User login
Mild OSA spontaneously resolves in about one-third of young children
CORONADO, CALIF. – results from a single-center study showed.
“OSA affects up to 6% of the pediatric population, and diagnosis of young children can be particularly challenging due to the heterogeneity of presenting symptoms,” Douglas C. von Allmen, MD, said at the Triological Society’s Combined Sections Meeting. “While school-age children may present with snoring, that’s less common in the younger population. Up to one-quarter of infants may have noisy breathing, which may mimic obstructive events throughout the first 3 years of life. Additionally, long-term clinical implications of mild sleep apnea in very young children is unclear.”
According to Dr. von Allmen, a fifth-year otolaryngology resident at the University of Cincinnati, management strategies of children with OSA can include a period of observation, particularly when there’s an absence of concerning findings on polysomnography (PSG), such as hypoventilation or significant hypoxia, or when the primary etiology of the OSA is unknown. “Additionally, few studies at this point have attempted to characterize the natural history of mild OSA in pediatric patients under 3 years of age,” he said.
In an effort to assess the effects of observation on the PSG outcomes of children under 3 years with mild OSA, Dr. Von Allmen and his colleagues performed a retrospective review of 26 children who had an overnight PSG with a follow-up PSG performed 3-12 months later. They excluded patients with neuromuscular disease, tracheostomy, or interstitial lung disease. All PSGs were performed at the Cincinnati Children’s Hospital Medical Center between 2012 and 2017 and were scored by a board-certified sleep physician. The researchers defined mild OSA as at least one, but fewer than five, events per hour. The mean age of the 26 patients was 7 months, 65% were male, 92% were white, and their median body mass index was in the 39th percentile. Comorbidities include laryngomalacia (40%), cardiac disease (40%), allergies (34%), asthma (23%), and Down syndrome (11%).
Between baseline and follow-up, the apnea-hypoapnea index (AHI) trended downward from 4.3 to 3.4 events per hour (P = .19), the obstructive AHI decreased significantly from 2.7 to 1.3 events per hour (P = .013), while the central apnea index also trended downward from 1.4 to 1.2 events per hour (P = .60). The oxyhemoglobin nadir and sleep efficiency did not change significantly, but there was a decrease in the arousal index (from 14.7 to 13 events per hour; P = .027) and in the percentage of REM sleep (from 33% to 30%; P = .008).
As for postobservation OSA severity outcomes, eight patients (31%) resolved spontaneously, one patient progressed from mild to moderate OSA, and the rest remained in their mild OSA state. Subanalysis revealed that OSA resolution rate was 36% in patients with laryngomalacia, compared with 27% in those with no laryngomalacia, a difference that did not reach statistical significance (P = .98).
Dr. von Allmen pointed out that the study cohort had comorbidities which may have contributed to the persistence of OSA. He also acknowledged certain limitations of the study, including its retrospective nature, the potential for selection bias, the small sample size, and the fact that it did not include a control sample of normal children. “The presence of laryngomalacia did not affect the resolution rate in our cohort, but we’ll need larger studies to better elucidate the factors that do affect persistent disease and to identify the optimal timing of intervention in children with mild OSA,” he said.
Dr. von Allmen reported having no financial disclosures. The study received a resident research award at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: von Allmen DC et al. Triological CSM, Abstracts.
CORONADO, CALIF. – results from a single-center study showed.
“OSA affects up to 6% of the pediatric population, and diagnosis of young children can be particularly challenging due to the heterogeneity of presenting symptoms,” Douglas C. von Allmen, MD, said at the Triological Society’s Combined Sections Meeting. “While school-age children may present with snoring, that’s less common in the younger population. Up to one-quarter of infants may have noisy breathing, which may mimic obstructive events throughout the first 3 years of life. Additionally, long-term clinical implications of mild sleep apnea in very young children is unclear.”
According to Dr. von Allmen, a fifth-year otolaryngology resident at the University of Cincinnati, management strategies of children with OSA can include a period of observation, particularly when there’s an absence of concerning findings on polysomnography (PSG), such as hypoventilation or significant hypoxia, or when the primary etiology of the OSA is unknown. “Additionally, few studies at this point have attempted to characterize the natural history of mild OSA in pediatric patients under 3 years of age,” he said.
In an effort to assess the effects of observation on the PSG outcomes of children under 3 years with mild OSA, Dr. Von Allmen and his colleagues performed a retrospective review of 26 children who had an overnight PSG with a follow-up PSG performed 3-12 months later. They excluded patients with neuromuscular disease, tracheostomy, or interstitial lung disease. All PSGs were performed at the Cincinnati Children’s Hospital Medical Center between 2012 and 2017 and were scored by a board-certified sleep physician. The researchers defined mild OSA as at least one, but fewer than five, events per hour. The mean age of the 26 patients was 7 months, 65% were male, 92% were white, and their median body mass index was in the 39th percentile. Comorbidities include laryngomalacia (40%), cardiac disease (40%), allergies (34%), asthma (23%), and Down syndrome (11%).
Between baseline and follow-up, the apnea-hypoapnea index (AHI) trended downward from 4.3 to 3.4 events per hour (P = .19), the obstructive AHI decreased significantly from 2.7 to 1.3 events per hour (P = .013), while the central apnea index also trended downward from 1.4 to 1.2 events per hour (P = .60). The oxyhemoglobin nadir and sleep efficiency did not change significantly, but there was a decrease in the arousal index (from 14.7 to 13 events per hour; P = .027) and in the percentage of REM sleep (from 33% to 30%; P = .008).
As for postobservation OSA severity outcomes, eight patients (31%) resolved spontaneously, one patient progressed from mild to moderate OSA, and the rest remained in their mild OSA state. Subanalysis revealed that OSA resolution rate was 36% in patients with laryngomalacia, compared with 27% in those with no laryngomalacia, a difference that did not reach statistical significance (P = .98).
Dr. von Allmen pointed out that the study cohort had comorbidities which may have contributed to the persistence of OSA. He also acknowledged certain limitations of the study, including its retrospective nature, the potential for selection bias, the small sample size, and the fact that it did not include a control sample of normal children. “The presence of laryngomalacia did not affect the resolution rate in our cohort, but we’ll need larger studies to better elucidate the factors that do affect persistent disease and to identify the optimal timing of intervention in children with mild OSA,” he said.
Dr. von Allmen reported having no financial disclosures. The study received a resident research award at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: von Allmen DC et al. Triological CSM, Abstracts.
CORONADO, CALIF. – results from a single-center study showed.
“OSA affects up to 6% of the pediatric population, and diagnosis of young children can be particularly challenging due to the heterogeneity of presenting symptoms,” Douglas C. von Allmen, MD, said at the Triological Society’s Combined Sections Meeting. “While school-age children may present with snoring, that’s less common in the younger population. Up to one-quarter of infants may have noisy breathing, which may mimic obstructive events throughout the first 3 years of life. Additionally, long-term clinical implications of mild sleep apnea in very young children is unclear.”
According to Dr. von Allmen, a fifth-year otolaryngology resident at the University of Cincinnati, management strategies of children with OSA can include a period of observation, particularly when there’s an absence of concerning findings on polysomnography (PSG), such as hypoventilation or significant hypoxia, or when the primary etiology of the OSA is unknown. “Additionally, few studies at this point have attempted to characterize the natural history of mild OSA in pediatric patients under 3 years of age,” he said.
In an effort to assess the effects of observation on the PSG outcomes of children under 3 years with mild OSA, Dr. Von Allmen and his colleagues performed a retrospective review of 26 children who had an overnight PSG with a follow-up PSG performed 3-12 months later. They excluded patients with neuromuscular disease, tracheostomy, or interstitial lung disease. All PSGs were performed at the Cincinnati Children’s Hospital Medical Center between 2012 and 2017 and were scored by a board-certified sleep physician. The researchers defined mild OSA as at least one, but fewer than five, events per hour. The mean age of the 26 patients was 7 months, 65% were male, 92% were white, and their median body mass index was in the 39th percentile. Comorbidities include laryngomalacia (40%), cardiac disease (40%), allergies (34%), asthma (23%), and Down syndrome (11%).
Between baseline and follow-up, the apnea-hypoapnea index (AHI) trended downward from 4.3 to 3.4 events per hour (P = .19), the obstructive AHI decreased significantly from 2.7 to 1.3 events per hour (P = .013), while the central apnea index also trended downward from 1.4 to 1.2 events per hour (P = .60). The oxyhemoglobin nadir and sleep efficiency did not change significantly, but there was a decrease in the arousal index (from 14.7 to 13 events per hour; P = .027) and in the percentage of REM sleep (from 33% to 30%; P = .008).
As for postobservation OSA severity outcomes, eight patients (31%) resolved spontaneously, one patient progressed from mild to moderate OSA, and the rest remained in their mild OSA state. Subanalysis revealed that OSA resolution rate was 36% in patients with laryngomalacia, compared with 27% in those with no laryngomalacia, a difference that did not reach statistical significance (P = .98).
Dr. von Allmen pointed out that the study cohort had comorbidities which may have contributed to the persistence of OSA. He also acknowledged certain limitations of the study, including its retrospective nature, the potential for selection bias, the small sample size, and the fact that it did not include a control sample of normal children. “The presence of laryngomalacia did not affect the resolution rate in our cohort, but we’ll need larger studies to better elucidate the factors that do affect persistent disease and to identify the optimal timing of intervention in children with mild OSA,” he said.
Dr. von Allmen reported having no financial disclosures. The study received a resident research award at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: von Allmen DC et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Comorbidities may contribute to the persistence of OSA in young children.
Major finding: OSA spontaneously resolved in 31% of patients.
Study details: A retrospective analysis of 26 children under age 3 years.
Disclosures: The researchers reported having no financial disclosures.
Source: Von Allmen et al. Triological CSM, Abstracts.
Asthma patients with sinusitis, polyps fare poorly after sinus surgery
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
REPORTING FROM THE TRIOLOGICAL CSWM
Key clinical point: Patients with asthma and the most severe form of chronic rhinosinusitis fare poorly on quality of life measures following sinus surgery.
Major finding: QOL in patients who had asthma and eosinophilic CRS with nasal polyps declined significantly at the 1- and 2-year analysis (P less than .03).
Study details: A single-center review of 457 patients with CRS who underwent sinus surgery.
Disclosures: The researchers reported having no financial disclosures.
Diagnosing OSA: Polysomnography beats Fitbit, apps
CORONADO, CALIF. –
“Currently, the gold standard for diagnosis is a polysomnography (PSG), which is basically a sleep study test,” one of the study authors, Daniel Chung, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “While it is very thorough, it is expensive, intrusive, and gives data on only 1 night of sleep and cannot give information on a patient’s sleep pattern over several days. Using wrist-worn devices such as Fitbit products or smartphone applications such as Sleep Cycle would be much cheaper alternatives with little distraction that can provide continuous data over several days if accurate.”
According to Dr. Chung, an otolaryngology head and neck surgery resident in the department of surgery at the George Washington University, Washington, previous studies about this topic are inconclusive.
“Most have been studied only in the pediatric population, and in general, show that Fitbit overestimates PSG measurements,” he said. “Also, prior studies have looked into 20-60 patients, depending on the study.”
In an effort to address these limitations, he and his colleagues prospectively evaluated 180 adult patients who were already scheduled to undergo a PSG with or without CPAP testing from the sleep lab. The overnight test was performed with a Fitbit Alta HR on their wrist of choice and a smartphone at their bedside. Each smartphone was randomly assigned and had a popular sleep application installed, with Sleep as Android on the Android phone and Sleep Cycle on the Apple iPhone. For the main outcomes of interest, the researchers collected the total sleep time, sleep efficiency, and apnea-hypopnea index (AHI) from the PSG and compared them with their equivalents from Fitbit and smartphone applications. For statistical analysis, they performed Bland-Altman plots, paired t-tests, and regression lines to assess R2, slope, and Y-intercept.
Dr. Chung and his colleagues found that both Fitbit Alta HR (P = .0014) and smartphone applications (P less than .0001) significantly overestimated the total sleep time, compared with PSG, while moderate correlation for sleep efficiency was observed between PSG and Fitbit (r = 0.38). Other findings of note were that the number of times awake recorded by Fitbit significantly underestimated the PSG AHI (P less than .0001), the snoring noise measurements from Sleep as Android had strong associations with PSG AHI (R2 = 0.43), and those from Sleep Cycle had modest associations with PSG AHI (R2 = 0.12). However, Bland-Altman plots showed wide limits of agreement for total sleep time and sleep efficiency, though as sleep efficiency approached 100%, the discrepancy between the PSG and Fitbit decreased.
“While there are various tools out right now that claim to measure sleep in a more comforting setting, we cannot recommend any product to act as a screening device or replacement for the PSG at this time,” Dr. Chung said.
He acknowledged certain limitations of the study, including the fact that Fitbit is unable to fully record sleep data if the wearer is asleep for fewer than 3 hours, which can happen in patients with severe OSA. In addition, the smartphone applications require the user to begin and conclude the recording. “This was performed only by the sleep technicians and not the patients, and can be a significant source of error, particularly with the Sleep as Android application, as it had a less intuitive interface,” he noted. “This lead to multiple unusable data points. There were also incidences where the Fitbit Alta HR failed to record sleep data even though it was fully charged and correctly placed, which led to only a smaller number of patients having both measurements at the same time.”
Dr. Chung reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: Chung D et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“Currently, the gold standard for diagnosis is a polysomnography (PSG), which is basically a sleep study test,” one of the study authors, Daniel Chung, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “While it is very thorough, it is expensive, intrusive, and gives data on only 1 night of sleep and cannot give information on a patient’s sleep pattern over several days. Using wrist-worn devices such as Fitbit products or smartphone applications such as Sleep Cycle would be much cheaper alternatives with little distraction that can provide continuous data over several days if accurate.”
According to Dr. Chung, an otolaryngology head and neck surgery resident in the department of surgery at the George Washington University, Washington, previous studies about this topic are inconclusive.
“Most have been studied only in the pediatric population, and in general, show that Fitbit overestimates PSG measurements,” he said. “Also, prior studies have looked into 20-60 patients, depending on the study.”
In an effort to address these limitations, he and his colleagues prospectively evaluated 180 adult patients who were already scheduled to undergo a PSG with or without CPAP testing from the sleep lab. The overnight test was performed with a Fitbit Alta HR on their wrist of choice and a smartphone at their bedside. Each smartphone was randomly assigned and had a popular sleep application installed, with Sleep as Android on the Android phone and Sleep Cycle on the Apple iPhone. For the main outcomes of interest, the researchers collected the total sleep time, sleep efficiency, and apnea-hypopnea index (AHI) from the PSG and compared them with their equivalents from Fitbit and smartphone applications. For statistical analysis, they performed Bland-Altman plots, paired t-tests, and regression lines to assess R2, slope, and Y-intercept.
Dr. Chung and his colleagues found that both Fitbit Alta HR (P = .0014) and smartphone applications (P less than .0001) significantly overestimated the total sleep time, compared with PSG, while moderate correlation for sleep efficiency was observed between PSG and Fitbit (r = 0.38). Other findings of note were that the number of times awake recorded by Fitbit significantly underestimated the PSG AHI (P less than .0001), the snoring noise measurements from Sleep as Android had strong associations with PSG AHI (R2 = 0.43), and those from Sleep Cycle had modest associations with PSG AHI (R2 = 0.12). However, Bland-Altman plots showed wide limits of agreement for total sleep time and sleep efficiency, though as sleep efficiency approached 100%, the discrepancy between the PSG and Fitbit decreased.
“While there are various tools out right now that claim to measure sleep in a more comforting setting, we cannot recommend any product to act as a screening device or replacement for the PSG at this time,” Dr. Chung said.
He acknowledged certain limitations of the study, including the fact that Fitbit is unable to fully record sleep data if the wearer is asleep for fewer than 3 hours, which can happen in patients with severe OSA. In addition, the smartphone applications require the user to begin and conclude the recording. “This was performed only by the sleep technicians and not the patients, and can be a significant source of error, particularly with the Sleep as Android application, as it had a less intuitive interface,” he noted. “This lead to multiple unusable data points. There were also incidences where the Fitbit Alta HR failed to record sleep data even though it was fully charged and correctly placed, which led to only a smaller number of patients having both measurements at the same time.”
Dr. Chung reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: Chung D et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“Currently, the gold standard for diagnosis is a polysomnography (PSG), which is basically a sleep study test,” one of the study authors, Daniel Chung, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “While it is very thorough, it is expensive, intrusive, and gives data on only 1 night of sleep and cannot give information on a patient’s sleep pattern over several days. Using wrist-worn devices such as Fitbit products or smartphone applications such as Sleep Cycle would be much cheaper alternatives with little distraction that can provide continuous data over several days if accurate.”
According to Dr. Chung, an otolaryngology head and neck surgery resident in the department of surgery at the George Washington University, Washington, previous studies about this topic are inconclusive.
“Most have been studied only in the pediatric population, and in general, show that Fitbit overestimates PSG measurements,” he said. “Also, prior studies have looked into 20-60 patients, depending on the study.”
In an effort to address these limitations, he and his colleagues prospectively evaluated 180 adult patients who were already scheduled to undergo a PSG with or without CPAP testing from the sleep lab. The overnight test was performed with a Fitbit Alta HR on their wrist of choice and a smartphone at their bedside. Each smartphone was randomly assigned and had a popular sleep application installed, with Sleep as Android on the Android phone and Sleep Cycle on the Apple iPhone. For the main outcomes of interest, the researchers collected the total sleep time, sleep efficiency, and apnea-hypopnea index (AHI) from the PSG and compared them with their equivalents from Fitbit and smartphone applications. For statistical analysis, they performed Bland-Altman plots, paired t-tests, and regression lines to assess R2, slope, and Y-intercept.
Dr. Chung and his colleagues found that both Fitbit Alta HR (P = .0014) and smartphone applications (P less than .0001) significantly overestimated the total sleep time, compared with PSG, while moderate correlation for sleep efficiency was observed between PSG and Fitbit (r = 0.38). Other findings of note were that the number of times awake recorded by Fitbit significantly underestimated the PSG AHI (P less than .0001), the snoring noise measurements from Sleep as Android had strong associations with PSG AHI (R2 = 0.43), and those from Sleep Cycle had modest associations with PSG AHI (R2 = 0.12). However, Bland-Altman plots showed wide limits of agreement for total sleep time and sleep efficiency, though as sleep efficiency approached 100%, the discrepancy between the PSG and Fitbit decreased.
“While there are various tools out right now that claim to measure sleep in a more comforting setting, we cannot recommend any product to act as a screening device or replacement for the PSG at this time,” Dr. Chung said.
He acknowledged certain limitations of the study, including the fact that Fitbit is unable to fully record sleep data if the wearer is asleep for fewer than 3 hours, which can happen in patients with severe OSA. In addition, the smartphone applications require the user to begin and conclude the recording. “This was performed only by the sleep technicians and not the patients, and can be a significant source of error, particularly with the Sleep as Android application, as it had a less intuitive interface,” he noted. “This lead to multiple unusable data points. There were also incidences where the Fitbit Alta HR failed to record sleep data even though it was fully charged and correctly placed, which led to only a smaller number of patients having both measurements at the same time.”
Dr. Chung reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: Chung D et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: To date, no wrist-worn device or smartphone app can be recommended as a replacement for polysomnography to diagnose OSA.
Major finding: Both Fitbit Alta HR (P = .0014) and smart phone applications (P less than .0001) significantly overestimated the total sleep time, compared to PSG.
Study details: A prospective study of 180 adult patients.
Disclosures: Dr. Chung reported having no financial disclosures.
Source: Chung D et al. Triological CSM, Abstracts.
SNOT-22 may help identify patients with undiagnosed OSA
CORONADO, CALIF. – results from a retrospective analysis demonstrated.
“We know based on experience and prior studies that there is significant overlap in symptoms for obstructive sleep apnea and chronic rhinosinusitis [CRS], which are two common conditions in the general population,” one of the study authors, David W. Jang, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Therefore, it is important to identify patients with undiagnosed OSA who may present to the physician with nose- and sinus-related symptoms.”
Dr. Jang, assistant professor of rhinology and endoscopic skull surgery in the department of surgery at Duke University, Durham, N.C., and his colleagues conducted a 3-year retrospective analysis of 165 adults who presented with a rhinologic chief complaint and completed the SNOT-22 survey. The researchers compared SNOT-22 survey results between patients with untreated OSA confirmed on polysomnography without chronic rhinosinusitis and a control group of CRS patients. A chi-square test with Bonferroni correction was used for analysis.
Of the 165 patients, 41 met criteria for untreated OSA, based on a mean apnea-hypopnea index of 29.3, while 124 were included in the CRS control group. Sleep and psychological domain scores were not significantly different between the two groups, although patients in the OSA group were more likely to choose a sleep-related symptom as their most important complaint (MIC) (P less than .001). As for the cardinal symptoms of CRS, nasal discharge and loss of smell were significantly higher in the CRS group (P less than .001), while facial pain and nasal obstruction were not significantly different (P = .117 and P = .198, respectively). Facial pain and nasal obstruction were the most common MICs in the rhinologic domain for OSA patients; thick nasal discharge and postnasal discharge were the most common MICs reported by patients in the CRS group.
“It was surprising that, for the cardinal symptoms of CRS, only two of the four were significantly worse for the CRS group and predictive of CRS [nasal discharge and loss of smell],” Dr. Jang said. “Nasal obstruction and facial pain scores were similar between the two groups. Also, there was no significant difference in each of the sleep-related questions when comparing the CRS and OSA groups.”
He concluded that the findings further underscore the “significant overlap in symptoms between CRS and OSA. The SNOT-22 questionnaire may help identify patients with undiagnosed OSA.”
Dr. Jang acknowledged certain limitations of the study, including its retrospective design and relatively small sample size. He reported receiving research funding from Olympus.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – results from a retrospective analysis demonstrated.
“We know based on experience and prior studies that there is significant overlap in symptoms for obstructive sleep apnea and chronic rhinosinusitis [CRS], which are two common conditions in the general population,” one of the study authors, David W. Jang, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Therefore, it is important to identify patients with undiagnosed OSA who may present to the physician with nose- and sinus-related symptoms.”
Dr. Jang, assistant professor of rhinology and endoscopic skull surgery in the department of surgery at Duke University, Durham, N.C., and his colleagues conducted a 3-year retrospective analysis of 165 adults who presented with a rhinologic chief complaint and completed the SNOT-22 survey. The researchers compared SNOT-22 survey results between patients with untreated OSA confirmed on polysomnography without chronic rhinosinusitis and a control group of CRS patients. A chi-square test with Bonferroni correction was used for analysis.
Of the 165 patients, 41 met criteria for untreated OSA, based on a mean apnea-hypopnea index of 29.3, while 124 were included in the CRS control group. Sleep and psychological domain scores were not significantly different between the two groups, although patients in the OSA group were more likely to choose a sleep-related symptom as their most important complaint (MIC) (P less than .001). As for the cardinal symptoms of CRS, nasal discharge and loss of smell were significantly higher in the CRS group (P less than .001), while facial pain and nasal obstruction were not significantly different (P = .117 and P = .198, respectively). Facial pain and nasal obstruction were the most common MICs in the rhinologic domain for OSA patients; thick nasal discharge and postnasal discharge were the most common MICs reported by patients in the CRS group.
“It was surprising that, for the cardinal symptoms of CRS, only two of the four were significantly worse for the CRS group and predictive of CRS [nasal discharge and loss of smell],” Dr. Jang said. “Nasal obstruction and facial pain scores were similar between the two groups. Also, there was no significant difference in each of the sleep-related questions when comparing the CRS and OSA groups.”
He concluded that the findings further underscore the “significant overlap in symptoms between CRS and OSA. The SNOT-22 questionnaire may help identify patients with undiagnosed OSA.”
Dr. Jang acknowledged certain limitations of the study, including its retrospective design and relatively small sample size. He reported receiving research funding from Olympus.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – results from a retrospective analysis demonstrated.
“We know based on experience and prior studies that there is significant overlap in symptoms for obstructive sleep apnea and chronic rhinosinusitis [CRS], which are two common conditions in the general population,” one of the study authors, David W. Jang, MD, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Therefore, it is important to identify patients with undiagnosed OSA who may present to the physician with nose- and sinus-related symptoms.”
Dr. Jang, assistant professor of rhinology and endoscopic skull surgery in the department of surgery at Duke University, Durham, N.C., and his colleagues conducted a 3-year retrospective analysis of 165 adults who presented with a rhinologic chief complaint and completed the SNOT-22 survey. The researchers compared SNOT-22 survey results between patients with untreated OSA confirmed on polysomnography without chronic rhinosinusitis and a control group of CRS patients. A chi-square test with Bonferroni correction was used for analysis.
Of the 165 patients, 41 met criteria for untreated OSA, based on a mean apnea-hypopnea index of 29.3, while 124 were included in the CRS control group. Sleep and psychological domain scores were not significantly different between the two groups, although patients in the OSA group were more likely to choose a sleep-related symptom as their most important complaint (MIC) (P less than .001). As for the cardinal symptoms of CRS, nasal discharge and loss of smell were significantly higher in the CRS group (P less than .001), while facial pain and nasal obstruction were not significantly different (P = .117 and P = .198, respectively). Facial pain and nasal obstruction were the most common MICs in the rhinologic domain for OSA patients; thick nasal discharge and postnasal discharge were the most common MICs reported by patients in the CRS group.
“It was surprising that, for the cardinal symptoms of CRS, only two of the four were significantly worse for the CRS group and predictive of CRS [nasal discharge and loss of smell],” Dr. Jang said. “Nasal obstruction and facial pain scores were similar between the two groups. Also, there was no significant difference in each of the sleep-related questions when comparing the CRS and OSA groups.”
He concluded that the findings further underscore the “significant overlap in symptoms between CRS and OSA. The SNOT-22 questionnaire may help identify patients with undiagnosed OSA.”
Dr. Jang acknowledged certain limitations of the study, including its retrospective design and relatively small sample size. He reported receiving research funding from Olympus.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Obstructive sleep apnea (OSA) should be suspected in patients with sleep dysfunction as their primary complaint without the significant nasal drainage and anosmia that characterizes chronic rhinosinusitis.
Major finding: Sleep and psychological domain scores on the SNOT-22 were not significantly different between patients with chronic rhinosinusitis and those with OSA, although OSA patients were more likely to choose a sleep-related symptom as their most important complaint (P less than .001).
Study details: A retrospective analysis of 165 adults who presented with a rhinologic chief complaint and completed the SNOT-22 survey.
Disclosures: Dr. Jang reported receiving research funding from Olympus.
Clinical trial: Magnetic Resonance Imaging in Obstructive Sleep Apnea
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
What’s the Buzz? Treatment Strategies in Chronic Subjective Tinnitus
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7
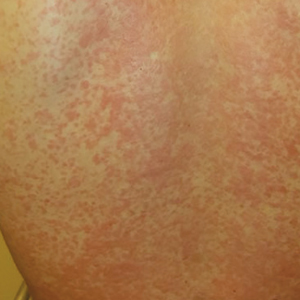
Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7

Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7

Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
Man, 25, With Sinus Pain, Sore Throat, and Rash
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.

One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.

The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11

The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.

One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.

The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11

The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
Emerging CPAP options show sustained benefits
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
REPORTING FROM SLEEP 2018
Key clinical point: Alternative treatments have shown sustained improvement in sleep apnea symptoms.
Major finding: The negative-pressure device improved oxygen flow in OSA to 64% vs. 25% for continuous-positive airway pressure.
Data source: Multiple abstracts presented at Sleep 2018 and published studies, including ADHERE study of 326 patients and a multicenter study of 430 patients using the upper airway stimulation device; a trial of 67 patients using the nasopharyngeal airway stent; and 101 patients in the artificial intelligence study.
Disclosures: Dr. Kushida disclosed a relationship with Seven Dreamers Laboratories. Dr. Kram is a paid adviser for Sommetrics. Dr. Remmers is founder of Zephyr Sleep Technologies. Dr. Strollo is an investigator in the STAR trial, supported by Inspire Medical Systems.
After warning, codeine use after tonsillectomy drops, doesn’t stop
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
FROM PEDIATRICS
Location matters when it comes to thyroidectomy rates
Thyroidectomy rates differ widely across the United States, according to a cross-sectional analysis of Medicare beneficiaries, but researchers aren’t sure what’s driving the variation.
There was a 6.2-fold difference in thyroidectomy rates across U.S. hospital referral regions in 2014, ranging from 22 to 139 per 100,000 Medicare beneficiaries. The national average was 60 procedures per 100,000 Medicare beneficiaries, David O. Francis, MD, of the University of Wisconsin, Madison, and his coauthors, reported (JAMA Otolaryngol Head Neck Surg. 2017 Oct 12. doi: 10.1001/jamaoto.2017.1746).
The researchers conducted a cross-sectional analysis of 15,888 Medicare beneficiaries aged 65 years and older who underwent a thyroidectomy in 2014. Of the thyroidectomies performed, 7,056 were partial and 8,382 were total thyroidectomies. They compared the frequency of partial and total thyroidectomies to total prostatectomy rates (high variation) and hospitalizations for hip fractures (low variation).
The stark variation in thyroidectomy outpaced those in hip fracture hospitalization (2.2-fold variation) and radical prostatectomy (5.6-fold variation) across U.S. hospital referral regions.
Higher rates of thyroidectomy were seen in Southern, Central, and certain urban regions of the United States.
But the variation in rates did not correlate with health care availability, socioeconomic status, or the availability of surgeons. This suggests that variation is caused by something other than disease burden. The researchers speculated that the “variability in thyroid surgery rates in areas with similar access to surgical services largely relates to local beliefs and practice patterns.”
The researchers also noted that the findings, which are based on Medicare data, may not be generalizable to young patients who account for more than half of all thyroidectomies performed in the United States.
The study was funded by the Department of Veterans Affairs and the Dartmouth Institute for Health Policy & Clinical Practice, with salary support from the National Institutes of Health. The researchers reported having no relevant conflicts of interest.
Thyroidectomy rates differ widely across the United States, according to a cross-sectional analysis of Medicare beneficiaries, but researchers aren’t sure what’s driving the variation.
There was a 6.2-fold difference in thyroidectomy rates across U.S. hospital referral regions in 2014, ranging from 22 to 139 per 100,000 Medicare beneficiaries. The national average was 60 procedures per 100,000 Medicare beneficiaries, David O. Francis, MD, of the University of Wisconsin, Madison, and his coauthors, reported (JAMA Otolaryngol Head Neck Surg. 2017 Oct 12. doi: 10.1001/jamaoto.2017.1746).
The researchers conducted a cross-sectional analysis of 15,888 Medicare beneficiaries aged 65 years and older who underwent a thyroidectomy in 2014. Of the thyroidectomies performed, 7,056 were partial and 8,382 were total thyroidectomies. They compared the frequency of partial and total thyroidectomies to total prostatectomy rates (high variation) and hospitalizations for hip fractures (low variation).
The stark variation in thyroidectomy outpaced those in hip fracture hospitalization (2.2-fold variation) and radical prostatectomy (5.6-fold variation) across U.S. hospital referral regions.
Higher rates of thyroidectomy were seen in Southern, Central, and certain urban regions of the United States.
But the variation in rates did not correlate with health care availability, socioeconomic status, or the availability of surgeons. This suggests that variation is caused by something other than disease burden. The researchers speculated that the “variability in thyroid surgery rates in areas with similar access to surgical services largely relates to local beliefs and practice patterns.”
The researchers also noted that the findings, which are based on Medicare data, may not be generalizable to young patients who account for more than half of all thyroidectomies performed in the United States.
The study was funded by the Department of Veterans Affairs and the Dartmouth Institute for Health Policy & Clinical Practice, with salary support from the National Institutes of Health. The researchers reported having no relevant conflicts of interest.
Thyroidectomy rates differ widely across the United States, according to a cross-sectional analysis of Medicare beneficiaries, but researchers aren’t sure what’s driving the variation.
There was a 6.2-fold difference in thyroidectomy rates across U.S. hospital referral regions in 2014, ranging from 22 to 139 per 100,000 Medicare beneficiaries. The national average was 60 procedures per 100,000 Medicare beneficiaries, David O. Francis, MD, of the University of Wisconsin, Madison, and his coauthors, reported (JAMA Otolaryngol Head Neck Surg. 2017 Oct 12. doi: 10.1001/jamaoto.2017.1746).
The researchers conducted a cross-sectional analysis of 15,888 Medicare beneficiaries aged 65 years and older who underwent a thyroidectomy in 2014. Of the thyroidectomies performed, 7,056 were partial and 8,382 were total thyroidectomies. They compared the frequency of partial and total thyroidectomies to total prostatectomy rates (high variation) and hospitalizations for hip fractures (low variation).
The stark variation in thyroidectomy outpaced those in hip fracture hospitalization (2.2-fold variation) and radical prostatectomy (5.6-fold variation) across U.S. hospital referral regions.
Higher rates of thyroidectomy were seen in Southern, Central, and certain urban regions of the United States.
But the variation in rates did not correlate with health care availability, socioeconomic status, or the availability of surgeons. This suggests that variation is caused by something other than disease burden. The researchers speculated that the “variability in thyroid surgery rates in areas with similar access to surgical services largely relates to local beliefs and practice patterns.”
The researchers also noted that the findings, which are based on Medicare data, may not be generalizable to young patients who account for more than half of all thyroidectomies performed in the United States.
The study was funded by the Department of Veterans Affairs and the Dartmouth Institute for Health Policy & Clinical Practice, with salary support from the National Institutes of Health. The researchers reported having no relevant conflicts of interest.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Key clinical point:
Major finding: Thyroidectomy rates vary 6.2-fold across U.S. hospital referral regions.
Data source: A cross-sectional analysis of Medicare data for 15,888 patients in 2014.
Disclosures: This study was funded by the Department of Veterans Affairs and the Dartmouth Institute for Health Policy & Clinical Practice, with salary support from the National Institutes of Health. The researchers reported having no relevant conflicts of interest.