User login
First prospective study finds pregnancies with Sjögren’s to be largely safe
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
2023 Update on genetics in fetal growth
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):
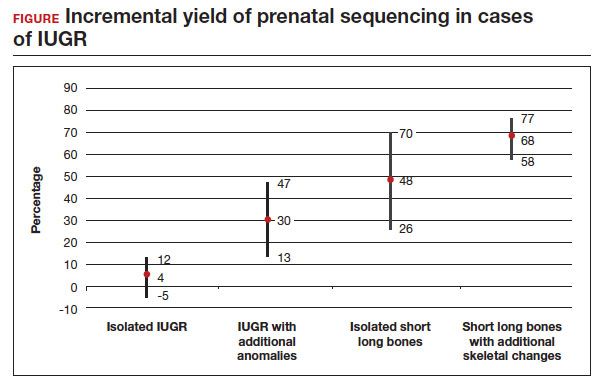
- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
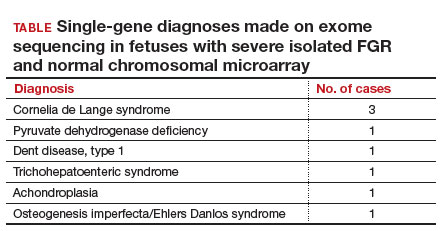
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.
To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
Investigating the etiology of recurrent pregnancy loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
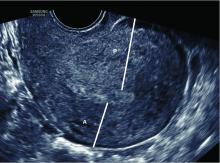
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.
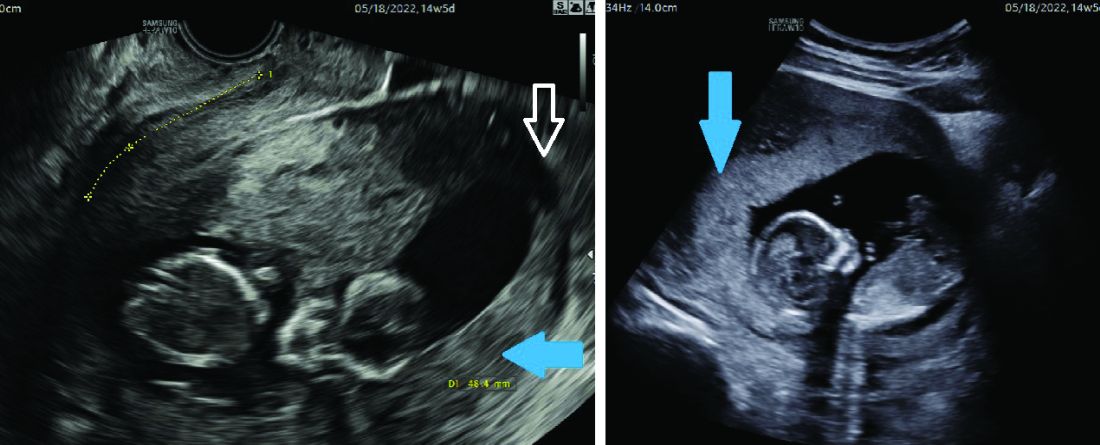
Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
With attention to the timing of loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Maternal health clinic teams with legal services to aid patients
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ACOG 2023
First in utero cerebrovascular surgery success
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
FROM STROKE
Scheduled bleeding may boost tolerability of hormone implants
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACOG 2023
Once-daily nifedipine sufficient for hypertension in pregnancy
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
AT ACOG 2023
Over half of pregnant patients not properly screened for thyroid disease
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
BALTIMORE – Less than half of the pregnant patients who met the criteria for thyroid screening were actually screened by their clinician, according to a retrospective cohort study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists in Baltimore. Those who met criteria and did receive screening had higher live birth rates and lower miscarriage rates than those who met the criteria but did not undergo screening, the study found.
“These results suggest that improving thyroid screening adherence may lead to improved pregnancy outcomes,” lead author Allan Dong, MD, of Advocate Lutheran General Hospital in Des Plaines, Ill., told attendees. “However, following targeted screening guidelines can be difficult for clinicians. In practice, universal screening for diabetes and pregnancy may provide more comprehensive screening coverage and potentially lead to improved outcomes.”
Instead of universal screening for thyroid disease, ACOG and the American Thyroid Association recommend targeted screening of high-risk patients, though ATA’s criteria are substantially broader than ACOG’s. But, Dr. Dong told attendees, “guidelines are only beneficial if they are followed appropriately,” and Ob.Gyns. have limited time to screen for risk factors in the midst of other clinical priorities. So he aimed to learn whether Ob.Gyns. were following the guidelines of either organization in screening people at higher risk for thyroid disease.
Dr. Dong and his coauthor, Melisa Lott, DO, reviewed the charts of all 1,025 patients who presented at their institution for new obstetrical visits in 2020 to determine which ones had risk factors that would qualify them for screening under ATA or ACOG guidelines. ACOG’s screening criteria included having a personal or family history of thyroid disease or type 1 diabetes, or there being clinical suspicion for thyroid disease. ATA’s screening criteria included the following:
- Personal or family history of thyroid disease.
- History of head or neck radiation.
- History of a prior thyroid surgery.
- Over age 30.
- Any autoimmune disease.
- A body mass index greater than 40 kg/m2.
- History of pregnancy loss, preterm delivery, or infertility.
- Recently used amiodarone lithium or iodine-based contrast.
- Lived in an area of known iodine deficiency.
- Clinical suspicion of thyroid disease.
ATA screening criteria identified four times as many patients requiring screening than did ACOG criteria, Dr. Dong noted. Of the 198 patients who met ACOG’s criteria, 43.9% were screened with thyroid function testing. Meanwhile, 826 patients – including all those who met ACOG’s criteria – met ATA’s criteria for screening, but only 13.1% of them underwent thyroid function testing.
Live birth rates were significantly higher among patients who met ATA criteria and were screened (92.6%) than among patients who met ATA criteria but were not screened (83.3%, P = .006). Similarly, the miscarriage rate was 4.6% in patients who met ATA criteria and were screened, compared to 12.4% in patients who met the criteria but did not undergo thyroid function testing (P = .009).
“A similar difference, although not statistically significant, was noted when comparing patients who were screened appropriately per ACOG criteria with those who met criteria for screening but were not screened,” Dr. Dong told attendees. “However, our study was underpowered to detect this difference due to the lower number of patients who meet criteria for screening under ACOG guidelines.”
The researchers did not find any significant difference in preterm delivery rates.
Anna Whelan, MD, of Women & Infants Hospital of Brown University, Providence, R.I., was not involved in the study but viewed the poster and pointed out that many of the patients, if seen by a primary care provider prior to pregnancy, would likely have been screened by their PCP. The rate of underscreening therefore suggests that patients “are not getting good, consistent primary care because there’s a lack of primary care physicians,” Dr. Whelan said in an interview.
In addition, she added, “maybe not all obstetricians and those providing care, such as midwives and other providers, are aware of the [ATA] guidelines on who should be screened.” She added that additional education about thyroid screening guidelines might be helpful for providers.
Dr. Dong reported being a stock shareholder in 3M, AbbVie, General Electric, Johnson & Johnson, Medtronic, Pfizer, and Viking Therapeutics. Dr. Whelan had no disclosures.
FROM ACOG 2023
Would you prescribe antenatal steroids to a pregnant patient at high risk for delivering at 22 weeks’ gestation?
For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2
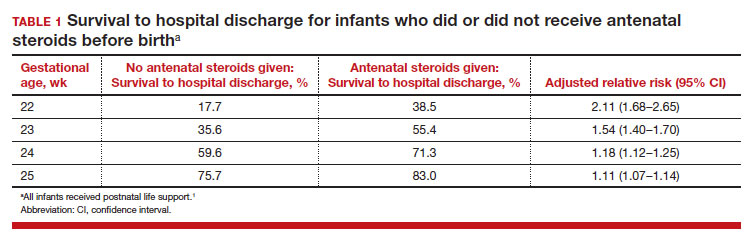
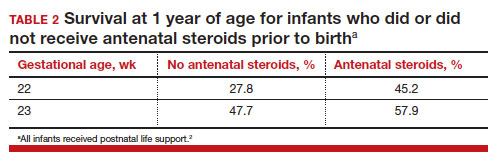
The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5
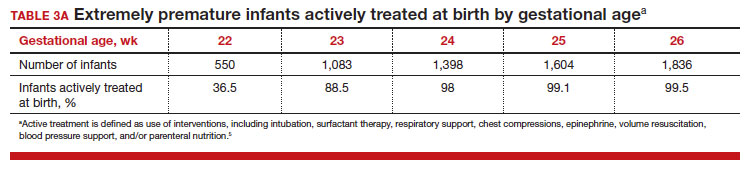
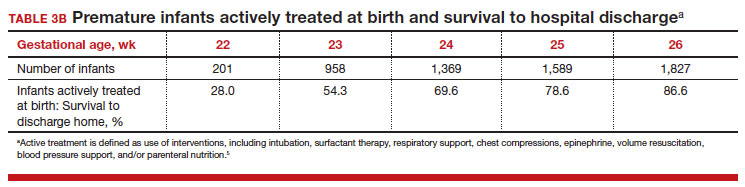
Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.

For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2


The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5


Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●

For many decades, the limit of newborn viability was at approximately 24 weeks’ gestation. Recent advances in pregnancy and neonatal care suggest that the new limit of viability is 22 (22 weeks and 0 days to 22 weeks and 6 days) or 23 (23 weeks and 0 days to 23 weeks and 6 days) weeks of gestation. In addition, data from observational cohort studies indicate that for infants born at 22 and 23 weeks’ gestation, survival is dependent on a course of antenatal steroids administered prior to birth plus intensive respiratory and cardiovascular support at delivery and in the neonatal intensive care unit (NICU).
Antenatal steroids: Critical for survival at 22 and 23 weeks of gestation
Most studies of birth outcomes at 22 and 23 weeks’ gestation rely on observational cohorts where unmeasured differences among the maternal-fetal dyads that received or did not receive a specific treatment confounds the interpretation of the data. However, data from multiple large observational cohorts suggest that between 22 and 24 weeks of gestation, completion of a course of antenatal steroids will optimize infant outcomes. Particularly noteworthy was the observation that the incremental survival benefit of antenatal steroids was greatest at 22 and 23 weeks’ gestation (TABLE 1).1 Similar results have been reported by Rossi and colleagues (TABLE 2).2


The importance of a completed course of antenatal steroids before birth was confirmed in another cohort study of 431 infants born in 2016 to 2019 at 22 weeks and 0 days’ to 23 weeks and 6 days’ gestation.3 Survival to discharge occurred in 53.9% of infants who received a full course of antenatal steroids before birth and 35.5% among those who did not receive antenatal steroids..3 Survival to discharge without major neonatal morbidities was 26.9% in those who received a full course of antenatal steroids and 10% among those who did not. In this cohort, major neonatal morbidities included severe intracranial hemorrhage, cystic periventricular leukomalacia, severe bronchopulmonary dysplasia, surgical necrotizing enterocolitis, or severe retinopathy of prematurity requiring treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal steroids prior to 22 weeks and 0 days gestation.4 However, some neonatologists might recommend that antenatal steroids be given starting at 21 weeks and 5 days of gestation if birth is anticipated in the 22nd week of gestation and the patient prefers aggressive treatment of the newborn.
Active respiratory and cardiovascular support improves newborn outcomes
To maximize survival, infants born at 22 and 23 weeks’ gestation always require intensive active treatment at birth and in the following days in the NICU. Active treatment may include respiratory support, surfactant treatment, pressors, closure of a patent ductus arteriosus, transfusion of red blood cells, and parenteral nutrition. In one observational cohort study, active treatment at birth was not routinely provided at 22 and 23 weeks’ gestation but was routinely provided at later gestational ages (TABLE 3A).5 Not surprisingly, active treatment, especially at early gestational ages, is associated with improved survival to discharge. For example, at 22 weeks’ gestation, survival to discharge in infants who received or did not receive intensive active treatment was 28% and 0%, respectively.5 However, specific clinical characteristics of the pregnant patient and newborn may have influenced which infants were actively treated, confounding interpretation of the observation. In this cohort of extremely premature newborns, survival to hospital discharge increased substantially between 22 weeks and 26 weeks of gestational age (TABLE 3B).5


Many of the surviving infants needed chronic support treatment. Among surviving infants born at 22 weeks and 26 weeks, chronic support treatments were being used by 22.6% and 10.6% of infants, respectively, 2 years after birth.5 For surviving infants born at 22 weeks, the specific chronic support treatments included gastrostomy or feeding tube (19.4%), oxygen (9.7%), pulse oximeter (9.7%), and/or tracheostomy (3.2%). For surviving infants born at 26 weeks’ gestation, the specific chronic support treatments included gastrotomy or feeding tube (8.5%), pulse oximeter (4.4%), oxygen (3.2%), tracheostomy (2.3%), an apnea monitor (1.5%), and/or ventilator or continuous positive airway pressure (1.1%).5
Continue to: Evolving improvement in infant outcomes...
Evolving improvement in infant outcomes
In 1963, Jacqueline Bouvier Kennedy went into preterm labor at 34 weeks of gestation and delivered her son Patrick at a community facility. Due to severe respiratory distress syndrome, Patrick was transferred to the Boston Children’s Hospital, and he died shortly thereafter.6 Sixty years later, due to advances in obstetric and neonatal care, death from respiratory distress syndrome at 34 weeks of gestation is uncommon in the United States.
Infant outcomes following birth at 22 and 23 weeks’ gestation continue to improve. An observational cohort study from Sweden reported that at 22 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 10% and 30%, respectively.7 Similarly, at 23 weeks’ gestation, the percentage of live-born infants who survived to 1-year post birth in 2004 to 2007 and 2014 to 2016 was 52% and 61%, respectively.7 However, most of the surviving infants in this cohort had one or more major neonatal morbidities, including intraventricular hemorrhage grade 3 or 4; periventricular leukomalacia; necrotizing enterocolitis; retinopathy of prematurity grade 3, 4, or 5; or severe bronchopulmonary dysplasia.7
In a cohort of infants born in Japan at 22 to 24 weeks of gestation, there was a notable decrease in major neurodisability at 3 years of age for births occurring in 2 epochs, 2003 to 2007 and 2008 to 2012.8 When comparing outcomes in 2003 to 2007 versus 2008 to 2012, the change in rate of various major complications included the following: cerebral palsy (15.9% vs 9.5%), visual impairment (13.6% vs 4.4%), blindness (4.8% vs 1.3%), and hearing impairment (2.6% vs 1.0%). In contrast, the rate of cognitive impairment, defined as less than 70% of standard test performance for chronological age, was similar in the 2 time periods (36.5% and 37.9%, respectively).8 Based on data reported between 2000 and 2020, a systematic review and meta-analysis by Backes and colleagues concluded that there has been substantial improvement in the survival of infants born at 22 weeks of gestation.9
The small baby unit
A feature of modern medicine is the relentless evolution of new clinical subspecialties and sub-subspecialties. NICUs evolved from newborn nurseries to serve the needs of the most severely ill newborns, with care provided by a cadre of highly trained subspecialized neonatologists and neonatal nurses. A new era is dawning, with some NICUs developing a sub-subspecialized small baby unit to care for infants born between 22 and 26 weeks of gestation. These units often are staffed by clinicians with a specific interest in optimizing the care of extremely preterm infants, providing continuity of care over a long hospitalization.10 The benefits of a small baby unit may include:
- relentless standardization and adherence to the best intensive care practices
- daily use of checklists
- strict adherence to central line care
- timely extubation and transition to continuous positive airway pressure
- adherence to breastfeeding guidelines
- limiting the number of clinicians responsible for the patient
- promotion of kangaroo care
- avoidance of noxious stimuli.10,11
Continue to: Ethical and clinical issues...
Ethical and clinical issues
Providing clinical care to infants born at the edge of viability is challenging and raises many ethical and clinical concerns.12,13 For an infant born at the edge of viability, clinicians and parents do not want to initiate a care process that improves survival but results in an extremely poor quality of life. At the same time, clinicians and parents do not want to withhold care that could help an extremely premature newborn survive and thrive. Consequently, the counseling process is complex and requires coordination between the obstetrical and neonatology disciplines, involving physicians and nurses from both. A primary consideration in deciding to institute active treatment at birth is the preference of the pregnant patient and the patient’s trusted family members. A thorough discussion of these issues is beyond the scope of this editorial. ACOG provides detailed advice about the approach to counseling patients who face the possibility of a periviable birth.14
To help standardize the counseling process, institutions may find it helpful to recommend that clinicians consistently use a calculator to provide newborn outcome data to patients. The National Institute of Child Health and Human Development’s Extremely Preterm Birth Outcomes calculator uses the following inputs:
- gestational age
- estimated birth weight
- sex
- singleton/multiple gestation
- antenatal steroid treatment.
It also provides the following outputs as percentages:
- survival with active treatment at birth
- survival without active treatment at birth
- profound neurodevelopmental impairment
- moderate to severe neurodevelopmental impairment
- blindness
- deafness
- moderate to severe cerebral palsy
- cognitive developmental delay.15
A full assessment of all known clinical factors should influence the interpretation of the output from the clinical calculator. An alternativeis to use data from the Vermont Oxford Network. NICUs with sufficient clinical volume may prefer to use their own outcome data in the counseling process.
Institutions and clinical teams may improve the consistency of the counseling process by identifying criteria for 3 main treatment options:
- clinical situations where active treatment at birth is not generally offered (eg, <22 weeks’ gestation)
- clinical situations where active treatment at birth is almost always routinely provided (eg, >25 weeks’ gestation)
- clinical situations where patient preferences are especially important in guiding the use of active treatment.
Most institutions do not routinely offer active treatment of the newborn at a gestational age of less than 22 weeks and 0 days. Instead, comfort care often is provided for these newborns. Most institutions routinely provide active treatment at birth beginning at 24 or 25 weeks’ gestation unless unique risk factors or comorbidities warrant not providing active treatment (TABLE 3A). Some professional societies recommend setting a threshold for recommending active treatment at birth. For example, the British Association of Perinatal Medicine recommends that if there is 50% or higher probability of survival without severe disability, active treatment at birth should be considered because it is in the best interest of the newborn.16 In the hours and days following birth, the clinical course of the newborn greatly influences the treatment plan and care goals. After the initial resuscitation, if the clinical condition of an extremely preterm infant worsens and the prognosis is grim, a pivot to palliative care may be considered.
Final thoughts
Periviability is the earliest stage of fetal development where there is a reasonable chance, but not a high likelihood, of survival outside the womb. For decades, the threshold for periviability was approximately 24 weeks of gestation. With current obstetrical and neonatal practice, the new threshold for periviability is 22 to 23 weeks of gestation, but death prior to hospital discharge occurs in approximately half of these newborns. For the survivors, lifelong neurodevelopmental complications and pulmonary disease are common. Obstetricians play a key role in counseling patients who are at risk of giving birth before 24 weeks of gestation. Given the challenges faced by an infant born at 22 and 23 weeks’ gestation, pregnant patients and trusted family members should approach the decision to actively resuscitate the newborn with caution. However, if the clinical team, patient, and trusted family members agree to pursue active treatment, completion of a course of antenatal steroids and appropriate respiratory and cardiovascular support at birth are key to improving long-term outcomes. ●
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks gestation. JAMA Network Open. 2018;E183235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks [published online November 28, 2021]. Am J Perinatol. doi:10.1055/s-004-1740062
- Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Network Open. 2022;5:E2233331.
- Use of antenatal corticosteroids at 22 weeks of gestation. ACOG website. Published September 2021. Accessed April 10, 2023. https://www.acog .org/clinical/clinical-guidance/practice -advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation
- Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248-263.
- The tragic death of Patrick, JFK and Jackie’s newborn son, in 1963. Irish Central website. Published November 6, 2022. Accessed April 10, 2023. https://www.irishcentral.com/roots/history /tragic-death-patrick-kennedy-jfk-jackie
- Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;32:1188-1199.
- Kono Y, Yonemoto N, Nakanishi H, et al. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatrics Open. 2018;2:E000211.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Morris M, Cleary JP, Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:E1007-E1015.
- Fathi O, Nelin LD, Shephard EG, et al. Development of a small baby unit to improve outcomes for the extremely premature infant. J Perinatology. 2002;42:157-164.
- Lantos JD. Ethical issues in treatment of babies born at 22 weeks of gestation. Arch Dis Child. 2021;106:1155-1157.
- Shinwell ES. Ethics of birth at the limit of viability: the risky business of prediction. Neonatology. 2015;107:317-320.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No 6. periviable birth. Obstet Gynecol. 2017;E187-E199.
- Extremely preterm birth outcomes tool. NICHD website. Updated March 2, 2020. Accessed April 10, 2023. https://www.nichd.nih.gov/research /supported/EPBO/use#
- Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. 2020;105:F232-F239.
News & Perspectives from Ob.Gyn. News
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.