroidectomized patients.
“It’s hard to understand how the adverse cardiovascular effects could last 2 decades after the end of hyperthyroidism,” one physician commented.
The Finnish national study was funded by the Pirkanmaa Hospital Research Fund. Dr. Metso reported having no financial conflicts.
Major Finding: Patients with surgically treated hyperthyroidism had a significant 17% increased risk of cardiovascular hospitalization during a median 10.5 years of follow-up, compared with controls without thyroid disease. The risk was increased only for selected types of cardiovascular disease.
Data Source: This was a retrospective population-based cohort study of 4,334 hyperthyroid patients treated surgically during 1986-2007 at any Finnish hospital, along with 12,991 matched controls.
Disclosures: This Finnish national study was funded by the Pirkanmaa Hospital District Research Fund. Dr. Metso reported having no financial conflicts.
REPLACE: *Natpara Is Effective in Hypoparathyroidism
HOUSTON – Hypoparathyroidism may soon lose its status as the only endocrine deficiency disease for which the missing hormone isn’t available as an approved replacement therapy.
That change may come as a result of the positive findings of the REPLACE study of the investigational recombinant human parathyroid hormone (1-84), known as Natpara.
REPLACE was a double-blind, randomized, multinational, dose-escalation clinical trial of 134 adults with hypoparathyroidism. After an average 10-week stabilization period in which participants’ oral calcium and active vitamin D doses were adjusted to maintain optimal biochemical control of their disease as defined by serum calcium concentrations of 8.0-9.0 mg/dL for 2 weeks, they were randomized 2:1 to once-daily self-injected subcutaneous recombinant human parathyroid hormone (rhPTH) or placebo for 24 weeks.
The starting dose of rhPTH was 50 mcg. Subjects underwent staged reductions in their calcium and vitamin D supplementation while maintaining a serum calcium level in the target range. In order to accomplish this, patients could be up-titrated to 75 mcg and if need be 100 mcg per day over 6-8 weeks, Dr. Michael Mannstadt explained at the annual meeting of the Endocrine Society.
The primary end point in the REPLACE study was at least a 50% reduction in oral calcium and vitamin D requirements at week 24 compared with baseline, along with maintenance of serum calcium in the target range. This combined end point was achieved in 53% of patients on rhPTH, compared with 2% of controls, reported Dr. Mannstadt, of Harvard Medical School and Massachusetts General Hospital, both in Boston.
At week 24, 43% of patients in the rhPTH arm no longer required vitamin D supplementation and could be maintained on an oral calcium dose of 500 mg/day or less of calcipotriol. This secondary end point was achieved in only 5% of controls.
The mean dose of active vitamin D was reduced by 78% compared with baseline in the rhPTH group and by 30% in controls. The mean dose of oral calcium was lowered by 52% in the rhPTH group, compared with a 5.7% increase over baseline in controls.
Natpara was generally well tolerated, with adverse event rates and profiles similar to those of the placebo arm. An ongoing extension phase of the REPLACE trial is looking at the effects of treatment beyond 24 weeks.
The REPLACE participants were similar to the patients typically encountered in clinical practice. They averaged 48 years of age and had a 13-year history of hypoparathyroidism; 78% were women; and their hormone deficiency resulted from surgery in three-quarters of cases.
Symptoms of hypoparathyroidism include paresthesias, uncontrollable muscle spasms, fatigue, laryngospasm, depression, and even seizures. The impetus for developing Natpara as the first replacement therapy for hypothyroidism stems from the high rate of serious complications resulting from current therapy, which entails large doses of calcium and vitamin D. High-dose calcium supplementation can result in major swings in serum calcium levels and, long term, as calcium builds up extraskeletally, in coronary artery calcification, renal damage, and neurocognitive abnormalities, Dr. Mannstadt noted.
This study was sponsored by NPS Pharmaceuticals. Dr. Mannstadt reported that he is on the company’s advisory board.
*CORRECTION 8/2/12: Natpara was misspelled in the headline in a previous version of this story. This version reflects the corrected headline.
HOUSTON – Hypoparathyroidism may soon lose its status as the only endocrine deficiency disease for which the missing hormone isn’t available as an approved replacement therapy.
That change may come as a result of the positive findings of the REPLACE study of the investigational recombinant human parathyroid hormone (1-84), known as Natpara.
REPLACE was a double-blind, randomized, multinational, dose-escalation clinical trial of 134 adults with hypoparathyroidism. After an average 10-week stabilization period in which participants’ oral calcium and active vitamin D doses were adjusted to maintain optimal biochemical control of their disease as defined by serum calcium concentrations of 8.0-9.0 mg/dL for 2 weeks, they were randomized 2:1 to once-daily self-injected subcutaneous recombinant human parathyroid hormone (rhPTH) or placebo for 24 weeks.
The starting dose of rhPTH was 50 mcg. Subjects underwent staged reductions in their calcium and vitamin D supplementation while maintaining a serum calcium level in the target range. In order to accomplish this, patients could be up-titrated to 75 mcg and if need be 100 mcg per day over 6-8 weeks, Dr. Michael Mannstadt explained at the annual meeting of the Endocrine Society.
The primary end point in the REPLACE study was at least a 50% reduction in oral calcium and vitamin D requirements at week 24 compared with baseline, along with maintenance of serum calcium in the target range. This combined end point was achieved in 53% of patients on rhPTH, compared with 2% of controls, reported Dr. Mannstadt, of Harvard Medical School and Massachusetts General Hospital, both in Boston.
At week 24, 43% of patients in the rhPTH arm no longer required vitamin D supplementation and could be maintained on an oral calcium dose of 500 mg/day or less of calcipotriol. This secondary end point was achieved in only 5% of controls.
The mean dose of active vitamin D was reduced by 78% compared with baseline in the rhPTH group and by 30% in controls. The mean dose of oral calcium was lowered by 52% in the rhPTH group, compared with a 5.7% increase over baseline in controls.
Natpara was generally well tolerated, with adverse event rates and profiles similar to those of the placebo arm. An ongoing extension phase of the REPLACE trial is looking at the effects of treatment beyond 24 weeks.
The REPLACE participants were similar to the patients typically encountered in clinical practice. They averaged 48 years of age and had a 13-year history of hypoparathyroidism; 78% were women; and their hormone deficiency resulted from surgery in three-quarters of cases.
Symptoms of hypoparathyroidism include paresthesias, uncontrollable muscle spasms, fatigue, laryngospasm, depression, and even seizures. The impetus for developing Natpara as the first replacement therapy for hypothyroidism stems from the high rate of serious complications resulting from current therapy, which entails large doses of calcium and vitamin D. High-dose calcium supplementation can result in major swings in serum calcium levels and, long term, as calcium builds up extraskeletally, in coronary artery calcification, renal damage, and neurocognitive abnormalities, Dr. Mannstadt noted.
This study was sponsored by NPS Pharmaceuticals. Dr. Mannstadt reported that he is on the company’s advisory board.
*CORRECTION 8/2/12: Natpara was misspelled in the headline in a previous version of this story. This version reflects the corrected headline.
HOUSTON – Hypoparathyroidism may soon lose its status as the only endocrine deficiency disease for which the missing hormone isn’t available as an approved replacement therapy.
That change may come as a result of the positive findings of the REPLACE study of the investigational recombinant human parathyroid hormone (1-84), known as Natpara.
REPLACE was a double-blind, randomized, multinational, dose-escalation clinical trial of 134 adults with hypoparathyroidism. After an average 10-week stabilization period in which participants’ oral calcium and active vitamin D doses were adjusted to maintain optimal biochemical control of their disease as defined by serum calcium concentrations of 8.0-9.0 mg/dL for 2 weeks, they were randomized 2:1 to once-daily self-injected subcutaneous recombinant human parathyroid hormone (rhPTH) or placebo for 24 weeks.
The starting dose of rhPTH was 50 mcg. Subjects underwent staged reductions in their calcium and vitamin D supplementation while maintaining a serum calcium level in the target range. In order to accomplish this, patients could be up-titrated to 75 mcg and if need be 100 mcg per day over 6-8 weeks, Dr. Michael Mannstadt explained at the annual meeting of the Endocrine Society.
The primary end point in the REPLACE study was at least a 50% reduction in oral calcium and vitamin D requirements at week 24 compared with baseline, along with maintenance of serum calcium in the target range. This combined end point was achieved in 53% of patients on rhPTH, compared with 2% of controls, reported Dr. Mannstadt, of Harvard Medical School and Massachusetts General Hospital, both in Boston.
At week 24, 43% of patients in the rhPTH arm no longer required vitamin D supplementation and could be maintained on an oral calcium dose of 500 mg/day or less of calcipotriol. This secondary end point was achieved in only 5% of controls.
The mean dose of active vitamin D was reduced by 78% compared with baseline in the rhPTH group and by 30% in controls. The mean dose of oral calcium was lowered by 52% in the rhPTH group, compared with a 5.7% increase over baseline in controls.
Natpara was generally well tolerated, with adverse event rates and profiles similar to those of the placebo arm. An ongoing extension phase of the REPLACE trial is looking at the effects of treatment beyond 24 weeks.
The REPLACE participants were similar to the patients typically encountered in clinical practice. They averaged 48 years of age and had a 13-year history of hypoparathyroidism; 78% were women; and their hormone deficiency resulted from surgery in three-quarters of cases.
Symptoms of hypoparathyroidism include paresthesias, uncontrollable muscle spasms, fatigue, laryngospasm, depression, and even seizures. The impetus for developing Natpara as the first replacement therapy for hypothyroidism stems from the high rate of serious complications resulting from current therapy, which entails large doses of calcium and vitamin D. High-dose calcium supplementation can result in major swings in serum calcium levels and, long term, as calcium builds up extraskeletally, in coronary artery calcification, renal damage, and neurocognitive abnormalities, Dr. Mannstadt noted.
This study was sponsored by NPS Pharmaceuticals. Dr. Mannstadt reported that he is on the company’s advisory board.
*CORRECTION 8/2/12: Natpara was misspelled in the headline in a previous version of this story. This version reflects the corrected headline.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Myopathy Presenting at Initiation of Statins May Have Another Cause
DESTIN, FLA. – Myopathy that occurs following initiation of statin therapy certainly may result from the drug, but don’t be too quick to rule out other causes.
In one case described by Dr. Robert Wortmann at the Congress of Clinical Rheumatology, the problem was actually profound hypothyroidism.
The 58-year-old patient, a physician who presented with muscle weakness of 6 months’ duration, had started taking a statin 1 month prior as treatment for hyperlipidemia. The patient’s creatine phosphokinase (CPK) values ranged from 1,100 to 1,500 U/L, and both the muscle weakness and CPK elevations persisted at 3 months after discontinuation of the statin.
Statins, as well as fibric acid derivatives and niacin, are important considerations in patients with myopathic symptoms. They can be associated with asymptomatic "hyper-CPK-emia"; transient myalgia and cramps with increased or normal CPK levels; persistent myalgia and cramps or weakness with increased or normal CPK levels; and rhabdomyolysis with myoglobinuria, renal failure, and, when these are unrecognized and untreated, death, said Dr. Wortmann, professor of medicine at Dartmouth Medical School, Lebanon, N.H.
Statin myopathies can occur due to various triggers.
"We know that when you take a statin and interfere with cholesterol synthesis, you can interfere with membrane formation, and this can lead to specific membrane effects and damage and myopathy," he explained, adding that certain genetic polymorphisms – particularly those associated with statin metabolism – also seem to put some people at greater risk for statin myopathies, as does the unmasking of underlying metabolic disorders by statins in some patients.
A particularly interesting statin myopathy is immune-mediated necrotizing myopathy, Dr. Wortmann said.
This potentially painful and debilitating myopathy appears to be associated with anti-HMG CoA reductase antibodies, and it is believed to be immune mediated because it responds to immunosuppressive therapy, he noted.
Patients with this necrotizing myopathy typically require multiple drugs given simultaneously to control the disease.
In the case presented by Dr. Wortmann, however, the problem was much simpler.
"I share this with you because I walked right by this. This was my mistake," he said, explaining that he had arranged for the physician to undergo an electromyogram and muscle biopsy.
"Fortunately, he went and saw his primary care physician again before we could get those done, [who] checked his [thyroid stimulating hormone] and it was way up there. He treated the hypothyroidism, his CPK went back to normal, his muscle symptoms went away, and he was continued on his statin," Dr. Wortmann said.
The most common musculoskeletal manifestation of hypothyroidism is carpal tunnel syndrome, but it can also be associated with sensory or sensorimotor polyneuropathy, asymptomatic hyper-CPK-emia, and myopathy. Myopathy is usually proximal, and may involve myalgias and cramps, with normal or high CPK levels, he noted, adding that although one older case report involved a patient with levels as high as 21,000 U/L, most are in the 700- to 1,200-U/L range.
"So hypothyroidism is a good thing to screen for if you see someone with muscle weakness, because if you pick it up, it’s easy [to manage]," he said. "The take-home message is that just because you see a patient who’s taking a drug [then] experience a side effect that may be found from that drug, it doesn’t mean that’s where it’s coming from."
Dr. Wortmann reported that he had no financial conflicts of interest that were relevant to his presentation.
DESTIN, FLA. – Myopathy that occurs following initiation of statin therapy certainly may result from the drug, but don’t be too quick to rule out other causes.
In one case described by Dr. Robert Wortmann at the Congress of Clinical Rheumatology, the problem was actually profound hypothyroidism.
The 58-year-old patient, a physician who presented with muscle weakness of 6 months’ duration, had started taking a statin 1 month prior as treatment for hyperlipidemia. The patient’s creatine phosphokinase (CPK) values ranged from 1,100 to 1,500 U/L, and both the muscle weakness and CPK elevations persisted at 3 months after discontinuation of the statin.
Statins, as well as fibric acid derivatives and niacin, are important considerations in patients with myopathic symptoms. They can be associated with asymptomatic "hyper-CPK-emia"; transient myalgia and cramps with increased or normal CPK levels; persistent myalgia and cramps or weakness with increased or normal CPK levels; and rhabdomyolysis with myoglobinuria, renal failure, and, when these are unrecognized and untreated, death, said Dr. Wortmann, professor of medicine at Dartmouth Medical School, Lebanon, N.H.
Statin myopathies can occur due to various triggers.
"We know that when you take a statin and interfere with cholesterol synthesis, you can interfere with membrane formation, and this can lead to specific membrane effects and damage and myopathy," he explained, adding that certain genetic polymorphisms – particularly those associated with statin metabolism – also seem to put some people at greater risk for statin myopathies, as does the unmasking of underlying metabolic disorders by statins in some patients.
A particularly interesting statin myopathy is immune-mediated necrotizing myopathy, Dr. Wortmann said.
This potentially painful and debilitating myopathy appears to be associated with anti-HMG CoA reductase antibodies, and it is believed to be immune mediated because it responds to immunosuppressive therapy, he noted.
Patients with this necrotizing myopathy typically require multiple drugs given simultaneously to control the disease.
In the case presented by Dr. Wortmann, however, the problem was much simpler.
"I share this with you because I walked right by this. This was my mistake," he said, explaining that he had arranged for the physician to undergo an electromyogram and muscle biopsy.
"Fortunately, he went and saw his primary care physician again before we could get those done, [who] checked his [thyroid stimulating hormone] and it was way up there. He treated the hypothyroidism, his CPK went back to normal, his muscle symptoms went away, and he was continued on his statin," Dr. Wortmann said.
The most common musculoskeletal manifestation of hypothyroidism is carpal tunnel syndrome, but it can also be associated with sensory or sensorimotor polyneuropathy, asymptomatic hyper-CPK-emia, and myopathy. Myopathy is usually proximal, and may involve myalgias and cramps, with normal or high CPK levels, he noted, adding that although one older case report involved a patient with levels as high as 21,000 U/L, most are in the 700- to 1,200-U/L range.
"So hypothyroidism is a good thing to screen for if you see someone with muscle weakness, because if you pick it up, it’s easy [to manage]," he said. "The take-home message is that just because you see a patient who’s taking a drug [then] experience a side effect that may be found from that drug, it doesn’t mean that’s where it’s coming from."
Dr. Wortmann reported that he had no financial conflicts of interest that were relevant to his presentation.
DESTIN, FLA. – Myopathy that occurs following initiation of statin therapy certainly may result from the drug, but don’t be too quick to rule out other causes.
In one case described by Dr. Robert Wortmann at the Congress of Clinical Rheumatology, the problem was actually profound hypothyroidism.
The 58-year-old patient, a physician who presented with muscle weakness of 6 months’ duration, had started taking a statin 1 month prior as treatment for hyperlipidemia. The patient’s creatine phosphokinase (CPK) values ranged from 1,100 to 1,500 U/L, and both the muscle weakness and CPK elevations persisted at 3 months after discontinuation of the statin.
Statins, as well as fibric acid derivatives and niacin, are important considerations in patients with myopathic symptoms. They can be associated with asymptomatic "hyper-CPK-emia"; transient myalgia and cramps with increased or normal CPK levels; persistent myalgia and cramps or weakness with increased or normal CPK levels; and rhabdomyolysis with myoglobinuria, renal failure, and, when these are unrecognized and untreated, death, said Dr. Wortmann, professor of medicine at Dartmouth Medical School, Lebanon, N.H.
Statin myopathies can occur due to various triggers.
"We know that when you take a statin and interfere with cholesterol synthesis, you can interfere with membrane formation, and this can lead to specific membrane effects and damage and myopathy," he explained, adding that certain genetic polymorphisms – particularly those associated with statin metabolism – also seem to put some people at greater risk for statin myopathies, as does the unmasking of underlying metabolic disorders by statins in some patients.
A particularly interesting statin myopathy is immune-mediated necrotizing myopathy, Dr. Wortmann said.
This potentially painful and debilitating myopathy appears to be associated with anti-HMG CoA reductase antibodies, and it is believed to be immune mediated because it responds to immunosuppressive therapy, he noted.
Patients with this necrotizing myopathy typically require multiple drugs given simultaneously to control the disease.
In the case presented by Dr. Wortmann, however, the problem was much simpler.
"I share this with you because I walked right by this. This was my mistake," he said, explaining that he had arranged for the physician to undergo an electromyogram and muscle biopsy.
"Fortunately, he went and saw his primary care physician again before we could get those done, [who] checked his [thyroid stimulating hormone] and it was way up there. He treated the hypothyroidism, his CPK went back to normal, his muscle symptoms went away, and he was continued on his statin," Dr. Wortmann said.
The most common musculoskeletal manifestation of hypothyroidism is carpal tunnel syndrome, but it can also be associated with sensory or sensorimotor polyneuropathy, asymptomatic hyper-CPK-emia, and myopathy. Myopathy is usually proximal, and may involve myalgias and cramps, with normal or high CPK levels, he noted, adding that although one older case report involved a patient with levels as high as 21,000 U/L, most are in the 700- to 1,200-U/L range.
"So hypothyroidism is a good thing to screen for if you see someone with muscle weakness, because if you pick it up, it’s easy [to manage]," he said. "The take-home message is that just because you see a patient who’s taking a drug [then] experience a side effect that may be found from that drug, it doesn’t mean that’s where it’s coming from."
Dr. Wortmann reported that he had no financial conflicts of interest that were relevant to his presentation.
EXPERT ANALYSIS FROM THE CONGRESS OF CLINICAL RHEUMATOLOGY