User login
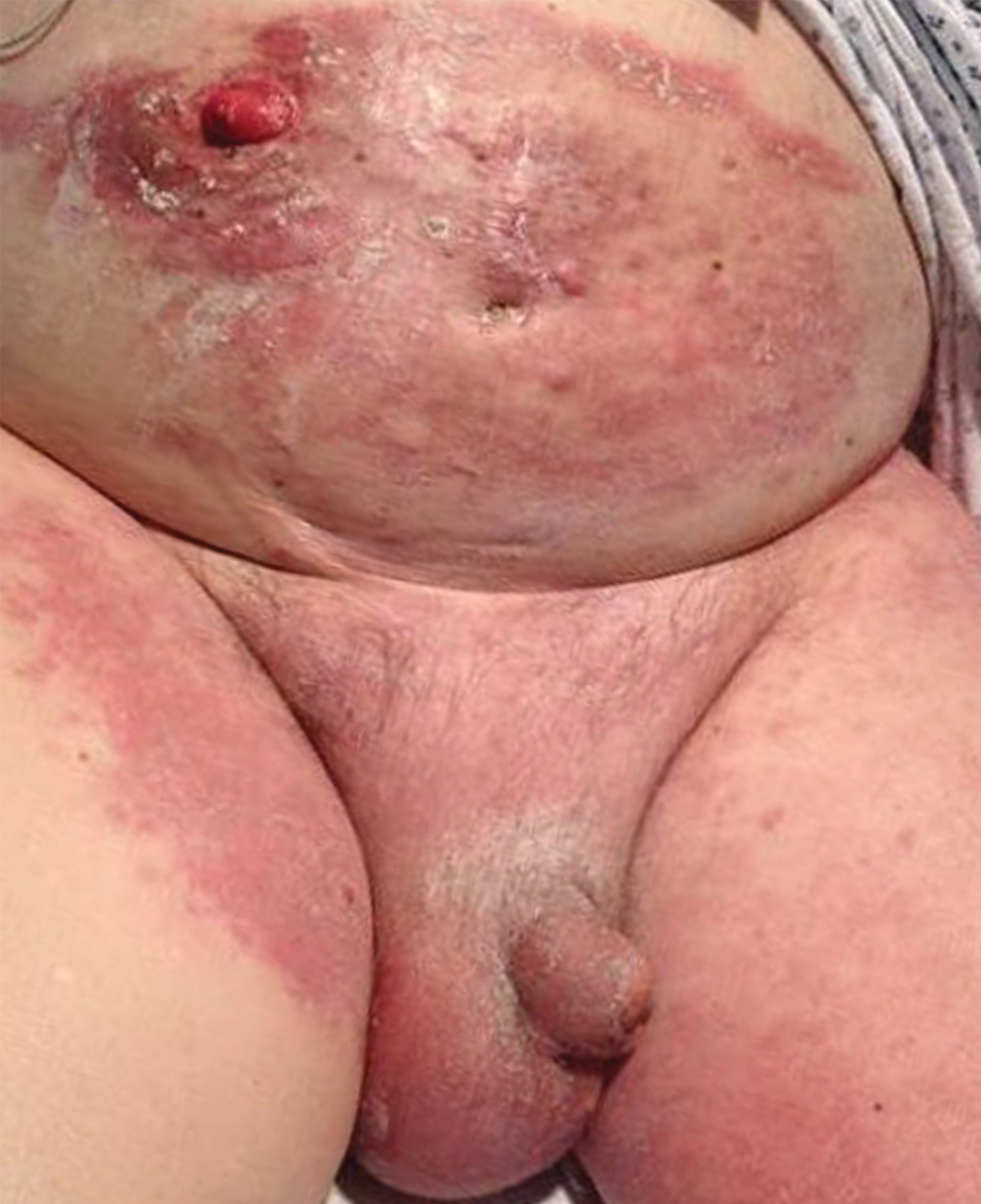
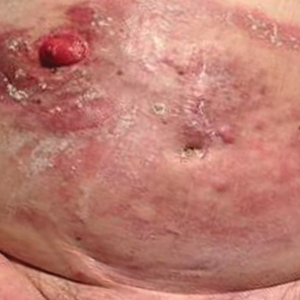
Erythematous Plaques and Nodules on the Abdomen and Groin
The Diagnosis: Inflammatory Urothelial Carcinoma
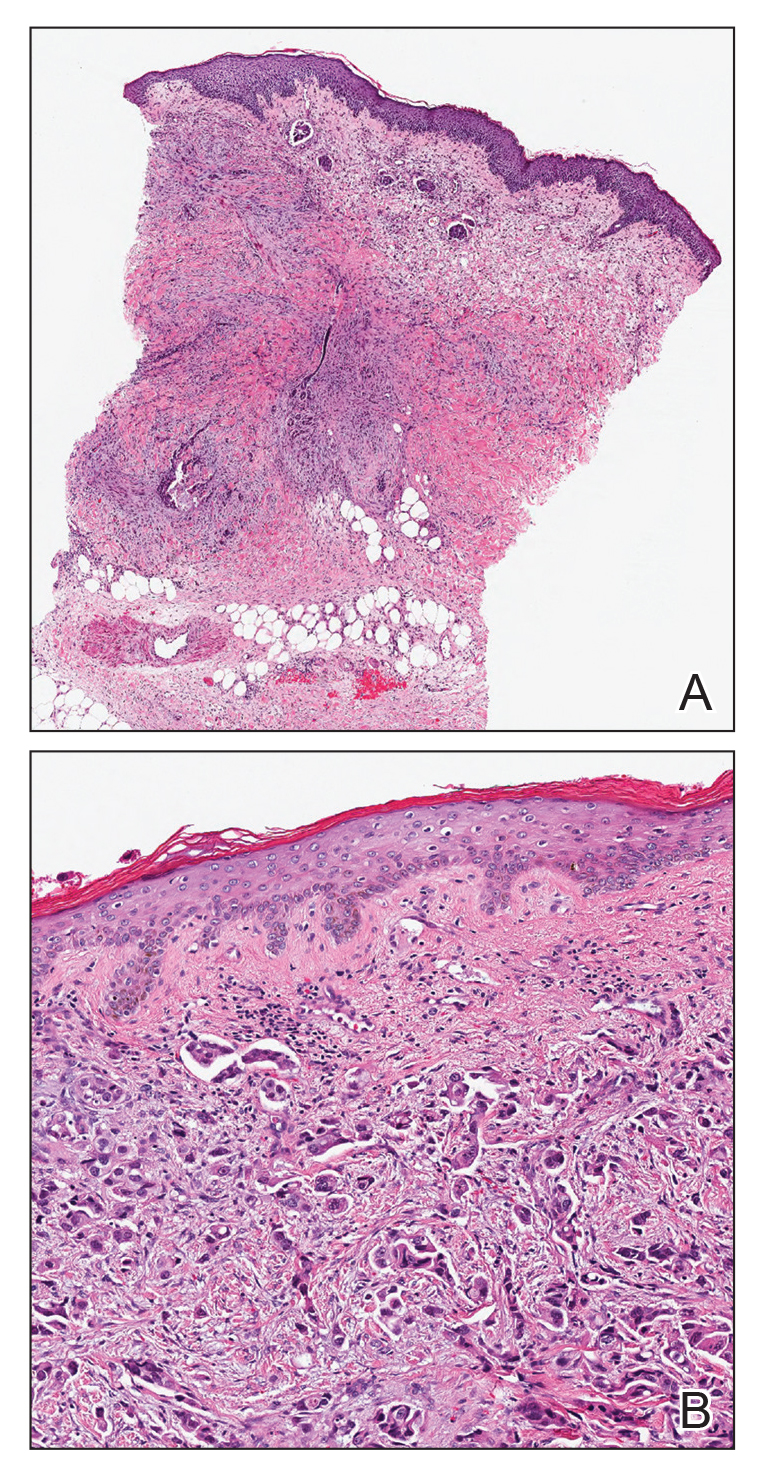
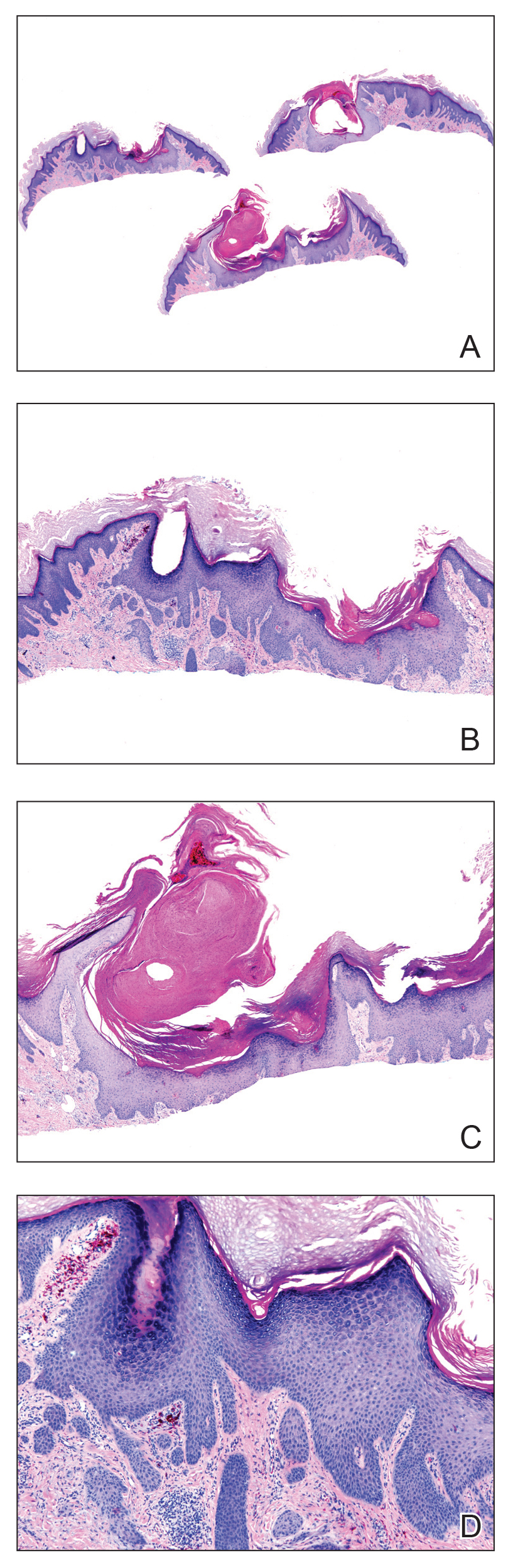
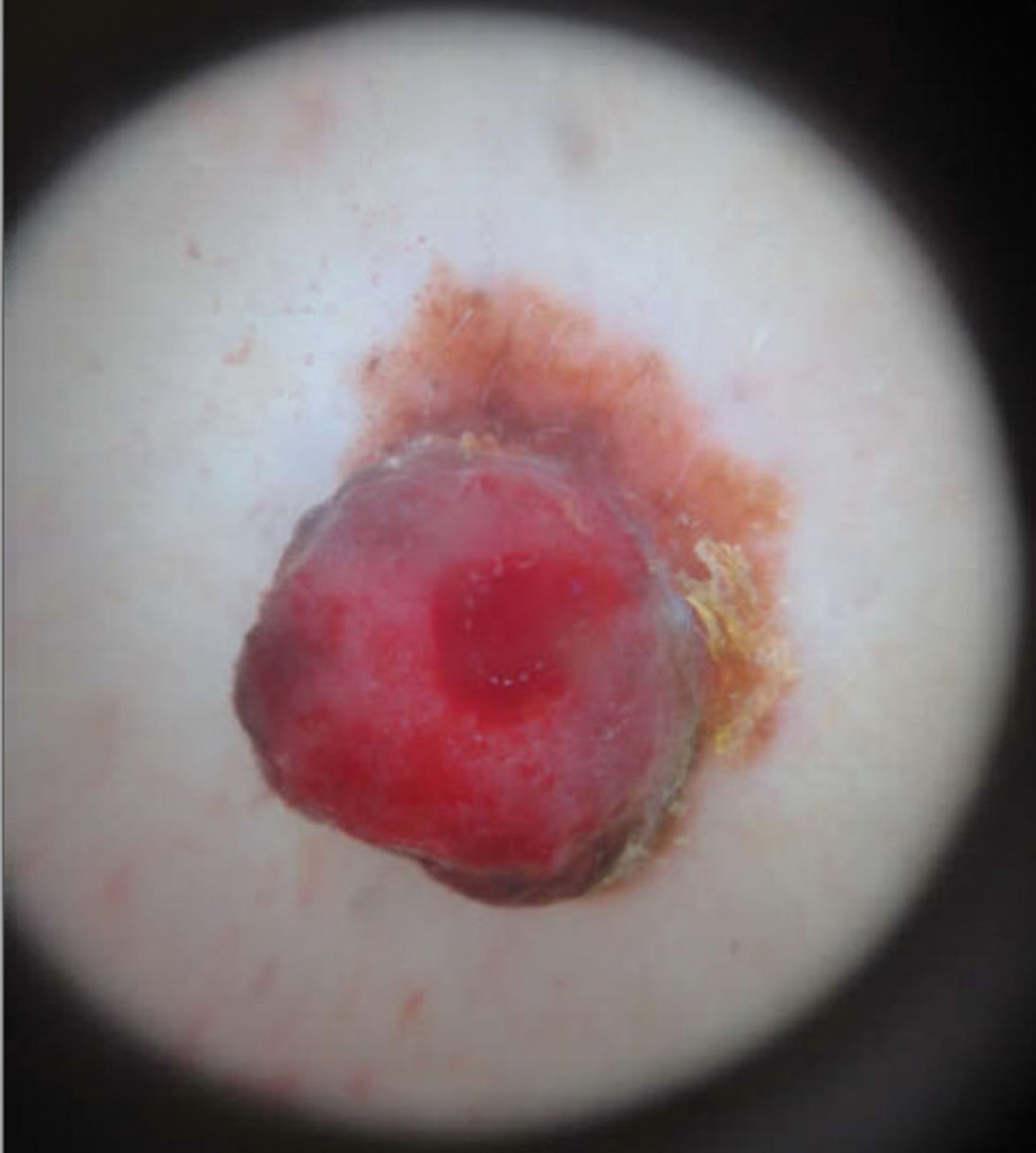
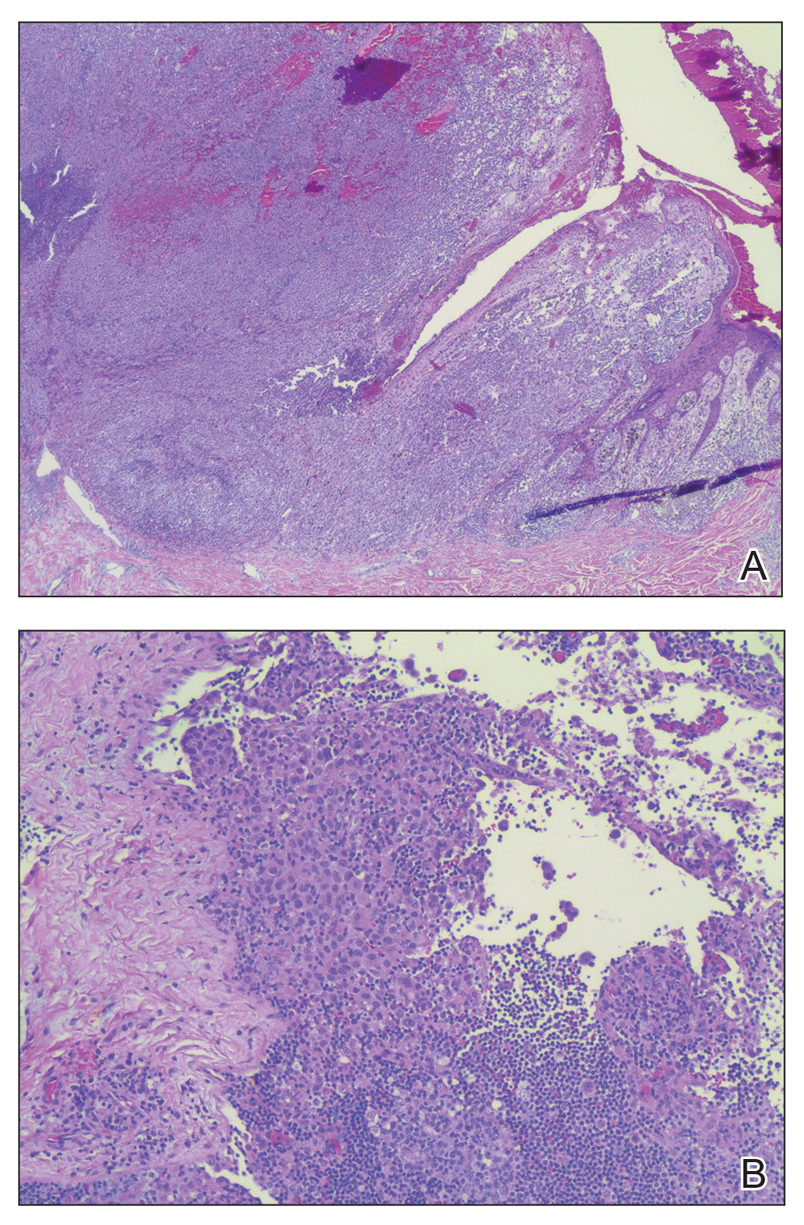
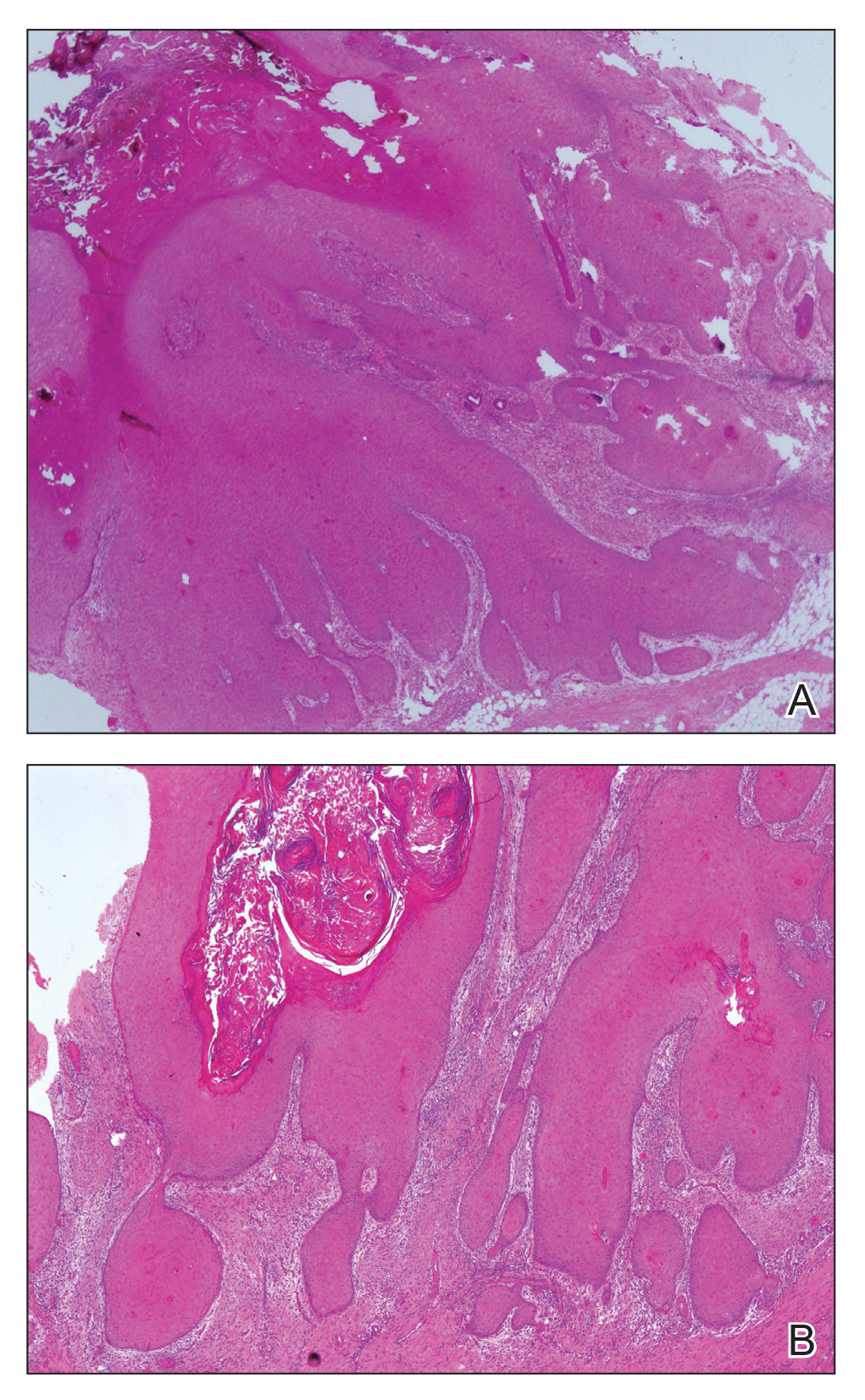
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
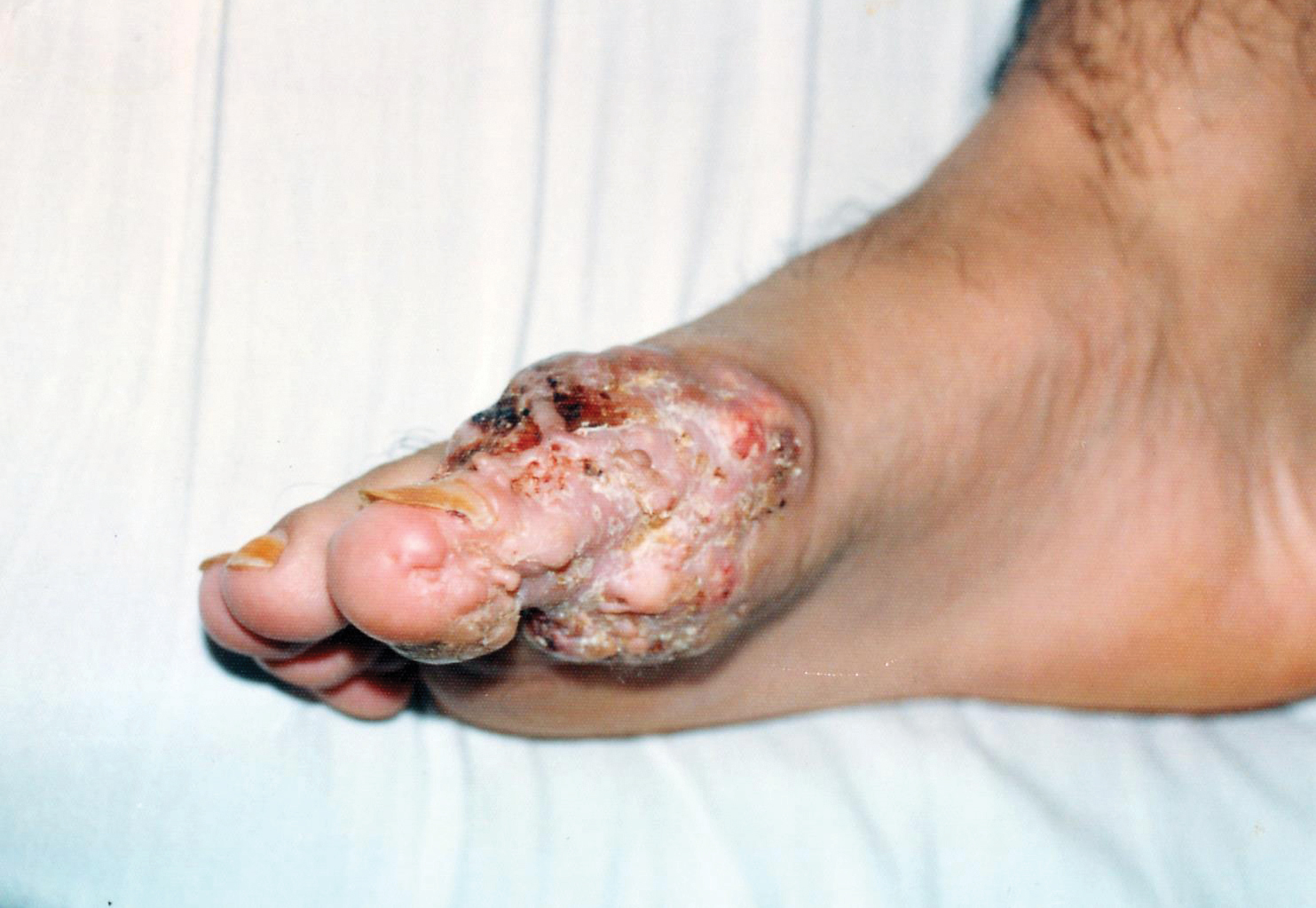
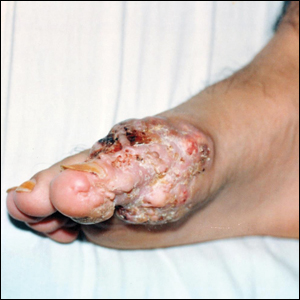
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
An 82-year-old man presented with acute abdominal pain and distension as well as an abdominal rash of 4 months' duration that was expanding despite treatment with topical miconazole. He had a history of melanoma and bladder cancer treated with cystoprostatectomy. He previously was diagnosed with candidiasis of his urostomy and was taking oral fluconazole. Physical examination revealed a large, well-demarcated, erythematous, smooth plaque covering the entire abdomen, scrotum, penis, inguinal folds, and bilateral upper thighs, with several satellite plaques and firm nodules clustered around the umbilicus. An 8-mm punch biopsy of a periumbilical nodule was performed.
Basal Cell Carcinoma Arising in Nevus Sebaceous During Pregnancy
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
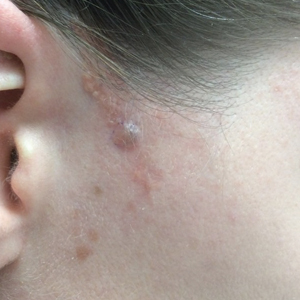
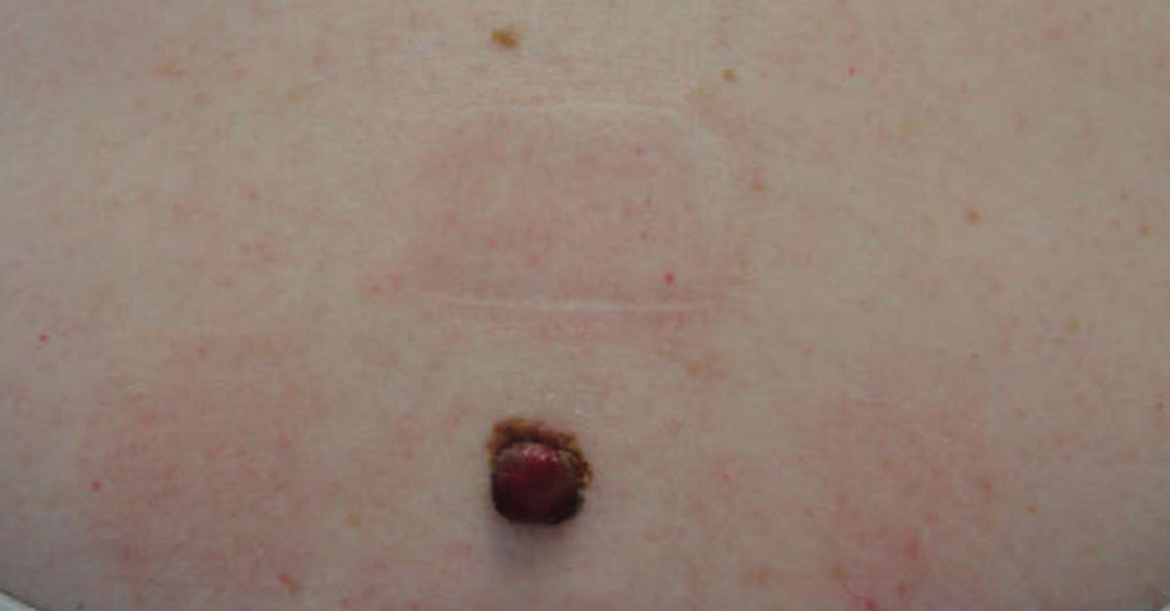
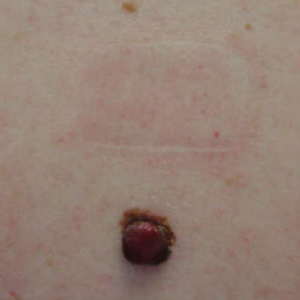
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
Practice Points
- Benign neoplasms arise more frequently in nevus sebaceous (NS) lesions than do malignant neoplasms.
- The hormonal changes that occur during pregnancy and puberty appear to play a role in the development of neoplasms in NS lesions.
- Monitoring NS lesions more closely during periods of hormonal change may help diagnose malignant transformations in these patients.
Multiple Keratoacanthomas Arising Within Red Tattoo Pigment
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
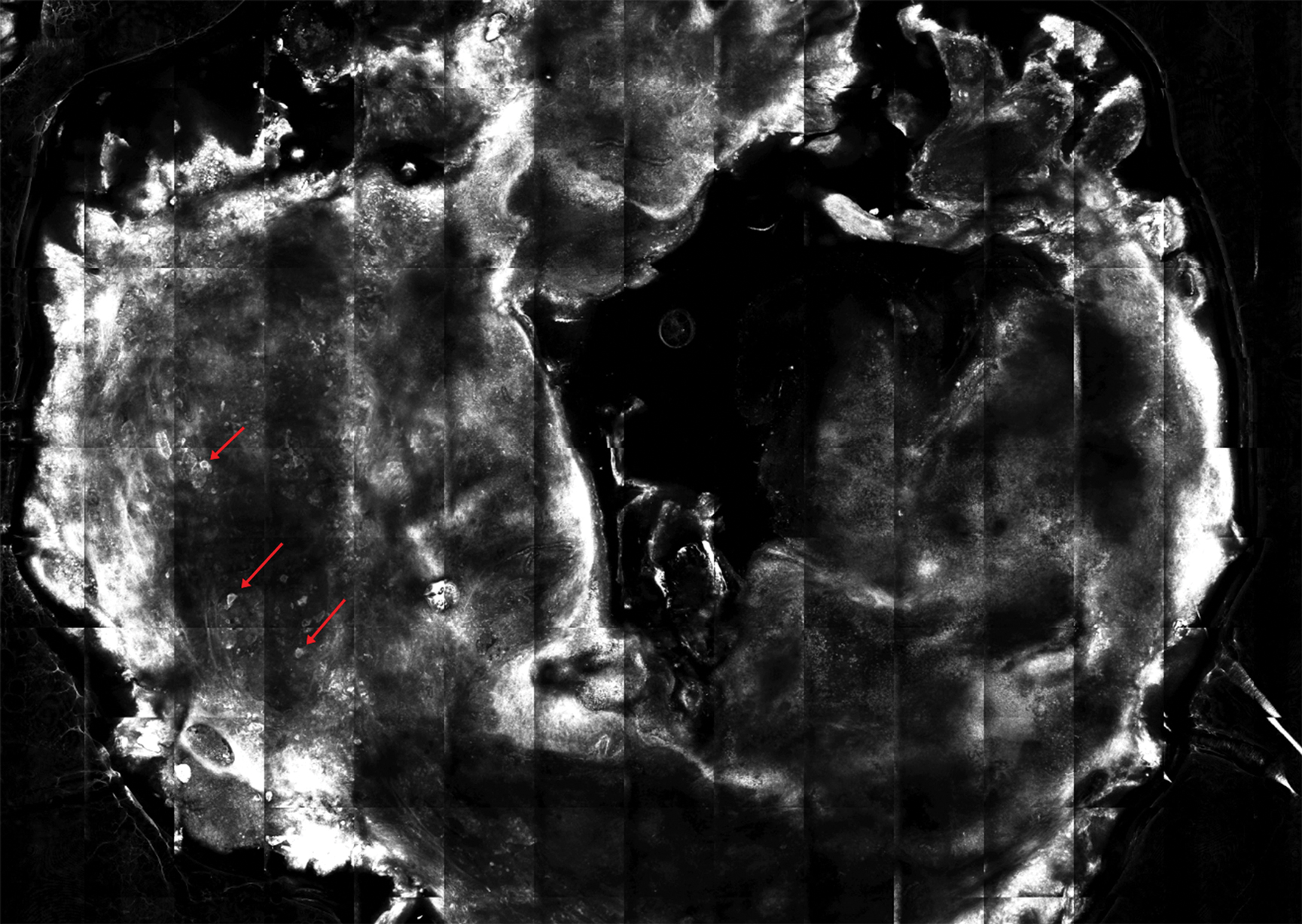
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
Practice Points
- Tattoo reactions range from infectious and inflammatory dermatoses to the development of malignant neoplasms.
- Red pigment is the most common cause of adverse tattoo reactions.
- The management of tattoo-associated keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) has not been widely published, but they can be approached similarly to nontattoo-associated KA-SCCs.
Skin Scores: A Review of Clinical Scoring Systems in Dermatology
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
Resident Pearls
- Mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis can be estimated by calculating the SCORTEN at the end of days 1 and 3 of hospitalization.
- The Psoriasis Epidemiology Screening Tool (PEST) assists with triaging which patients with psoriasis should be evaluated for psoriatic arthritis by a rheumatologist.
- The ALT-70 score is helpful to support one’s diagnosis of cellulitis or pseudocellulitis.
- The Mohs appropriate use criteria (AUC) score 270 different clinical scenarios as appropriate, uncertain, or inappropriate for Mohs micrographic surgery.
Lifetime indoor tanning raises risk of cutaneous squamous cell carcinoma
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
FROM JAMA DERMATOLOGY
Twitter Chat: Skin Cancer

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.
Reflectance Confocal Microscopy to Facilitate Knifeless Skin Cancer Management
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Laser treatment of basal cell carcinoma continues to be refined
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
EXPERT ANALYSIS FROM MOAS 2019
Sniffing Out Malignant Melanoma: A Case of Canine Olfactory Detection
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
Practice Points
- Physiologic and pathologic processes produce volatile organic compounds in the skin and other tissues.
- Malignant melanocytes release unique volatile organic compounds (VOCs) as well as differing combinations and quantities of VOCs as compared to normal melanocytes.
- Volatile organic compounds released at the skin’s surface can be detected by various methods, including canine olfaction; therefore, unusual canine behavior toward skin lesions should not be ignored.
Unusually Early-Onset Plantar Verrucous Carcinoma
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
Practice Points
- Verrucous carcinoma (VC) frequently is observed at 3 particular anatomic sites: the oral cavity, the anogenital area, and on the plantar surface.
- Plantar VC is rare, with a male predominance and most patients presenting in the fifth to sixth decades of life.
- Differentiating VS from benign tumors may be difficult, especially if only superficial biopsies are taken. Multiple biopsies and a close clinical correlation are required before a definite diagnosis is possible.