User login
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
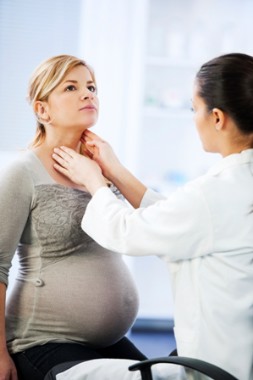
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
When treating women with thyroid dysfunction during and after pregnancy, clinicians should use caution interpreting serum-free thyroxine levels, use propylthiouracil as the first-line drug during for hyperthyroidism in the first trimester, and advise breastfeeding women to maintain a daily intake of 250 mcg of iodine to ensure breast milk provides 100 mcg of iodine/day to the infant.
These mark some of the changes the Endocrine Society made to its 2007 Clinical Practice Guideline (CPG) for the management of thyroid disease during pregnancy and the postpartum.
"Pregnancy may affect the course of thyroid diseases and conversely, thyroid diseases may affect the course of pregnancy," Dr. Leslie De Groot, lead researcher from the University of Rhode Island, Kingston, said in a prepared statement. "Pregnant women may be under the care of multiple health care professionals including obstetricians, nurse midwives, family practitioners and endocrinologists making the development of guidelines all the more critical."
In order to update the Endocrine Society’s 2007 CPG, Dr. De Groot and a task force of 12 other experts reviewed existing medical literature on the topic and followed the approach of the U.S. Preventive Services Task Force and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to evaluate the strength of each recommendation. The effort, published online in the Aug. 12 issue of the Journal of Clinical Endocrinology & Metabolism, included collaboration with the Asia and Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society (J. Clin. Endocrinol. Metab. 2012 Aug. 1;97:2543-65 [doi: 10.1210/jc.2011-2803]).
"At present, with the exception of studies on iodide nutrition, only a few prospective, randomized intervention trials have been published in this area [of thyroid dysfunction during pregnancy]," the authors wrote. "We are aware of large-scale prospective intervention trials that are ongoing. Nevertheless, in the past decade many high-quality studies have modified older dogmas and profoundly changed the ways in which these patients are managed."
Key recommendations in the 2012 clinical practice guideline that differ from the 2007 version include the following:
• Use caution in the interpretation of serum-free T4 levels during pregnancy. "Each laboratory should establish trimester-specific reference ranges for pregnant women if using a free T4 assay," the authors wrote in a supplemental index in which they summarized changes between the 2007 and 2012 versions of the guideline. "The non-pregnant total T4 range (5-12 mcg/dL or 50-150 nmol/L) can be adapted in the second and third trimesters by multiplying this range by 1.5-fold. Alternatively, the free thyroxine index (‘adjusted T4’) appears to be a reliable assay during pregnancy."
• Use propylthiouracil (PTU), if available, as the first-line drug for treatment of hyperthyroidism during the first trimester. This is because of the possible association of methimazole (MMI) with congenital abnormalities. MMI "may also be prescribed if PTU is not available or if a patient cannot tolerate or has an adverse response to PTU," the authors wrote. "Recent analyses reported by the FDA indicate that PTU may rarely be associated with severe liver toxicity. For this reason, we recommend that clinicians should change treatment of patients from PTU to MMI after the completion of first trimester. Available data indicate that MMI and PTU are equally efficacious in treatment of pregnant women."
• Breastfeeding women should maintain a daily intake of 250 mcg of iodine. This ensures that breast milk provides 100 mcg iodine/day to the infant. "These changes are in response to recent publications indicating that some vitamin-mineral preparations used during pregnancy may not provide adequate iodine intake, and that iodine supplements should be continued during breastfeeding," the authors explained.
• Measure thyroid receptor antibodies (TRAb) before 22 weeks’ gestational age in a subset of mothers. This includes mothers with either current Graves’ disease, a history of Graves’ disease and treatment with 131-I (radioiodine) or thyroidectomy before pregnancy, a previous neonate with Graves’ disease, or previously elevated TRAb. This approach is recommended because thyroid receptor antibodies "freely cross the placenta and can stimulate or inhibit the fetal thyroid," the authors wrote. "Women who have negative TRAb and do not require antithyroid drugs have a very low risk of fetal or neonatal thyroid dysfunction. This change makes more explicit the timing and indications for measurement of TRAb in pregnancy."
The authors could not reach agreement on universal screening recommendations for all newly pregnant women. Some recommend screening of all pregnant women for serum TSH abnormalities by the 9th week or at the time of their first visit while others recommended neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit.
Despite their differences on universal screening recommendations, the authors unanimously agreed that clinicians should perform targeted screening of high-risk women during the prenatal and perinatal periods. This includes women over age 30 years and those with a family history or autoimmune thyroid disease or hypothyroidism; a goiter; thyroid antibodies, primarily thyroid peroxidase antibodies; symptoms or clinical signs suggestive of thyroid hypofunction; type 1 diabetes mellitus, or other autoimmune disorders; those with infertility; a prior history of preterm delivery; prior therapeutic head or neck irradiation or prior thyroid surgery; and those currently receiving levothyroxine replacement.
No member of the task force disclosed relevant financial conflicts of interest.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM