User login
Voclosporin linked to threefold increase in lupus nephritis remission
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Treatment with the investigational calcineurin inhibitor voclosporin is associated with a significant, threefold-higher rate of complete remission for lupus nephritis, compared with the current standard of care, according to 48-week data from the AURA-LV (Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study.
In a company release, manufacturer Aurinia Pharmaceuticals presented the results of the international phase IIb controlled trial involving 265 patients with active lupus nephritis from 20 countries, which they say has now met its primary and secondary endpoints.
After 48 weeks, 49% of patients in the low-dose group and 40% in the high-dose group had achieved complete remission, compared with 24% in the control group (P less than .001 for low-dose vs. control; P = .026 for high-dose vs. control). This represented a threefold-higher remission rate for the low-dose group (odds ratio, 3.21) and a twofold-higher rate for the high-dose group (OR, 2.1), compared with controls.
Complete remission was defined as a composite endpoint that included urine protein/creatinine ratio of 0.5 mg/mg or less; normal, stable renal function; presence of sustained, low dose steroids; and no administration of rescue medications.
Partial remission – defined as at least a 50% reduction in urine protein/creatinine ratio with no concomitant use of rescue medication – was seen in 68% of patients in the low-dose voclosporin group, 72% of patients in the high-dose group, and 48% of patients in the control arm (P = .007 for low-dose vs. control; P = .002 for high-dose vs. control).
Investigators said there were no unexpected safety signals from voclosporin, but there were three deaths and one malignancy reported in the control arm after the study treatment period ended.
Brad Rovin, MD, of Ohio State University in Columbus said in the company’s statement that current treatments for this severe complication of systemic lupus erythematosus are toxic, and complete renal response rates are low.
“The AURA trial’s long-term results convincingly demonstrate that the addition of voclosporin to standard of care treatment is superior to standard of care alone,” Dr. Rovin said. “This is an impressive renal response rate, and these results may shift the treatment paradigm of lupus nephritis.”
Aurinia’s chief medical officer, Neil Solomons, MD, said in the release that the results provide the company with confidence that they can now execute a successful phase III program.
The 24-week results of the study – in which all primary and the 24-week secondary endpoints were also met – were released in November 2016.
Key clinical point:
Major finding: Nearly half of patients treated with the lower dose of investigational calcineurin inhibitor voclosporin achieved complete remission compared to one-quarter of patients in the control group.
Data source: The AURA-LV phase IIb controlled study involving 265 patients with active lupus nephritis.
Disclosures: The results were presented in a company release from manufacturer Aurinia Pharmaceuticals.
Porcelain heart in a uremic patient
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
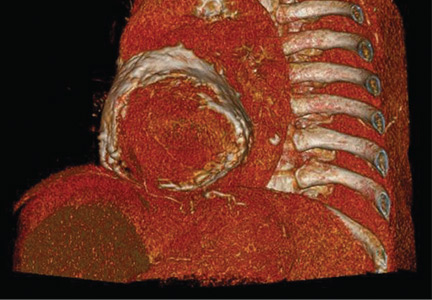
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
Acid-base disturbances
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
In reply: Acid-base disturbances
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
Weakness and pain in arms and legs • dark urine • history of vertebral osteomyelitis • Dx?
THE CASE
A 76-year-old Caucasian woman presented to the emergency department with a 7-day history of weakness and pain in her arms and legs. She had a history of Candida albicans vertebral osteomyelitis that had been treated for 3 months with fluconazole; non-Hodgkin lymphoma that had been in remission for 6 months; diabetes mellitus; hyperlipidemia; and hypothyroidism. The woman had dark urine, but denied chills, fever, respiratory symptoms, bowel or bladder leakage, falls/trauma, or grapefruit juice intake.
Her current medications included oral fluconazole 400 mg/d, simvastatin 20 mg/d, levothyroxine 88 mcg/d, pregabalin 75 mg/d, metformin 1000 mg twice daily, 6 units of subcutaneous insulin glargine at bedtime, and 2 units of insulin lispro with each meal. During the examination, we noted marked proximal muscle weakness, significant tenderness in all extremities, and diminished deep tendon reflexes. The patient had no saddle anesthesia, impaired rectal tone, or sensory abnormalities.
THE DIAGNOSIS
Magnetic resonance imaging of the patient’s spine confirmed multilevel discitis and osteomyelitis (T7-T9, L5-S1) with no cord compression. Laboratory data included a creatinine level of 1.42 mg/dL (the patient’s baseline was 0.8 mg/dL); a creatine kinase (CK) level of 8876 U/L (normal range, 0-220 U/L); a thyroid-stimulating hormone (TSH) level of 9.35 mIU/L (normal range, 0.4-5.5 mIU/L); and an erythrocyte sedimentation rate of 27 mm/hr (normal range, 0-31 mm/hr).
The patient received aggressive fluid hydration, orally and intravenously. On Day 2, the patient’s serum myoglobin level was 14,301 ng/mL (normal range, 30-90 ng/mL) and her aldolase level was 87.6 U/L (normal range, 1.5-8.5 U/L).
Zeroing in on the cause. There were no signs of drug abuse or use of other non-statin culprit medications that could have caused the patient’s rhabdomyolysis. She also did not describe any triggers of rhabdomyolysis, such as trauma, viral infection, metabolic disturbances, or temperature dysregulation. We believed the most likely cause of our patient’s signs and symptoms was statin-induced rhabdomyolysis, likely due to an interaction between simvastatin and fluconazole. We considered hypothyroidism-induced rhabdomyolysis, but thought it was unlikely because the patient had a mildly increased TSH level on admission, and one would expect to see levels higher than 100 mIU/L.1-3
We also considered viral myositis in the differential, but it was an unlikely culprit because the patient lacked any history of fever or respiratory or gastrointestinal symptoms. And while paraneoplastic polymyositis could have caused the patient’s weakness, the marked muscle pain and acute kidney injury were far more suggestive of rhabdomyolysis.
DISCUSSION
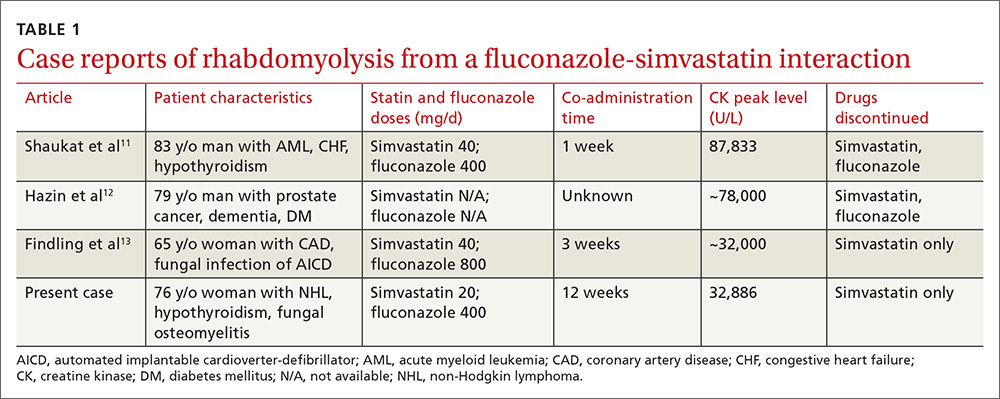
Rhabdomyolysis is a serious complication of statin treatment. Both higher statin doses and pharmacokinetic factors can raise statin levels, leading to this serious muscle-related syndrome.4,5 Co-administration of statins with drugs that are strong inhibitors of cytochrome P450 (CYP) 3A4 (the main cytochrome P450 isoform that metabolizes most statins) can increase statin levels several fold.6,7 The trigger for our patient’s statin-induced rhabdomyolysis was fluconazole, a known moderate inhibitor of CYP3A4, which is comparatively weaker than certain potent azoles like itraconazole or ketoconazole.7-10 Doses of fluconazole generally ≥200 mg/d are needed to produce clinical interactions with CYP3A4 substrates.7 There are only 3 reported cases of fluconazole-simvastatin–induced rhabdomyolysis (TABLE 1).11-13
The Food and Drug Administration advises against simvastatin co-prescription with itraconazole and ketoconazole, but doesn’t mention fluconazole in its Drug Safety communication on simvastatin.14
Lexicomp places the simvastatin-fluconazole drug interaction into category C, which means that the agents can interact in a clinically significant manner (and a monitoring plan should be implemented), but that the benefits of concomitant use usually outweigh the risks.15
How our patient’s case differs from previous cases
Several features distinguish our patient’s scenario from previous cases. First, unlike other cases in which both drugs were stopped, only simvastatin was discontinued in our patient. Simvastatin and fluconazole have a half-life of 3 hours6 and 32 hours,7 respectively, suggesting that when simvastatin has fully cleared, fluconazole’s concentration will not even have halved. Thus, fluconazole was safely continued to treat the patient’s osteomyelitis.
Second, compared to previous case reports, our patient was taking a lower dose of simvastatin (20 mg). A 20-mg dose can make the drug interaction easier to miss; pharmacists are more likely to inform the physician of a potential drug interaction when the dose of a statin is ≥40 mg compared to when it is <40 mg (odds ratio=1.89; 95% confidence interval, 0.98-3.63).16
Researchers involved in the British randomized trial SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) sought to evaluate any added benefit to a higher dose of simvastatin in post-myocardial infarction patients. Among approximately 12,000 patients in the trial, there were 7 cases of rhabdomyolysis for the 80-mg simvastatin group and none for the 20-mg group.5 Another large case-control study showed that a 40-mg simvastatin dose was 5 times more likely to cause rhabdomyolysis than a 20-mg dose.17 Yet, based on our patient’s case, even 20 mg/d simvastatin should not decrease physician suspicion for rhabdomyolysis if patients are also taking a CYP3A4 inhibitor.
Third, the simvastatin-fluconazole co-administration time in our patient was 12 weeks, which is longer than previously reported (TABLE 111-13). Azole inhibition of CYP450 occurs relatively rapidly, but that does not mean that rhabdomyolysis will always occur immediately. For example, in cases of statin monotherapy, rhabdomyolysis secondary to statin biochemical toxicity can occur up to 1050 (mean=348) days after the drug’s initiation.18
Avoiding a drug-drug interaction in your patient
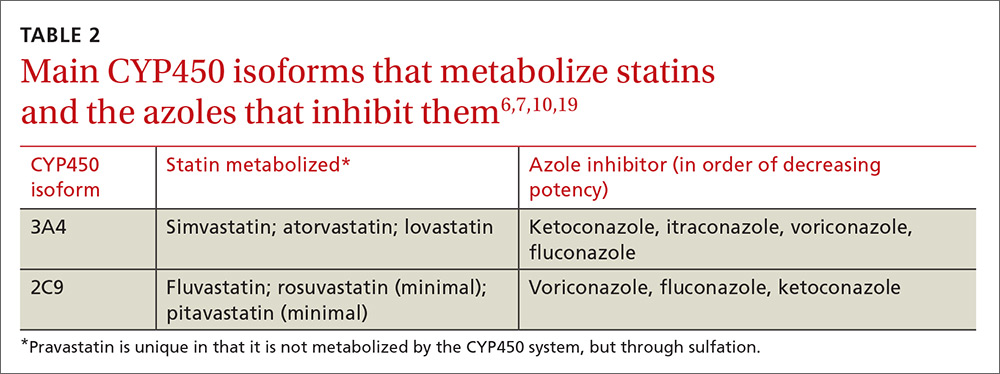
Physicians can use pharmacokinetic profiles to choose among different statins and azoles to help avoid a drug interaction (TABLE 26,7,10,19). Pravastatin’s serum concentration, for example, is not influenced by CYP3A4 inhibitors such as itraconazole11 because pravastatin is metabolized by sulfation6 and not by the CYP450 system. Rosuvastatin and pitavastatin are minimally metabolized by the CYP450 system.19,20
Among approximately 2700 statin-treated outpatients,4 the prevalence of potentially harmful statin interactions with other drugs (including CYP3A4 inhibitors), was significantly higher among patients treated with simvastatin or atorvastatin (CYP3A4-metabolized statins), than among patients treated with fluvastatin (CYP2C9-metabolized statin) or pravastatin (metabolized by sulfation). Apart from drug-drug interactions, other risk factors for statin-induced rhabdomyolysis include use of lipophilic statins, advanced age, and female gender.21
We discontinued our patient’s simvastatin on the day she was admitted to the hospital, but continued with the fluconazole throughout her hospitalization. Her CK level continued to rise, peaked on hospital Day 3 at 32,886 U/L, and then progressively decreased. The patient’s weakness and pain improved and her acute kidney injury resolved with hydration. She was discharged on hospital Day 7 on oral fluconazole, but no statin, and her muscle symptoms have since resolved.
THE TAKEAWAY
When hyperlipidemic patients have to take an azole for an extended period (eg, cancer prophylaxis or chronic osteomyelitis) and the azole is a strong CYP450 inhibitor (eg, itraconazole), switching to a statin that is not primarily metabolized by the CYP450 system (eg, pravastatin, pitavastatin) is wise. If the azole is a moderate CYP450 inhibitor (eg, fluconazole), we suggest that therapy should be closely monitored. In the case of short-term azole treatment (eg, such as for oral candidiasis), the statin should be stopped or the dose reduced by at least 50% (eg, from 40 or 20 mg to 10 mg).6
Prescriber knowledge is sometimes a limiting factor in identifying clinically significant interactions.22 This is especially pertinent in a case like this one, where a lower statin dose may result in a lower chance of the pharmacist alerting the prescribing physician16 and when an azole is used that is a comparatively weaker CYP450 inhibitor than other azoles such as itraconazole. Even in the era of electronic medical records, approximately 90% of drug interaction alerts are overridden by physicians, and alert fatigue is pronounced.23
The intricacies and pharmacokinetic principles of this case should contribute to greater provider familiarity with even low-dose simvastatin-fluconazole interactions and help prevent iatrogenic complications such as rhabdomyolysis.
1. Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocr J. 2003;50:221-223.
2. Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with a strikingly elevated serum creatine kinase level. Muscle Nerve. 2002;26:141-144.
3. Barahona MJ, Mauri A, Sucunza N, et al. Hypothyroidism as a cause of rhabdomyolysis. Endocr J. 2002;49:621-623.
4. Rätz Bravo AE, Tchambaz L, Krähenbühl-Melcher A, et al. Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf. 2005;28:263-275.
5. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
6. Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111:390-400.
7. Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111-180.
8. Malhotra B, Dickins M, Alvey C, et al. Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br J Clin Pharmacol. 2011;72:263-269.
9. US Food and Drug Administration. Drug development and drug interactions: Table of substrates, inhibitors and inducers. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm. Accessed February 9, 2017.
10. Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805-1808.
11. Shaukat A, Benekli M, Vladutiu GD, et al. Simvastatin-fluconazole causing rhabdomyolysis. Ann Pharmacother. 2003;37:1032-1035.
12. Hazin R, Abuzetun JY, Suker M, et al. Rhabdomyolysis induced by simvastatin-fluconazole combination. J Natl Med Assoc. 2008;100:444-446.
13. Findling O, Meier N, Sellner J, et al. Clinical reasoning: rhabdomyolysis after combined treatment with simvastatin and fluconazole. Neurology. 2008;71:e34-e37.
14. US Food and Drug Administration. FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed February 1, 2017.
15. Wolters Kluwer. Lexicomp online. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed February 9, 2017.
16. Molden E, Skovlund E, Braathen P. Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf. 2008;31:587-596.
17. Parkin L, Paul C, Herbison GP. Simvastatin dose and risk of rhabdomyolysis: nested case-control study based on national health and drug dispensing data. Int J Cardiol. 2014;174:83-89.
18. Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585-2590.
19. Saito Y. Pitavastatin: an overview. Atheroscler Suppl. 2011;12:271-276.
20. Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20:303-328.
21. Magni P, Macchi C, Morlotti B, et al. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med. 2015;26:82-88.
22. Ko Y, Malone DC, Skrepnek GH, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31:525-536.
23. Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20:489-493.
THE CASE
A 76-year-old Caucasian woman presented to the emergency department with a 7-day history of weakness and pain in her arms and legs. She had a history of Candida albicans vertebral osteomyelitis that had been treated for 3 months with fluconazole; non-Hodgkin lymphoma that had been in remission for 6 months; diabetes mellitus; hyperlipidemia; and hypothyroidism. The woman had dark urine, but denied chills, fever, respiratory symptoms, bowel or bladder leakage, falls/trauma, or grapefruit juice intake.
Her current medications included oral fluconazole 400 mg/d, simvastatin 20 mg/d, levothyroxine 88 mcg/d, pregabalin 75 mg/d, metformin 1000 mg twice daily, 6 units of subcutaneous insulin glargine at bedtime, and 2 units of insulin lispro with each meal. During the examination, we noted marked proximal muscle weakness, significant tenderness in all extremities, and diminished deep tendon reflexes. The patient had no saddle anesthesia, impaired rectal tone, or sensory abnormalities.
THE DIAGNOSIS
Magnetic resonance imaging of the patient’s spine confirmed multilevel discitis and osteomyelitis (T7-T9, L5-S1) with no cord compression. Laboratory data included a creatinine level of 1.42 mg/dL (the patient’s baseline was 0.8 mg/dL); a creatine kinase (CK) level of 8876 U/L (normal range, 0-220 U/L); a thyroid-stimulating hormone (TSH) level of 9.35 mIU/L (normal range, 0.4-5.5 mIU/L); and an erythrocyte sedimentation rate of 27 mm/hr (normal range, 0-31 mm/hr).
The patient received aggressive fluid hydration, orally and intravenously. On Day 2, the patient’s serum myoglobin level was 14,301 ng/mL (normal range, 30-90 ng/mL) and her aldolase level was 87.6 U/L (normal range, 1.5-8.5 U/L).
Zeroing in on the cause. There were no signs of drug abuse or use of other non-statin culprit medications that could have caused the patient’s rhabdomyolysis. She also did not describe any triggers of rhabdomyolysis, such as trauma, viral infection, metabolic disturbances, or temperature dysregulation. We believed the most likely cause of our patient’s signs and symptoms was statin-induced rhabdomyolysis, likely due to an interaction between simvastatin and fluconazole. We considered hypothyroidism-induced rhabdomyolysis, but thought it was unlikely because the patient had a mildly increased TSH level on admission, and one would expect to see levels higher than 100 mIU/L.1-3
We also considered viral myositis in the differential, but it was an unlikely culprit because the patient lacked any history of fever or respiratory or gastrointestinal symptoms. And while paraneoplastic polymyositis could have caused the patient’s weakness, the marked muscle pain and acute kidney injury were far more suggestive of rhabdomyolysis.
DISCUSSION
Rhabdomyolysis is a serious complication of statin treatment. Both higher statin doses and pharmacokinetic factors can raise statin levels, leading to this serious muscle-related syndrome.4,5 Co-administration of statins with drugs that are strong inhibitors of cytochrome P450 (CYP) 3A4 (the main cytochrome P450 isoform that metabolizes most statins) can increase statin levels several fold.6,7 The trigger for our patient’s statin-induced rhabdomyolysis was fluconazole, a known moderate inhibitor of CYP3A4, which is comparatively weaker than certain potent azoles like itraconazole or ketoconazole.7-10 Doses of fluconazole generally ≥200 mg/d are needed to produce clinical interactions with CYP3A4 substrates.7 There are only 3 reported cases of fluconazole-simvastatin–induced rhabdomyolysis (TABLE 1).11-13
The Food and Drug Administration advises against simvastatin co-prescription with itraconazole and ketoconazole, but doesn’t mention fluconazole in its Drug Safety communication on simvastatin.14
Lexicomp places the simvastatin-fluconazole drug interaction into category C, which means that the agents can interact in a clinically significant manner (and a monitoring plan should be implemented), but that the benefits of concomitant use usually outweigh the risks.15
How our patient’s case differs from previous cases
Several features distinguish our patient’s scenario from previous cases. First, unlike other cases in which both drugs were stopped, only simvastatin was discontinued in our patient. Simvastatin and fluconazole have a half-life of 3 hours6 and 32 hours,7 respectively, suggesting that when simvastatin has fully cleared, fluconazole’s concentration will not even have halved. Thus, fluconazole was safely continued to treat the patient’s osteomyelitis.
Second, compared to previous case reports, our patient was taking a lower dose of simvastatin (20 mg). A 20-mg dose can make the drug interaction easier to miss; pharmacists are more likely to inform the physician of a potential drug interaction when the dose of a statin is ≥40 mg compared to when it is <40 mg (odds ratio=1.89; 95% confidence interval, 0.98-3.63).16
Researchers involved in the British randomized trial SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) sought to evaluate any added benefit to a higher dose of simvastatin in post-myocardial infarction patients. Among approximately 12,000 patients in the trial, there were 7 cases of rhabdomyolysis for the 80-mg simvastatin group and none for the 20-mg group.5 Another large case-control study showed that a 40-mg simvastatin dose was 5 times more likely to cause rhabdomyolysis than a 20-mg dose.17 Yet, based on our patient’s case, even 20 mg/d simvastatin should not decrease physician suspicion for rhabdomyolysis if patients are also taking a CYP3A4 inhibitor.
Third, the simvastatin-fluconazole co-administration time in our patient was 12 weeks, which is longer than previously reported (TABLE 111-13). Azole inhibition of CYP450 occurs relatively rapidly, but that does not mean that rhabdomyolysis will always occur immediately. For example, in cases of statin monotherapy, rhabdomyolysis secondary to statin biochemical toxicity can occur up to 1050 (mean=348) days after the drug’s initiation.18
Avoiding a drug-drug interaction in your patient
Physicians can use pharmacokinetic profiles to choose among different statins and azoles to help avoid a drug interaction (TABLE 26,7,10,19). Pravastatin’s serum concentration, for example, is not influenced by CYP3A4 inhibitors such as itraconazole11 because pravastatin is metabolized by sulfation6 and not by the CYP450 system. Rosuvastatin and pitavastatin are minimally metabolized by the CYP450 system.19,20
Among approximately 2700 statin-treated outpatients,4 the prevalence of potentially harmful statin interactions with other drugs (including CYP3A4 inhibitors), was significantly higher among patients treated with simvastatin or atorvastatin (CYP3A4-metabolized statins), than among patients treated with fluvastatin (CYP2C9-metabolized statin) or pravastatin (metabolized by sulfation). Apart from drug-drug interactions, other risk factors for statin-induced rhabdomyolysis include use of lipophilic statins, advanced age, and female gender.21
We discontinued our patient’s simvastatin on the day she was admitted to the hospital, but continued with the fluconazole throughout her hospitalization. Her CK level continued to rise, peaked on hospital Day 3 at 32,886 U/L, and then progressively decreased. The patient’s weakness and pain improved and her acute kidney injury resolved with hydration. She was discharged on hospital Day 7 on oral fluconazole, but no statin, and her muscle symptoms have since resolved.
THE TAKEAWAY
When hyperlipidemic patients have to take an azole for an extended period (eg, cancer prophylaxis or chronic osteomyelitis) and the azole is a strong CYP450 inhibitor (eg, itraconazole), switching to a statin that is not primarily metabolized by the CYP450 system (eg, pravastatin, pitavastatin) is wise. If the azole is a moderate CYP450 inhibitor (eg, fluconazole), we suggest that therapy should be closely monitored. In the case of short-term azole treatment (eg, such as for oral candidiasis), the statin should be stopped or the dose reduced by at least 50% (eg, from 40 or 20 mg to 10 mg).6
Prescriber knowledge is sometimes a limiting factor in identifying clinically significant interactions.22 This is especially pertinent in a case like this one, where a lower statin dose may result in a lower chance of the pharmacist alerting the prescribing physician16 and when an azole is used that is a comparatively weaker CYP450 inhibitor than other azoles such as itraconazole. Even in the era of electronic medical records, approximately 90% of drug interaction alerts are overridden by physicians, and alert fatigue is pronounced.23
The intricacies and pharmacokinetic principles of this case should contribute to greater provider familiarity with even low-dose simvastatin-fluconazole interactions and help prevent iatrogenic complications such as rhabdomyolysis.
THE CASE
A 76-year-old Caucasian woman presented to the emergency department with a 7-day history of weakness and pain in her arms and legs. She had a history of Candida albicans vertebral osteomyelitis that had been treated for 3 months with fluconazole; non-Hodgkin lymphoma that had been in remission for 6 months; diabetes mellitus; hyperlipidemia; and hypothyroidism. The woman had dark urine, but denied chills, fever, respiratory symptoms, bowel or bladder leakage, falls/trauma, or grapefruit juice intake.
Her current medications included oral fluconazole 400 mg/d, simvastatin 20 mg/d, levothyroxine 88 mcg/d, pregabalin 75 mg/d, metformin 1000 mg twice daily, 6 units of subcutaneous insulin glargine at bedtime, and 2 units of insulin lispro with each meal. During the examination, we noted marked proximal muscle weakness, significant tenderness in all extremities, and diminished deep tendon reflexes. The patient had no saddle anesthesia, impaired rectal tone, or sensory abnormalities.
THE DIAGNOSIS
Magnetic resonance imaging of the patient’s spine confirmed multilevel discitis and osteomyelitis (T7-T9, L5-S1) with no cord compression. Laboratory data included a creatinine level of 1.42 mg/dL (the patient’s baseline was 0.8 mg/dL); a creatine kinase (CK) level of 8876 U/L (normal range, 0-220 U/L); a thyroid-stimulating hormone (TSH) level of 9.35 mIU/L (normal range, 0.4-5.5 mIU/L); and an erythrocyte sedimentation rate of 27 mm/hr (normal range, 0-31 mm/hr).
The patient received aggressive fluid hydration, orally and intravenously. On Day 2, the patient’s serum myoglobin level was 14,301 ng/mL (normal range, 30-90 ng/mL) and her aldolase level was 87.6 U/L (normal range, 1.5-8.5 U/L).
Zeroing in on the cause. There were no signs of drug abuse or use of other non-statin culprit medications that could have caused the patient’s rhabdomyolysis. She also did not describe any triggers of rhabdomyolysis, such as trauma, viral infection, metabolic disturbances, or temperature dysregulation. We believed the most likely cause of our patient’s signs and symptoms was statin-induced rhabdomyolysis, likely due to an interaction between simvastatin and fluconazole. We considered hypothyroidism-induced rhabdomyolysis, but thought it was unlikely because the patient had a mildly increased TSH level on admission, and one would expect to see levels higher than 100 mIU/L.1-3
We also considered viral myositis in the differential, but it was an unlikely culprit because the patient lacked any history of fever or respiratory or gastrointestinal symptoms. And while paraneoplastic polymyositis could have caused the patient’s weakness, the marked muscle pain and acute kidney injury were far more suggestive of rhabdomyolysis.
DISCUSSION
Rhabdomyolysis is a serious complication of statin treatment. Both higher statin doses and pharmacokinetic factors can raise statin levels, leading to this serious muscle-related syndrome.4,5 Co-administration of statins with drugs that are strong inhibitors of cytochrome P450 (CYP) 3A4 (the main cytochrome P450 isoform that metabolizes most statins) can increase statin levels several fold.6,7 The trigger for our patient’s statin-induced rhabdomyolysis was fluconazole, a known moderate inhibitor of CYP3A4, which is comparatively weaker than certain potent azoles like itraconazole or ketoconazole.7-10 Doses of fluconazole generally ≥200 mg/d are needed to produce clinical interactions with CYP3A4 substrates.7 There are only 3 reported cases of fluconazole-simvastatin–induced rhabdomyolysis (TABLE 1).11-13
The Food and Drug Administration advises against simvastatin co-prescription with itraconazole and ketoconazole, but doesn’t mention fluconazole in its Drug Safety communication on simvastatin.14
Lexicomp places the simvastatin-fluconazole drug interaction into category C, which means that the agents can interact in a clinically significant manner (and a monitoring plan should be implemented), but that the benefits of concomitant use usually outweigh the risks.15
How our patient’s case differs from previous cases
Several features distinguish our patient’s scenario from previous cases. First, unlike other cases in which both drugs were stopped, only simvastatin was discontinued in our patient. Simvastatin and fluconazole have a half-life of 3 hours6 and 32 hours,7 respectively, suggesting that when simvastatin has fully cleared, fluconazole’s concentration will not even have halved. Thus, fluconazole was safely continued to treat the patient’s osteomyelitis.
Second, compared to previous case reports, our patient was taking a lower dose of simvastatin (20 mg). A 20-mg dose can make the drug interaction easier to miss; pharmacists are more likely to inform the physician of a potential drug interaction when the dose of a statin is ≥40 mg compared to when it is <40 mg (odds ratio=1.89; 95% confidence interval, 0.98-3.63).16
Researchers involved in the British randomized trial SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) sought to evaluate any added benefit to a higher dose of simvastatin in post-myocardial infarction patients. Among approximately 12,000 patients in the trial, there were 7 cases of rhabdomyolysis for the 80-mg simvastatin group and none for the 20-mg group.5 Another large case-control study showed that a 40-mg simvastatin dose was 5 times more likely to cause rhabdomyolysis than a 20-mg dose.17 Yet, based on our patient’s case, even 20 mg/d simvastatin should not decrease physician suspicion for rhabdomyolysis if patients are also taking a CYP3A4 inhibitor.
Third, the simvastatin-fluconazole co-administration time in our patient was 12 weeks, which is longer than previously reported (TABLE 111-13). Azole inhibition of CYP450 occurs relatively rapidly, but that does not mean that rhabdomyolysis will always occur immediately. For example, in cases of statin monotherapy, rhabdomyolysis secondary to statin biochemical toxicity can occur up to 1050 (mean=348) days after the drug’s initiation.18
Avoiding a drug-drug interaction in your patient
Physicians can use pharmacokinetic profiles to choose among different statins and azoles to help avoid a drug interaction (TABLE 26,7,10,19). Pravastatin’s serum concentration, for example, is not influenced by CYP3A4 inhibitors such as itraconazole11 because pravastatin is metabolized by sulfation6 and not by the CYP450 system. Rosuvastatin and pitavastatin are minimally metabolized by the CYP450 system.19,20
Among approximately 2700 statin-treated outpatients,4 the prevalence of potentially harmful statin interactions with other drugs (including CYP3A4 inhibitors), was significantly higher among patients treated with simvastatin or atorvastatin (CYP3A4-metabolized statins), than among patients treated with fluvastatin (CYP2C9-metabolized statin) or pravastatin (metabolized by sulfation). Apart from drug-drug interactions, other risk factors for statin-induced rhabdomyolysis include use of lipophilic statins, advanced age, and female gender.21
We discontinued our patient’s simvastatin on the day she was admitted to the hospital, but continued with the fluconazole throughout her hospitalization. Her CK level continued to rise, peaked on hospital Day 3 at 32,886 U/L, and then progressively decreased. The patient’s weakness and pain improved and her acute kidney injury resolved with hydration. She was discharged on hospital Day 7 on oral fluconazole, but no statin, and her muscle symptoms have since resolved.
THE TAKEAWAY
When hyperlipidemic patients have to take an azole for an extended period (eg, cancer prophylaxis or chronic osteomyelitis) and the azole is a strong CYP450 inhibitor (eg, itraconazole), switching to a statin that is not primarily metabolized by the CYP450 system (eg, pravastatin, pitavastatin) is wise. If the azole is a moderate CYP450 inhibitor (eg, fluconazole), we suggest that therapy should be closely monitored. In the case of short-term azole treatment (eg, such as for oral candidiasis), the statin should be stopped or the dose reduced by at least 50% (eg, from 40 or 20 mg to 10 mg).6
Prescriber knowledge is sometimes a limiting factor in identifying clinically significant interactions.22 This is especially pertinent in a case like this one, where a lower statin dose may result in a lower chance of the pharmacist alerting the prescribing physician16 and when an azole is used that is a comparatively weaker CYP450 inhibitor than other azoles such as itraconazole. Even in the era of electronic medical records, approximately 90% of drug interaction alerts are overridden by physicians, and alert fatigue is pronounced.23
The intricacies and pharmacokinetic principles of this case should contribute to greater provider familiarity with even low-dose simvastatin-fluconazole interactions and help prevent iatrogenic complications such as rhabdomyolysis.
1. Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocr J. 2003;50:221-223.
2. Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with a strikingly elevated serum creatine kinase level. Muscle Nerve. 2002;26:141-144.
3. Barahona MJ, Mauri A, Sucunza N, et al. Hypothyroidism as a cause of rhabdomyolysis. Endocr J. 2002;49:621-623.
4. Rätz Bravo AE, Tchambaz L, Krähenbühl-Melcher A, et al. Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf. 2005;28:263-275.
5. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
6. Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111:390-400.
7. Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111-180.
8. Malhotra B, Dickins M, Alvey C, et al. Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br J Clin Pharmacol. 2011;72:263-269.
9. US Food and Drug Administration. Drug development and drug interactions: Table of substrates, inhibitors and inducers. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm. Accessed February 9, 2017.
10. Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805-1808.
11. Shaukat A, Benekli M, Vladutiu GD, et al. Simvastatin-fluconazole causing rhabdomyolysis. Ann Pharmacother. 2003;37:1032-1035.
12. Hazin R, Abuzetun JY, Suker M, et al. Rhabdomyolysis induced by simvastatin-fluconazole combination. J Natl Med Assoc. 2008;100:444-446.
13. Findling O, Meier N, Sellner J, et al. Clinical reasoning: rhabdomyolysis after combined treatment with simvastatin and fluconazole. Neurology. 2008;71:e34-e37.
14. US Food and Drug Administration. FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed February 1, 2017.
15. Wolters Kluwer. Lexicomp online. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed February 9, 2017.
16. Molden E, Skovlund E, Braathen P. Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf. 2008;31:587-596.
17. Parkin L, Paul C, Herbison GP. Simvastatin dose and risk of rhabdomyolysis: nested case-control study based on national health and drug dispensing data. Int J Cardiol. 2014;174:83-89.
18. Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585-2590.
19. Saito Y. Pitavastatin: an overview. Atheroscler Suppl. 2011;12:271-276.
20. Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20:303-328.
21. Magni P, Macchi C, Morlotti B, et al. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med. 2015;26:82-88.
22. Ko Y, Malone DC, Skrepnek GH, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31:525-536.
23. Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20:489-493.
1. Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocr J. 2003;50:221-223.
2. Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with a strikingly elevated serum creatine kinase level. Muscle Nerve. 2002;26:141-144.
3. Barahona MJ, Mauri A, Sucunza N, et al. Hypothyroidism as a cause of rhabdomyolysis. Endocr J. 2002;49:621-623.
4. Rätz Bravo AE, Tchambaz L, Krähenbühl-Melcher A, et al. Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf. 2005;28:263-275.
5. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
6. Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111:390-400.
7. Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111-180.
8. Malhotra B, Dickins M, Alvey C, et al. Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br J Clin Pharmacol. 2011;72:263-269.
9. US Food and Drug Administration. Drug development and drug interactions: Table of substrates, inhibitors and inducers. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm. Accessed February 9, 2017.
10. Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805-1808.
11. Shaukat A, Benekli M, Vladutiu GD, et al. Simvastatin-fluconazole causing rhabdomyolysis. Ann Pharmacother. 2003;37:1032-1035.
12. Hazin R, Abuzetun JY, Suker M, et al. Rhabdomyolysis induced by simvastatin-fluconazole combination. J Natl Med Assoc. 2008;100:444-446.
13. Findling O, Meier N, Sellner J, et al. Clinical reasoning: rhabdomyolysis after combined treatment with simvastatin and fluconazole. Neurology. 2008;71:e34-e37.
14. US Food and Drug Administration. FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed February 1, 2017.
15. Wolters Kluwer. Lexicomp online. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed February 9, 2017.
16. Molden E, Skovlund E, Braathen P. Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf. 2008;31:587-596.
17. Parkin L, Paul C, Herbison GP. Simvastatin dose and risk of rhabdomyolysis: nested case-control study based on national health and drug dispensing data. Int J Cardiol. 2014;174:83-89.
18. Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585-2590.
19. Saito Y. Pitavastatin: an overview. Atheroscler Suppl. 2011;12:271-276.
20. Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20:303-328.
21. Magni P, Macchi C, Morlotti B, et al. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med. 2015;26:82-88.
22. Ko Y, Malone DC, Skrepnek GH, et al. Prescribers’ knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31:525-536.
23. Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20:489-493.
Hyperkalemia in Adults: Review of a Common Electrolyte Imbalance
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5
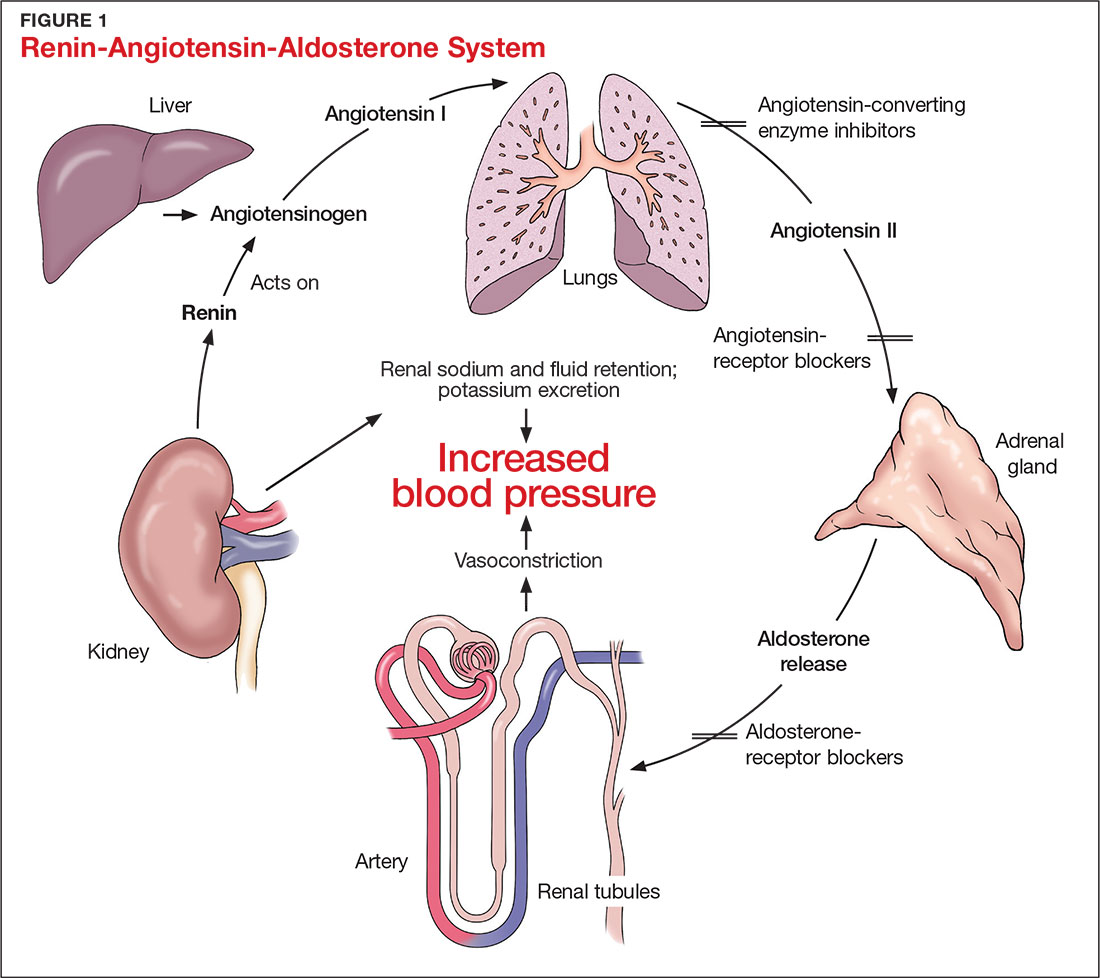
CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.
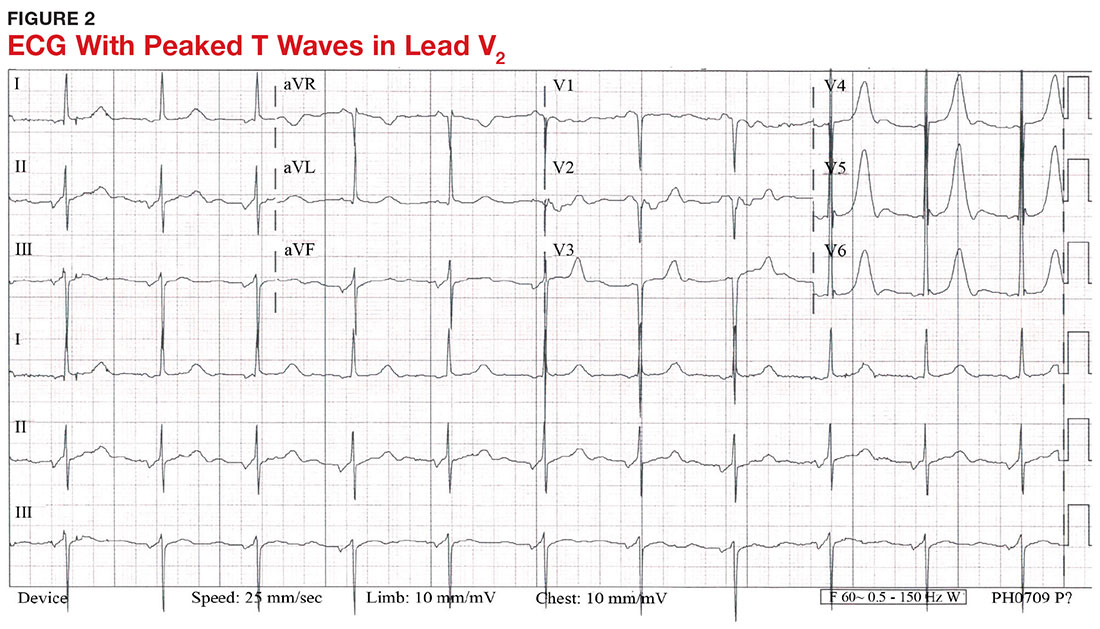
With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26
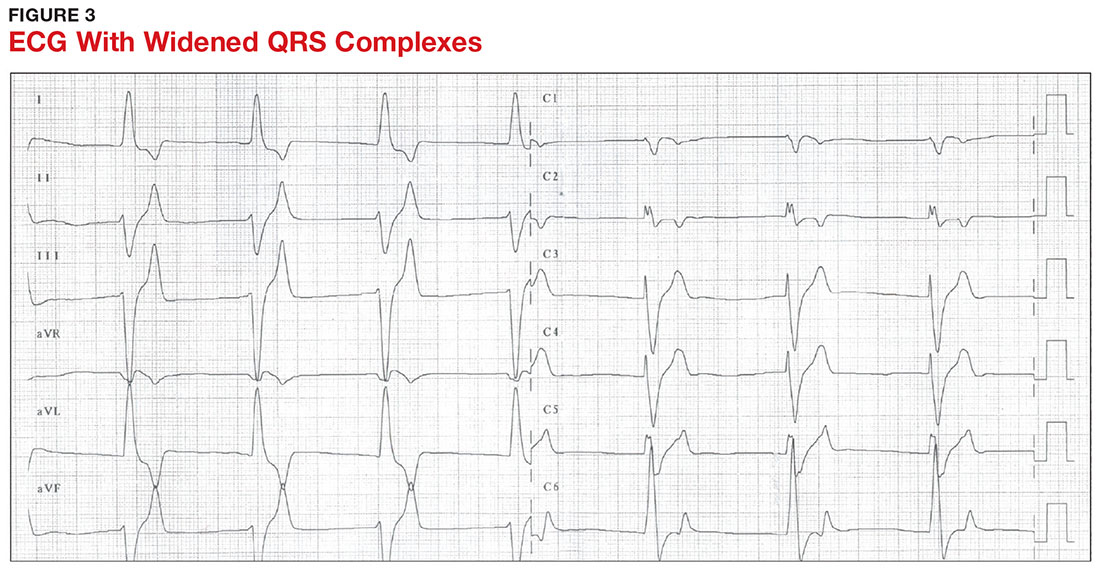
An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26
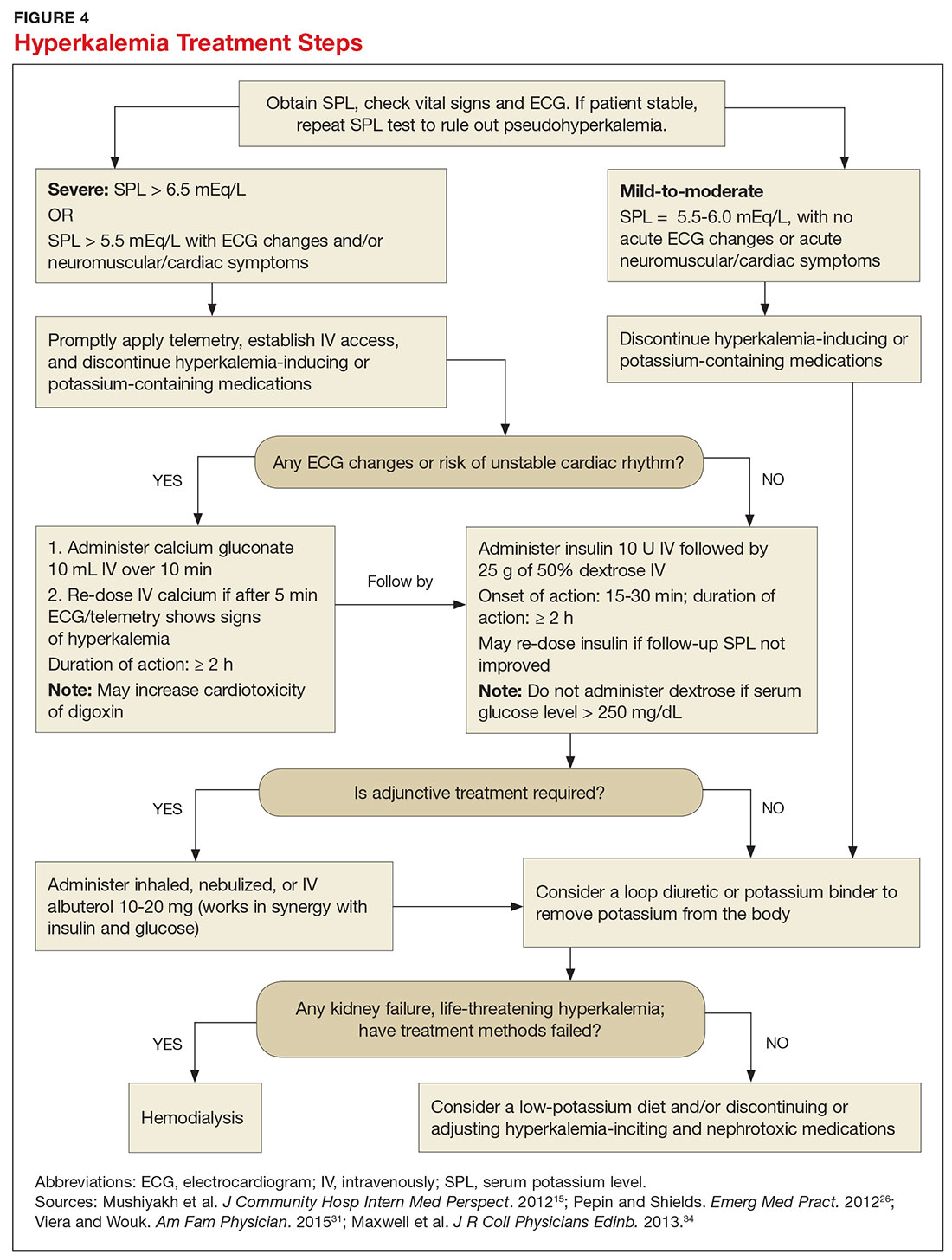
In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5

CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.

With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26

An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26

In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5

CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.

With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26

An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26

In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
AKI is common in young, critically ill adults
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Clinical question: What are the epidemiology, risk factors, and associated morbidity and mortality of acute kidney injury (AKI) in critically ill children and young adults?
Background: Adult studies on acute kidney injury have shown increasing mortality and morbidity when both plasma creatinine and urine output were used to diagnose AKI than when used alone. Studies of AKI in children have also been limited.
Setting: International (32 pediatric intensive care units across Asia, Australia, Europe, and North America).
Synopsis: 4,984 patients aged 3 months to 25 years with a predicted ICU stay of at least 48 hours were considered for enrollment, of which 4,683 were included in the final analysis. The primary outcome was 28-day mortality. Secondary outcomes were length of stay in the ICU, receipt and duration of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and renal-replacement therapy. A total of 26.9% of patients developed AKI in the first 7 days of an ICU admission. Severe AKI increased mortality by day 28 (adjusted odds ratio, 1.77; 95% confidence interval, 1.17-2.68) and was associated with increased use of renal-replacement therapy and mechanical ventilation and longer stays in the ICU. Urine output predicted mortality more accurately than did plasma creatinine, and using plasma creatinine alone failed to identify two-thirds of patients with low urine output.
Bottom line: In critically ill young patients, AKI is a common occurrence and is associated with both an increased morbidity and mortality. In a majority of patients with low urine output, plasma creatinine was a poor discriminant of renal function.
Citations: Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Eng J Med. 2017; 376 (1):11-20.
Dr. Rachoin is an assistant professor of clinical medicine and associate division head, Hospital Medicine, at Cooper Medical School at Rowan University. He works as a hospitalist at Cooper University Hospital in Camden, N.J.
Diabetes-Related Kidney Failure Drops Among Native Americans
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
Thrombotic microangiopathies: Similar presentations, different therapies
Our knowledge of the pathogenesis of thrombotic microangiopathies has greatly advanced in the last decade, improving the diagnosis and treatment of these diseases.
Many conditions involve thrombotic microangiopathies (Table 1). This article reviews the most common ones, ie, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, atypical hemolytic uremic syndrome, and antiphospholipid syndrome—their clinical features (focusing on the kidney), course, and management. Of note, although the diseases are similar, their pathogeneses and treatments differ.
DIFFERENT PATHWAYS TO MULTIORGAN THROMBOSIS
The thrombotic microangiopathies are multisystem disorders that can affect children and adults and often present with prominent renal and neurologic involvement. Endothelial injury is likely the inciting factor leading to thrombosis in the kidney and in many other organs. The causes variously include:
- Toxins from bacteria or drugs
- Abnormal complement activation, genetic or autoantibody-induced
- Procoagulant factors, eg, antiphospholipid antibodies
- Loss of anticoagulants, eg, from a defect of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13); ADAMTS13 is also known as von Willebrand factor-cleaving protease
- Severe hypertension.
The histopathologic features are similar in all the thrombotic microangiopathies. Laboratory findings include thrombocytopenia, microangiopathic hemolytic anemia (with schistocytes on the peripheral blood smear), and high serum lactate dehydrogenase (LDH) levels; these are also markers of treatment progress. Bilirubin may be elevated and haptoglobin absent. Renal biopsy reveals thrombi in the glomeruli and arterioles.
THROMBOTIC THROMBOCYTOPENIC PURPURA
A young woman with fever, bruising, and renal failure, then blindness
A 36-year-old black woman who had been previously healthy presents to her doctor with fever and bruising.
Her hematocrit is 28% (reference range 38%–46%), platelet count 15 x 109/L (150–450), and prothrombin and partial thromboplastin times are normal. Her peripheral blood smear shows microangiopathic hemolytic anemia with schistocytes.
Over the next few days, her urine output declines and she develops sudden blindness followed by decreased mental acuity. Blood is drawn and sent for ADAMTS13 assay. Treatment is started at once with daily therapeutic plasma exchange. The assay results, when they arrive, show marked ADAMTS13 reduction (< 5%). Over the ensuing weeks, her mental acuity improves, her vision returns, and her renal function improves.
ADAMTS13 deficiency is definitive
Thrombotic thrombocytopenic purpura is characterized by:
- Neurologic abnormalities and acute renal failure
- Thrombocytopenia and microangiopathic hemolytic anemia
- Histologic evidence of thrombotic microangiopathy
- Deficiency of von Willebrand factor-cleaving protease (ADAMTS13 < 10%).
von Willebrand factor forms ultralarge multimers in the circulation that interact with platelets; these are normally cleaved by ADAMTS13. With ADAMTS13 deficiency (from either a genetic mutation or autoantibodies), the ultralarge multimers lead to coagulation as blood flows through small vessels.1
In 2003, Tsai2 evaluated 127 patients over age 10 who had thrombocytopenia and microangiopathic hemolysis with no plausible cause or features suggestive of hemolytic uremic syndrome. All were severely deficient in ADAMTS13. Subsequently, thrombotic thrombocytopenic purpura has been defined by a severe actual or effective deficiency of ADAMTS13.
Prompt plasma exchange is critical
Although the ADAMTS13 assay is important for diagnosing thrombotic thrombocytopenic purpura, in suspected cases daily plasma exchange should be started promptly, before test results return. Plasma exchange removes autoantibodies to ADAMTS13 from the blood, removes circulating ultralarge von Willebrand factor multimers, and replaces the missing ADAMTS13. Untreated, the disease is progressive, with irreversible renal failure, neurologic deterioration, and a 90% mortality rate. Plasma exchange reduces the mortality rate to less than 15%. If another diagnosis is confirmed, plasma exchange can be stopped.
Plasma exchange has been shown in clinical trials to be superior to plasma infusion in normalizing platelet counts and reducing mortality.3,4 Mortality rates were comparable with different replacement fluids vs fresh-frozen plasma, including solvent or detergent-treated plasma, and cryo-poor (cryosupernatant) plasma.4 Antiplatelet therapy, platelet transfusions, and splenectomy are ineffective.
Glucocorticoids for early treatment
An appropriate strategy is to add a glucocorticoid to plasma-exchange therapy at once (oral prednisone 1 mg/kg per day or intravenous methylprednisolone 125 mg twice daily) and withdraw it after several days if it is determined that it is not needed. Steroids for suspected thrombotic thrombocytopenic purpura can be justified for several reasons:
- The results of the ADAMTS13 assay are usually delayed, so steroids provide coverage for other diagnoses.
- They are helpful if thrombotic thrombocytopenic purpura is idiopathic (which is true for most cases) and if the patient has a poor response to initial therapy with plasma exchange.
- They are indicated for patients whose platelet counts do not increase with several days of plasma exchange or whose thrombocytopenia recurs as plasma exchange is decreased.
Rituximab improves survival
Rituximab, a chimeric (half murine) monoclonal antibody against CD19 and CD20 B cells, suppresses antibody production by knocking out the precursors of antibody-producing cells.
Anecdotal reports and small studies involving a total of 42 patients have been published on the use of rituximab for thrombotic thrombocytopenic purpura. Courses of rituximab varied greatly, from 1 to 13 weekly doses at 375 mg/m2, with 4 doses being the most common. Complete remission occurred in 90% of cases.5,6 A typical study from 2014 involved 48 patients (30 of whom received rituximab) followed by severe ADAMTS13 deficiency during remission.7 Despite the small study size, the investigators found significantly improved relapse-free survival rates with rituximab treatment.
But rituximab can cost $25,000 for 2 doses of 1,000 mg, and this will most likely prohibit its routine use. The cost and insurance coverage vary with location and policies.
Based on such studies, a reasonable strategy is to treat thrombotic thrombocytopenic purpura with:
- Daily plasma exchange
- Steroids, at least until the diagnosis is certain
- Rituximab if warranted.
New targeted therapies
Caplacizumab, a humanized immunoglobulin that inhibits the interaction between ultralarge von Willebrand factor multimers and platelets, has the potential to change this strategy when it receives US Food and Drug Administration approval, which is expected soon.
Peyvandi et al8 randomized 75 patients with acquired thrombotic thrombocytopenic purpura to either subcutaneous caplacizumab 10 mg daily for 30 days or placebo. Both groups had daily plasma exchange. The treatment group had a 39% reduction in median time to normalization of platelets vs the placebo group, and 3 of 36 patients had exacerbations, compared with 11 of 39 patients in the placebo group. Although 8 patients relapsed within the first month after stopping caplacizumab, their cases were brought under control. There were also more bleeding episodes with caplacizumab (54% vs 38%), most being mild to moderate. Two patients in the placebo group died, but none in the treatment group.
The fact that platelet normalization occurred significantly faster with caplacizumab, even in some patients who had not yet had plasma exchange therapy initiated, has enormous clinical significance. The low platelet count in thrombotic thrombocytopenic purpura is a marker of susceptibility to rapid damage to the brain and kidneys, so correcting it quickly is critical.
Other strategies for new drug development include replacing the deficient ADAMTS13 with a recombinant molecule and blocking antibody production (the same mode of action as rituximab and glucocorticoids).9 Using all 3 strategies to treat thrombotic thrombocytopenic purpura may be the future standard of care.
HEMOLYTIC UREMIC SYNDROME
A child with sudden onset of bloody diarrhea and kidney failure
A 4-year-old girl plays with baby animals at a petting zoo and does not wash her hands immediately afterwards. Three days later, she develops fever, abdominal cramps, nausea, vomiting, and bloody diarrhea. Her pediatrician gives her antibiotics. On day 6, she develops ecchymoses on the extremities and lips, thrombocytopenia, low urine output, and seizures. Her stool tests positive for Escherichia coli O157:H7
Classic presentation: Young patient with bloody diarrhea
The classic presentation of hemolytic uremic syndrome is of a young patient with bloody diarrhea typically lasting 5 to 10 days. Kidney failure may follow, requiring dialysis in about 60% of patients for a mean of 10 days. About one-fourth of patients develop neurologic symptoms, and about the same fraction are left with long-term morbidity, eg, hypertension, proteinuria, and reduced glomerular filtration rate. The mortality rate is typically 4%10,11 but varies with the outbreak.
Histologically, the kidneys look identical to those in thrombotic thrombocytopenic purpura, with thrombi in glomeruli and small vessels.
E coli is the most common culprit, but other bacteria, including Shigella dysenteriae, and viruses are sometimes the cause. Fewer than 10% of children infected with Shiga toxin-positive E coli, also known as enterohemorrhagic E coli (O157:H7, O104:H4), develop hemolytic uremic syndrome.
Lessons from outbreaks
Petting zoos are a common source of transmission of pathogenic bacteria. Disease can be extremely serious: in 15 cases linked to a Florida petting zoo, 3 children died.
Other outbreaks involving pathogenic E coli have been tied to fresh vegetables and to undercooked hamburger at fast-food chains.
In Germany in 2011, more than 3,000 people acquired Shiga toxin nonhemolytic uremic syndrome due to E coli, and 16 of them died. In addition, 845 acquired hemolytic uremic syndrome, and 36 died. This outbreak was associated with the more virulent and less common O104:H4 strain, which has acquired a Shiga toxin-encoding phage. Patients were treated with quinolone antibiotics, which actually increase toxin production in this strain.12
Unusual in the German epidemic was that more adults were affected (88%), especially women (68% of cases).13 The source of infection was eventually found to be alfalfa sprouts, the seeds of which had been contaminated by E coli. Women did not harbor any intrinsic factor making them more susceptible; rather, they were more likely to eat salads.13
Supportive management
Supportive care is most important. Transfusion with packed red blood cells is indicated for hemoglobin below 6 g/dL. Hypertension should be controlled and dialysis provided. For central nervous system involvement or severe disease, plasma exchange is sometimes used.
Eculizumab was tried for a time as therapy but did not prove to be of benefit. Shiga toxin-binding agents have been developed, but by the time they are given it is too late in the disease process to help.
Antibiotics may harm; it is possible that they kill beneficial bacteria, allowing the Shiga toxin-producing E coli to better proliferate. Antimotility agents also are contraindicated. Other agents not recommended include urokinase, heparin, dipyridamole, and vincristine. Splenectomy is not advised.
The most important way to control hemolytic uremic syndrome is to prevent it by thoroughly cooking meat, cleaning fresh produce, and having children wash their hands after petting animals.
ATYPICAL HEMOLYTIC UREMIC SYNDROME
A young man in renal failure
A 28-year-old man has a history of “thrombotic thrombocytopenic purpura-hemolytic uremic syndrome” at age 12. He slowly progresses to end-stage renal disease and receives a renal transplant from his mother at age 20 that fails after 3 months. The renal transplant biopsy report at the time reads “thrombotic microangiopathy.” The patient’s brother also requires dialysis.
The patient’s complement values are low, especially C3. His father is offering him a kidney at this time, and the patient wants to know whether to proceed.
Normal ADAMTS13, no diarrhea
Hemolytic uremic syndrome without diarrhea is now called atypical hemolytic uremic syndrome. Patients have normal levels of ADAMTS13, do not have diarrhea, and have no evidence of Shiga toxin-producing E coli.
Continuous complement pathway activation
The complement system is part of the innate immune system, which provides immediate defense against infection and does not evolve as does the adaptive immune system. The classic complement pathway is activated by the C1 antibody-antigen complex. The alternative complement pathway leads to the same pathway via C3.14 Both pathways lead to the formation of C5 through C9 membrane attack complexes, which form channels across the membranes of target cells, leading to cell lysis and death.
The alternate pathway does not require an antibody trigger so is always active at a low level. Inhibitory factors (factor H, factor I, membrane cofactor protein, factor H-related proteins) are naturally present and slow it down at various steps. People who are born with an abnormal factor or, more commonly, develop antibodies against one of the factors, have uninhibited complement activation. If this happens in the blood vessels, massive coagulation and atypical hemolytic uremic syndrome ensues. The endothelial damage and clotting in the brain, kidney, and other organs are identical to that of hemolytic uremic syndrome caused by Shiga toxin.
Treat with eculizumab
Historically, atypical hemolytic uremic syndrome was treated with plasma exchange, which replaces defective complement regulatory proteins and removes inhibitory antibodies.
Understanding the complement pathways is key to developing drugs that target atypical hemolytic uremic syndrome, and about 60 are in the pipeline. The only one currently approved in the United States for atypical hemolytic uremic syndrome is eculizumab, a humanized monoclonal antibody that binds with high affinity to C5, blocking the end of the complement cascade and preventing formation of the membrane attack complex.15–18
The effects of eculizumab on atypical hemolytic uremic syndrome were studied in 2 prospective trials.19 Platelet counts rose rapidly within weeks of starting treatment, and kidney function improved. Benefits continued throughout the 64 weeks studied. There were no deaths among the 37 patients enrolled, and although these were single-arm trials, they provide evidence of dramatic benefit considering the high mortality risk of this disease.
Eculizumab is now considered the treatment of choice. It may be used empirically for patients with hemolytic uremic syndrome who test negative for Shiga toxin and antiphospholipid antibody, and who do not have a very low level of ADAMTS13. The big drawback of eculizumab is its high price,20–22 which varies by amount used, location, and pharmacy negotiation, but can be in the hundreds of thousands of dollars.
For a patient with atypical hemolytic uremic syndrome on dialysis, treatment with eculizumab should continue for 4 to 6 months if there are no extrarenal manifestations. But many patients continue to have the defect in the complement system, so the problem may recur.
Case revisited
For our patient considering a kidney transplant, many experts feel that a transplant can be done as long as platelet counts are monitored and treatment with eculizumab is restarted if needed. One can also make the case for waiting a few years for new oral drugs to become available before offering transplant.
ANTIPHOSPHOLIPID SYNDROME
A young woman with a history of thrombosis and miscarriages
A 27-year-old woman presents with arthralgias, low-grade fever, and malaise. She has a history of 3 spontaneous abortions and Raynaud phenomenon. Two years ago, she had deep vein thrombosis of the right calf after a long automobile trip.
She now has swollen metacarpophalangeal and proximal interphalangeal joints, livedo reticularis (a mottled venous pattern of the skin best seen under fluorescent light) of the legs and arms, and ankle edema (2-cm indentation).
Her blood pressure is 152/92 mm Hg. Laboratory values:
- White blood cell count 3.6 × 109/L (reference range 4.5–11.0)
- Hematocrit 24% (36%–47%)
- Platelet count 89 × 109/L (150–450)
- Urinalysis: protein 4+, heme 3+, red blood cells 8–15 per high-power field (< 3), red blood cell casts present
- Blood urea nitrogen 43 mg/dL (10–20)
- Creatinine 2.6 mg/dL (0.5–1.1).
- Prothrombin time 14.6 s (10–14)
- Partial thromboplastin time 85 s (25–35)
- Antinuclear antibody positive at 1:160
- Anti-double-stranded DNA and serum complement normal
- Syphilis serologic screening (VDRL) positive.
The patient has leukopenia, anemia, thrombocytopenia, hematuria, proteinuria, high blood urea nitrogen, and markedly elevated partial thromboplastin time. Although she has a positive antinuclear antibody test and renal dysfunction, her anti-dsDNA and serum complement tests are normal, making the diagnosis of systemic lupus erythematosus unlikely.
Consider antiphospholipid syndrome
In any patient with multiple pregnancy losses, lupus, or a history of thrombosis, antiphospholipid syndrome should be considered.
In a series of patients with antiphospholipid antibody who underwent kidney biopsy, more than half were men, indicating that, unlike lupus, this is not primarily a disease of young women.
Diagnosis based on specific criteria
Clinical criteria require at least one of the following in the patient’s history23:
- One or more episodes of arterial, venous, or small-vessel thrombosis
- Unexplained pregnancy morbidity (death of a fetus or neonate with normal morphology or 3 or more spontaneous abortions).
Serologic criteria for any of the following antiphospholipid antibodies require that at least one of the following tests be positive at least twice and at least 12 weeks apart:
- Anticardiolipin antibodies—high-titer immunoglobulin (Ig) G or IgM
- Autoantibodies for beta 2-glycoprotein
- Lupus anticoagulant—autoantibodies that increase clotting time in vitro and target beta 2-glycoprotein I and prothrombin (despite its name and actions in vitro, lupus anticoagulant functions as a coagulant).
As with the other thrombotic microangiopathies, patients with anticardiolipin syndrome have microthrombi in the glomeruli and blood vessels that are evident on kidney biopsy.
Suspect condition in likely groups
Antiphospholipid syndrome is surprisingly common.24 In a case-control study, de Groot et al25 found that 3.1% of patients under age 70 with a first episode of venous thrombosis and no known cancer were positive for lupus anticoagulant vs 0.9% of controls. In another case-control study, Urbanus et al26 found that 17% of women under age 50 with a stroke tested positive for lupus anticoagulant compared with less than 1% of controls. Because of such studies, it has become routine to test for anticardiolipin and lupus anticoagulant in young patients presenting with a stroke.
About 1% of women trying to have children have recurrent miscarriages, and of these, 10% to 15% have antiphospholipid antibody present.27–30
Pathogenesis
Patients with antiphospholipid syndrome have a much higher proportion of plasma beta 2-glycoprotein in the oxidized form than do healthy controls. The level is also higher than in patients with a different autoimmune disease whether or not they have antibodies against beta 2-glycoprotein 1. Although about 40% of patients with lupus have an anticardiolipin antibody, only a small percentage develop antiphospholipid syndrome with clotting.
It is thought that antiphospholipid syndrome involves initial injury to the endothelium, then potentiation of thrombus formation. Oxidized beta 2-glycoprotein complexes may bind to the endothelial cell surface, causing it to become the target of antibodies. The exact relationships between the factors are not yet understood.
The risk of a thrombotic event in an asymptomatic patient positive for all 3 factors—lupus anticoagulant, anticardiolipin antibody, and anti-beta 2-glycoprotein I antibody—is more than 5% per year.31
Manage thrombosis with anticoagulation
Khamashta et al,32 in a 1995 study, retrospectively studied patients with antiphospholipid antibodies and a history of thrombosis. Of 147 patients, 66 had idiopathic primary disease, 62 had systemic lupus, and 19 had “lupus-like” disease. Almost 70% (101 patients) had a recurrence of thrombosis, totaling 186 events. The mean time to recurrence was 12 months (range 2 weeks to 12 years). Recurrence rates were 0.01 events per patient per year with high-dose warfarin, 0.23 with low-dose warfarin, and 0.18 with aspirin. But the highest bleeding rates were in the 6 months after warfarin withdrawal; 29 patients had bleeding events, one-fourth of which were severe.
Standard therapy has become anticoagulation, starting with heparin or enoxaparin, then warfarin. There is inadequate evidence for the role of newer oral anticoagulant therapy.
A very high INR is not generally better than a moderately elevated level
For a time, it was thought that the international normalized ratio (INR) should be kept on the very high side to prevent thrombosis.
Crowther et al33 conducted a randomized, double-blind trial comparing moderate warfarin therapy (INR 2.0–3.0) and high-intensity warfarin therapy (INR 3.1–4.0) in antiphospholipid syndrome. Thrombosis actually recurred more frequently in the high-intensity therapy group (10.7% vs 3.4%), with no significant difference in major bleeding events.
A reasonable strategy is to keep the INR between 2.5 and 3.0, keeping in mind that values fluctuate in any individual patient. A higher goal often leads to excessive anticoagulation and bleeding. If the goal is too low, recurrent thrombosis becomes more likely. There are fewer data on the newer oral anticoagulants, but their role is likely to increase as reversal agents are developed.
Recommendations published in 2003 for treating antiphospholipid syndrome include34:
- Warfarin (INR 2.0–3.0) after the first thrombotic event
- Warfarin (INR 3.0–4.0) if a clot develops despite warfarin
- Warfarin (INR > 3.0) for an arterial event.
For the rare but catastrophic antiphospholipid syndrome in which thrombosis occurs in multiple organs, recommendations are for heparin plus steroids, with or without intravenous immunoglobulin and plasmapheresis. This approach has not always been successful, and the mortality rate is high.
Treatment of asymptomatic carriers is uncertain
Treatment of asymptomatic carriers of the antiphospholipid antibody is controversial. Evidence for management is scarce; some experts recommend aspirin therapy, but benefit has yet to be proven in clinical trials.
Canaud et al35 documented the role of activation of the kinase mammalian target of rapamycin (mTOR) in the vascular changes characteristic of antiphospholipid nephropathy. Postkidney transplant surveillance biopsies of patients with antiphospholipid antibodies showed vascular damage occurring over time (despite patients being asymptomatic) compared with other renal transplant patients. Patients with antiphospholipid antibodies who were treated with the immunosuppressive drug sirolimus were protected from developing these changes. Twelve years after transplant, 70% of patients with antiphospholipid antibodies taking sirolimus still had a functioning graft compared with 11% of untreated patients.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am Soc Nephrol 2003; 14:1072–1081.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Brunskill SJ, Tusold A, Benjamin S, Stanworth SJ, Murphy MF. A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus Med 2007; 17:17–35.
- Jasti S, Coyle T, Gentile T, Rosales L, Poiesz B. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher 2008; 23:151–156.
- Ling HT, Field JJ, Blinder MA. Sustained response with rituximab in patients with thrombotic thrombocytopenic purpura: a report of 13 cases and review of the literature. Am J Hematol 2009; 84:418–421.
- Hie M, Gay J, Galicier L, et al; French Thrombotic Microangiopathies Reference Centre. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood 2014; 124:204–210.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016; 374:511–522.
- Veyradier A. Von Willebrand factor—a new target for TTP treatment? N Engl J Med 2016; 374:583–585.
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med 1995; 333:364–368.
- Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J Infect Dis 2002; 186:493–500.
- Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 2011; 365:709–717.
- Frank C, Werber D, Cramer JP, et al; HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365:1771–1780.
- Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol 2012; 8:634–642.
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359–395.
- Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344:1058–1066.
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007; 25:1256–1264.
- Soliris (eculizumab). Prescribing information. Alexion Pharmaceuticals, Inc.
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med 2013; 368:2169–2181.
- Kim JJ, Waller SC, Reid CJ. Eculizumab in atypical haemolytic-uraemic syndrome allows cessation of plasma exchange and dialysis. Clin Kidney J 2012; 5:34–36.
- Povey H, Vundru R, Junglee N, Jibani M. Renal recovery with eculizumab in atypical hemolytic uremic syndrome following prolonged dialysis. Clin Nephrol 2014; 82:326–331.
- Gargau M, Azancot M, Ramos R, Sanchez-Corral P, Montero MA, Seron D. Early treatment with eculizumab may be beneficial in atypical haemolytic uraemic syndrome. Clin Kidney J 2012; 5:1–3.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044.
- de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost 2005; 3:1993–1997.
- Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 2009; 8:998–1005.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Stirrat GM. Recurrent miscarriage I: definition and epidemiology. Lancet 1990; 336:673–675.
- Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod 1995; 10:2001–2005.
- Yetman DL, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertil Steril 1996; 66:540–546.
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–4718.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995; 332:993–997.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Lockshin M, Tenedios F, Petri M, et al. Cardiac disease in the antiphospholipid syndrome: recommendations for treatment. Committee consensus report. Lupus 2003; 12:518–523.
- Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371:303–312.
Our knowledge of the pathogenesis of thrombotic microangiopathies has greatly advanced in the last decade, improving the diagnosis and treatment of these diseases.
Many conditions involve thrombotic microangiopathies (Table 1). This article reviews the most common ones, ie, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, atypical hemolytic uremic syndrome, and antiphospholipid syndrome—their clinical features (focusing on the kidney), course, and management. Of note, although the diseases are similar, their pathogeneses and treatments differ.
DIFFERENT PATHWAYS TO MULTIORGAN THROMBOSIS
The thrombotic microangiopathies are multisystem disorders that can affect children and adults and often present with prominent renal and neurologic involvement. Endothelial injury is likely the inciting factor leading to thrombosis in the kidney and in many other organs. The causes variously include:
- Toxins from bacteria or drugs
- Abnormal complement activation, genetic or autoantibody-induced
- Procoagulant factors, eg, antiphospholipid antibodies
- Loss of anticoagulants, eg, from a defect of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13); ADAMTS13 is also known as von Willebrand factor-cleaving protease
- Severe hypertension.
The histopathologic features are similar in all the thrombotic microangiopathies. Laboratory findings include thrombocytopenia, microangiopathic hemolytic anemia (with schistocytes on the peripheral blood smear), and high serum lactate dehydrogenase (LDH) levels; these are also markers of treatment progress. Bilirubin may be elevated and haptoglobin absent. Renal biopsy reveals thrombi in the glomeruli and arterioles.
THROMBOTIC THROMBOCYTOPENIC PURPURA
A young woman with fever, bruising, and renal failure, then blindness
A 36-year-old black woman who had been previously healthy presents to her doctor with fever and bruising.
Her hematocrit is 28% (reference range 38%–46%), platelet count 15 x 109/L (150–450), and prothrombin and partial thromboplastin times are normal. Her peripheral blood smear shows microangiopathic hemolytic anemia with schistocytes.
Over the next few days, her urine output declines and she develops sudden blindness followed by decreased mental acuity. Blood is drawn and sent for ADAMTS13 assay. Treatment is started at once with daily therapeutic plasma exchange. The assay results, when they arrive, show marked ADAMTS13 reduction (< 5%). Over the ensuing weeks, her mental acuity improves, her vision returns, and her renal function improves.
ADAMTS13 deficiency is definitive
Thrombotic thrombocytopenic purpura is characterized by:
- Neurologic abnormalities and acute renal failure
- Thrombocytopenia and microangiopathic hemolytic anemia
- Histologic evidence of thrombotic microangiopathy
- Deficiency of von Willebrand factor-cleaving protease (ADAMTS13 < 10%).
von Willebrand factor forms ultralarge multimers in the circulation that interact with platelets; these are normally cleaved by ADAMTS13. With ADAMTS13 deficiency (from either a genetic mutation or autoantibodies), the ultralarge multimers lead to coagulation as blood flows through small vessels.1
In 2003, Tsai2 evaluated 127 patients over age 10 who had thrombocytopenia and microangiopathic hemolysis with no plausible cause or features suggestive of hemolytic uremic syndrome. All were severely deficient in ADAMTS13. Subsequently, thrombotic thrombocytopenic purpura has been defined by a severe actual or effective deficiency of ADAMTS13.
Prompt plasma exchange is critical
Although the ADAMTS13 assay is important for diagnosing thrombotic thrombocytopenic purpura, in suspected cases daily plasma exchange should be started promptly, before test results return. Plasma exchange removes autoantibodies to ADAMTS13 from the blood, removes circulating ultralarge von Willebrand factor multimers, and replaces the missing ADAMTS13. Untreated, the disease is progressive, with irreversible renal failure, neurologic deterioration, and a 90% mortality rate. Plasma exchange reduces the mortality rate to less than 15%. If another diagnosis is confirmed, plasma exchange can be stopped.
Plasma exchange has been shown in clinical trials to be superior to plasma infusion in normalizing platelet counts and reducing mortality.3,4 Mortality rates were comparable with different replacement fluids vs fresh-frozen plasma, including solvent or detergent-treated plasma, and cryo-poor (cryosupernatant) plasma.4 Antiplatelet therapy, platelet transfusions, and splenectomy are ineffective.
Glucocorticoids for early treatment
An appropriate strategy is to add a glucocorticoid to plasma-exchange therapy at once (oral prednisone 1 mg/kg per day or intravenous methylprednisolone 125 mg twice daily) and withdraw it after several days if it is determined that it is not needed. Steroids for suspected thrombotic thrombocytopenic purpura can be justified for several reasons:
- The results of the ADAMTS13 assay are usually delayed, so steroids provide coverage for other diagnoses.
- They are helpful if thrombotic thrombocytopenic purpura is idiopathic (which is true for most cases) and if the patient has a poor response to initial therapy with plasma exchange.
- They are indicated for patients whose platelet counts do not increase with several days of plasma exchange or whose thrombocytopenia recurs as plasma exchange is decreased.
Rituximab improves survival
Rituximab, a chimeric (half murine) monoclonal antibody against CD19 and CD20 B cells, suppresses antibody production by knocking out the precursors of antibody-producing cells.
Anecdotal reports and small studies involving a total of 42 patients have been published on the use of rituximab for thrombotic thrombocytopenic purpura. Courses of rituximab varied greatly, from 1 to 13 weekly doses at 375 mg/m2, with 4 doses being the most common. Complete remission occurred in 90% of cases.5,6 A typical study from 2014 involved 48 patients (30 of whom received rituximab) followed by severe ADAMTS13 deficiency during remission.7 Despite the small study size, the investigators found significantly improved relapse-free survival rates with rituximab treatment.
But rituximab can cost $25,000 for 2 doses of 1,000 mg, and this will most likely prohibit its routine use. The cost and insurance coverage vary with location and policies.
Based on such studies, a reasonable strategy is to treat thrombotic thrombocytopenic purpura with:
- Daily plasma exchange
- Steroids, at least until the diagnosis is certain
- Rituximab if warranted.
New targeted therapies
Caplacizumab, a humanized immunoglobulin that inhibits the interaction between ultralarge von Willebrand factor multimers and platelets, has the potential to change this strategy when it receives US Food and Drug Administration approval, which is expected soon.
Peyvandi et al8 randomized 75 patients with acquired thrombotic thrombocytopenic purpura to either subcutaneous caplacizumab 10 mg daily for 30 days or placebo. Both groups had daily plasma exchange. The treatment group had a 39% reduction in median time to normalization of platelets vs the placebo group, and 3 of 36 patients had exacerbations, compared with 11 of 39 patients in the placebo group. Although 8 patients relapsed within the first month after stopping caplacizumab, their cases were brought under control. There were also more bleeding episodes with caplacizumab (54% vs 38%), most being mild to moderate. Two patients in the placebo group died, but none in the treatment group.
The fact that platelet normalization occurred significantly faster with caplacizumab, even in some patients who had not yet had plasma exchange therapy initiated, has enormous clinical significance. The low platelet count in thrombotic thrombocytopenic purpura is a marker of susceptibility to rapid damage to the brain and kidneys, so correcting it quickly is critical.
Other strategies for new drug development include replacing the deficient ADAMTS13 with a recombinant molecule and blocking antibody production (the same mode of action as rituximab and glucocorticoids).9 Using all 3 strategies to treat thrombotic thrombocytopenic purpura may be the future standard of care.
HEMOLYTIC UREMIC SYNDROME
A child with sudden onset of bloody diarrhea and kidney failure
A 4-year-old girl plays with baby animals at a petting zoo and does not wash her hands immediately afterwards. Three days later, she develops fever, abdominal cramps, nausea, vomiting, and bloody diarrhea. Her pediatrician gives her antibiotics. On day 6, she develops ecchymoses on the extremities and lips, thrombocytopenia, low urine output, and seizures. Her stool tests positive for Escherichia coli O157:H7
Classic presentation: Young patient with bloody diarrhea
The classic presentation of hemolytic uremic syndrome is of a young patient with bloody diarrhea typically lasting 5 to 10 days. Kidney failure may follow, requiring dialysis in about 60% of patients for a mean of 10 days. About one-fourth of patients develop neurologic symptoms, and about the same fraction are left with long-term morbidity, eg, hypertension, proteinuria, and reduced glomerular filtration rate. The mortality rate is typically 4%10,11 but varies with the outbreak.
Histologically, the kidneys look identical to those in thrombotic thrombocytopenic purpura, with thrombi in glomeruli and small vessels.
E coli is the most common culprit, but other bacteria, including Shigella dysenteriae, and viruses are sometimes the cause. Fewer than 10% of children infected with Shiga toxin-positive E coli, also known as enterohemorrhagic E coli (O157:H7, O104:H4), develop hemolytic uremic syndrome.
Lessons from outbreaks
Petting zoos are a common source of transmission of pathogenic bacteria. Disease can be extremely serious: in 15 cases linked to a Florida petting zoo, 3 children died.
Other outbreaks involving pathogenic E coli have been tied to fresh vegetables and to undercooked hamburger at fast-food chains.
In Germany in 2011, more than 3,000 people acquired Shiga toxin nonhemolytic uremic syndrome due to E coli, and 16 of them died. In addition, 845 acquired hemolytic uremic syndrome, and 36 died. This outbreak was associated with the more virulent and less common O104:H4 strain, which has acquired a Shiga toxin-encoding phage. Patients were treated with quinolone antibiotics, which actually increase toxin production in this strain.12
Unusual in the German epidemic was that more adults were affected (88%), especially women (68% of cases).13 The source of infection was eventually found to be alfalfa sprouts, the seeds of which had been contaminated by E coli. Women did not harbor any intrinsic factor making them more susceptible; rather, they were more likely to eat salads.13
Supportive management
Supportive care is most important. Transfusion with packed red blood cells is indicated for hemoglobin below 6 g/dL. Hypertension should be controlled and dialysis provided. For central nervous system involvement or severe disease, plasma exchange is sometimes used.
Eculizumab was tried for a time as therapy but did not prove to be of benefit. Shiga toxin-binding agents have been developed, but by the time they are given it is too late in the disease process to help.
Antibiotics may harm; it is possible that they kill beneficial bacteria, allowing the Shiga toxin-producing E coli to better proliferate. Antimotility agents also are contraindicated. Other agents not recommended include urokinase, heparin, dipyridamole, and vincristine. Splenectomy is not advised.
The most important way to control hemolytic uremic syndrome is to prevent it by thoroughly cooking meat, cleaning fresh produce, and having children wash their hands after petting animals.
ATYPICAL HEMOLYTIC UREMIC SYNDROME
A young man in renal failure
A 28-year-old man has a history of “thrombotic thrombocytopenic purpura-hemolytic uremic syndrome” at age 12. He slowly progresses to end-stage renal disease and receives a renal transplant from his mother at age 20 that fails after 3 months. The renal transplant biopsy report at the time reads “thrombotic microangiopathy.” The patient’s brother also requires dialysis.
The patient’s complement values are low, especially C3. His father is offering him a kidney at this time, and the patient wants to know whether to proceed.
Normal ADAMTS13, no diarrhea
Hemolytic uremic syndrome without diarrhea is now called atypical hemolytic uremic syndrome. Patients have normal levels of ADAMTS13, do not have diarrhea, and have no evidence of Shiga toxin-producing E coli.
Continuous complement pathway activation
The complement system is part of the innate immune system, which provides immediate defense against infection and does not evolve as does the adaptive immune system. The classic complement pathway is activated by the C1 antibody-antigen complex. The alternative complement pathway leads to the same pathway via C3.14 Both pathways lead to the formation of C5 through C9 membrane attack complexes, which form channels across the membranes of target cells, leading to cell lysis and death.
The alternate pathway does not require an antibody trigger so is always active at a low level. Inhibitory factors (factor H, factor I, membrane cofactor protein, factor H-related proteins) are naturally present and slow it down at various steps. People who are born with an abnormal factor or, more commonly, develop antibodies against one of the factors, have uninhibited complement activation. If this happens in the blood vessels, massive coagulation and atypical hemolytic uremic syndrome ensues. The endothelial damage and clotting in the brain, kidney, and other organs are identical to that of hemolytic uremic syndrome caused by Shiga toxin.
Treat with eculizumab
Historically, atypical hemolytic uremic syndrome was treated with plasma exchange, which replaces defective complement regulatory proteins and removes inhibitory antibodies.
Understanding the complement pathways is key to developing drugs that target atypical hemolytic uremic syndrome, and about 60 are in the pipeline. The only one currently approved in the United States for atypical hemolytic uremic syndrome is eculizumab, a humanized monoclonal antibody that binds with high affinity to C5, blocking the end of the complement cascade and preventing formation of the membrane attack complex.15–18
The effects of eculizumab on atypical hemolytic uremic syndrome were studied in 2 prospective trials.19 Platelet counts rose rapidly within weeks of starting treatment, and kidney function improved. Benefits continued throughout the 64 weeks studied. There were no deaths among the 37 patients enrolled, and although these were single-arm trials, they provide evidence of dramatic benefit considering the high mortality risk of this disease.
Eculizumab is now considered the treatment of choice. It may be used empirically for patients with hemolytic uremic syndrome who test negative for Shiga toxin and antiphospholipid antibody, and who do not have a very low level of ADAMTS13. The big drawback of eculizumab is its high price,20–22 which varies by amount used, location, and pharmacy negotiation, but can be in the hundreds of thousands of dollars.
For a patient with atypical hemolytic uremic syndrome on dialysis, treatment with eculizumab should continue for 4 to 6 months if there are no extrarenal manifestations. But many patients continue to have the defect in the complement system, so the problem may recur.
Case revisited
For our patient considering a kidney transplant, many experts feel that a transplant can be done as long as platelet counts are monitored and treatment with eculizumab is restarted if needed. One can also make the case for waiting a few years for new oral drugs to become available before offering transplant.
ANTIPHOSPHOLIPID SYNDROME
A young woman with a history of thrombosis and miscarriages
A 27-year-old woman presents with arthralgias, low-grade fever, and malaise. She has a history of 3 spontaneous abortions and Raynaud phenomenon. Two years ago, she had deep vein thrombosis of the right calf after a long automobile trip.
She now has swollen metacarpophalangeal and proximal interphalangeal joints, livedo reticularis (a mottled venous pattern of the skin best seen under fluorescent light) of the legs and arms, and ankle edema (2-cm indentation).
Her blood pressure is 152/92 mm Hg. Laboratory values:
- White blood cell count 3.6 × 109/L (reference range 4.5–11.0)
- Hematocrit 24% (36%–47%)
- Platelet count 89 × 109/L (150–450)
- Urinalysis: protein 4+, heme 3+, red blood cells 8–15 per high-power field (< 3), red blood cell casts present
- Blood urea nitrogen 43 mg/dL (10–20)
- Creatinine 2.6 mg/dL (0.5–1.1).
- Prothrombin time 14.6 s (10–14)
- Partial thromboplastin time 85 s (25–35)
- Antinuclear antibody positive at 1:160
- Anti-double-stranded DNA and serum complement normal
- Syphilis serologic screening (VDRL) positive.
The patient has leukopenia, anemia, thrombocytopenia, hematuria, proteinuria, high blood urea nitrogen, and markedly elevated partial thromboplastin time. Although she has a positive antinuclear antibody test and renal dysfunction, her anti-dsDNA and serum complement tests are normal, making the diagnosis of systemic lupus erythematosus unlikely.
Consider antiphospholipid syndrome
In any patient with multiple pregnancy losses, lupus, or a history of thrombosis, antiphospholipid syndrome should be considered.
In a series of patients with antiphospholipid antibody who underwent kidney biopsy, more than half were men, indicating that, unlike lupus, this is not primarily a disease of young women.
Diagnosis based on specific criteria
Clinical criteria require at least one of the following in the patient’s history23:
- One or more episodes of arterial, venous, or small-vessel thrombosis
- Unexplained pregnancy morbidity (death of a fetus or neonate with normal morphology or 3 or more spontaneous abortions).
Serologic criteria for any of the following antiphospholipid antibodies require that at least one of the following tests be positive at least twice and at least 12 weeks apart:
- Anticardiolipin antibodies—high-titer immunoglobulin (Ig) G or IgM
- Autoantibodies for beta 2-glycoprotein
- Lupus anticoagulant—autoantibodies that increase clotting time in vitro and target beta 2-glycoprotein I and prothrombin (despite its name and actions in vitro, lupus anticoagulant functions as a coagulant).
As with the other thrombotic microangiopathies, patients with anticardiolipin syndrome have microthrombi in the glomeruli and blood vessels that are evident on kidney biopsy.
Suspect condition in likely groups
Antiphospholipid syndrome is surprisingly common.24 In a case-control study, de Groot et al25 found that 3.1% of patients under age 70 with a first episode of venous thrombosis and no known cancer were positive for lupus anticoagulant vs 0.9% of controls. In another case-control study, Urbanus et al26 found that 17% of women under age 50 with a stroke tested positive for lupus anticoagulant compared with less than 1% of controls. Because of such studies, it has become routine to test for anticardiolipin and lupus anticoagulant in young patients presenting with a stroke.
About 1% of women trying to have children have recurrent miscarriages, and of these, 10% to 15% have antiphospholipid antibody present.27–30
Pathogenesis
Patients with antiphospholipid syndrome have a much higher proportion of plasma beta 2-glycoprotein in the oxidized form than do healthy controls. The level is also higher than in patients with a different autoimmune disease whether or not they have antibodies against beta 2-glycoprotein 1. Although about 40% of patients with lupus have an anticardiolipin antibody, only a small percentage develop antiphospholipid syndrome with clotting.
It is thought that antiphospholipid syndrome involves initial injury to the endothelium, then potentiation of thrombus formation. Oxidized beta 2-glycoprotein complexes may bind to the endothelial cell surface, causing it to become the target of antibodies. The exact relationships between the factors are not yet understood.
The risk of a thrombotic event in an asymptomatic patient positive for all 3 factors—lupus anticoagulant, anticardiolipin antibody, and anti-beta 2-glycoprotein I antibody—is more than 5% per year.31
Manage thrombosis with anticoagulation
Khamashta et al,32 in a 1995 study, retrospectively studied patients with antiphospholipid antibodies and a history of thrombosis. Of 147 patients, 66 had idiopathic primary disease, 62 had systemic lupus, and 19 had “lupus-like” disease. Almost 70% (101 patients) had a recurrence of thrombosis, totaling 186 events. The mean time to recurrence was 12 months (range 2 weeks to 12 years). Recurrence rates were 0.01 events per patient per year with high-dose warfarin, 0.23 with low-dose warfarin, and 0.18 with aspirin. But the highest bleeding rates were in the 6 months after warfarin withdrawal; 29 patients had bleeding events, one-fourth of which were severe.
Standard therapy has become anticoagulation, starting with heparin or enoxaparin, then warfarin. There is inadequate evidence for the role of newer oral anticoagulant therapy.
A very high INR is not generally better than a moderately elevated level
For a time, it was thought that the international normalized ratio (INR) should be kept on the very high side to prevent thrombosis.
Crowther et al33 conducted a randomized, double-blind trial comparing moderate warfarin therapy (INR 2.0–3.0) and high-intensity warfarin therapy (INR 3.1–4.0) in antiphospholipid syndrome. Thrombosis actually recurred more frequently in the high-intensity therapy group (10.7% vs 3.4%), with no significant difference in major bleeding events.
A reasonable strategy is to keep the INR between 2.5 and 3.0, keeping in mind that values fluctuate in any individual patient. A higher goal often leads to excessive anticoagulation and bleeding. If the goal is too low, recurrent thrombosis becomes more likely. There are fewer data on the newer oral anticoagulants, but their role is likely to increase as reversal agents are developed.
Recommendations published in 2003 for treating antiphospholipid syndrome include34:
- Warfarin (INR 2.0–3.0) after the first thrombotic event
- Warfarin (INR 3.0–4.0) if a clot develops despite warfarin
- Warfarin (INR > 3.0) for an arterial event.
For the rare but catastrophic antiphospholipid syndrome in which thrombosis occurs in multiple organs, recommendations are for heparin plus steroids, with or without intravenous immunoglobulin and plasmapheresis. This approach has not always been successful, and the mortality rate is high.
Treatment of asymptomatic carriers is uncertain
Treatment of asymptomatic carriers of the antiphospholipid antibody is controversial. Evidence for management is scarce; some experts recommend aspirin therapy, but benefit has yet to be proven in clinical trials.
Canaud et al35 documented the role of activation of the kinase mammalian target of rapamycin (mTOR) in the vascular changes characteristic of antiphospholipid nephropathy. Postkidney transplant surveillance biopsies of patients with antiphospholipid antibodies showed vascular damage occurring over time (despite patients being asymptomatic) compared with other renal transplant patients. Patients with antiphospholipid antibodies who were treated with the immunosuppressive drug sirolimus were protected from developing these changes. Twelve years after transplant, 70% of patients with antiphospholipid antibodies taking sirolimus still had a functioning graft compared with 11% of untreated patients.
Our knowledge of the pathogenesis of thrombotic microangiopathies has greatly advanced in the last decade, improving the diagnosis and treatment of these diseases.
Many conditions involve thrombotic microangiopathies (Table 1). This article reviews the most common ones, ie, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, atypical hemolytic uremic syndrome, and antiphospholipid syndrome—their clinical features (focusing on the kidney), course, and management. Of note, although the diseases are similar, their pathogeneses and treatments differ.
DIFFERENT PATHWAYS TO MULTIORGAN THROMBOSIS
The thrombotic microangiopathies are multisystem disorders that can affect children and adults and often present with prominent renal and neurologic involvement. Endothelial injury is likely the inciting factor leading to thrombosis in the kidney and in many other organs. The causes variously include:
- Toxins from bacteria or drugs
- Abnormal complement activation, genetic or autoantibody-induced
- Procoagulant factors, eg, antiphospholipid antibodies
- Loss of anticoagulants, eg, from a defect of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13); ADAMTS13 is also known as von Willebrand factor-cleaving protease
- Severe hypertension.
The histopathologic features are similar in all the thrombotic microangiopathies. Laboratory findings include thrombocytopenia, microangiopathic hemolytic anemia (with schistocytes on the peripheral blood smear), and high serum lactate dehydrogenase (LDH) levels; these are also markers of treatment progress. Bilirubin may be elevated and haptoglobin absent. Renal biopsy reveals thrombi in the glomeruli and arterioles.
THROMBOTIC THROMBOCYTOPENIC PURPURA
A young woman with fever, bruising, and renal failure, then blindness
A 36-year-old black woman who had been previously healthy presents to her doctor with fever and bruising.
Her hematocrit is 28% (reference range 38%–46%), platelet count 15 x 109/L (150–450), and prothrombin and partial thromboplastin times are normal. Her peripheral blood smear shows microangiopathic hemolytic anemia with schistocytes.
Over the next few days, her urine output declines and she develops sudden blindness followed by decreased mental acuity. Blood is drawn and sent for ADAMTS13 assay. Treatment is started at once with daily therapeutic plasma exchange. The assay results, when they arrive, show marked ADAMTS13 reduction (< 5%). Over the ensuing weeks, her mental acuity improves, her vision returns, and her renal function improves.
ADAMTS13 deficiency is definitive
Thrombotic thrombocytopenic purpura is characterized by:
- Neurologic abnormalities and acute renal failure
- Thrombocytopenia and microangiopathic hemolytic anemia
- Histologic evidence of thrombotic microangiopathy
- Deficiency of von Willebrand factor-cleaving protease (ADAMTS13 < 10%).
von Willebrand factor forms ultralarge multimers in the circulation that interact with platelets; these are normally cleaved by ADAMTS13. With ADAMTS13 deficiency (from either a genetic mutation or autoantibodies), the ultralarge multimers lead to coagulation as blood flows through small vessels.1
In 2003, Tsai2 evaluated 127 patients over age 10 who had thrombocytopenia and microangiopathic hemolysis with no plausible cause or features suggestive of hemolytic uremic syndrome. All were severely deficient in ADAMTS13. Subsequently, thrombotic thrombocytopenic purpura has been defined by a severe actual or effective deficiency of ADAMTS13.
Prompt plasma exchange is critical
Although the ADAMTS13 assay is important for diagnosing thrombotic thrombocytopenic purpura, in suspected cases daily plasma exchange should be started promptly, before test results return. Plasma exchange removes autoantibodies to ADAMTS13 from the blood, removes circulating ultralarge von Willebrand factor multimers, and replaces the missing ADAMTS13. Untreated, the disease is progressive, with irreversible renal failure, neurologic deterioration, and a 90% mortality rate. Plasma exchange reduces the mortality rate to less than 15%. If another diagnosis is confirmed, plasma exchange can be stopped.
Plasma exchange has been shown in clinical trials to be superior to plasma infusion in normalizing platelet counts and reducing mortality.3,4 Mortality rates were comparable with different replacement fluids vs fresh-frozen plasma, including solvent or detergent-treated plasma, and cryo-poor (cryosupernatant) plasma.4 Antiplatelet therapy, platelet transfusions, and splenectomy are ineffective.
Glucocorticoids for early treatment
An appropriate strategy is to add a glucocorticoid to plasma-exchange therapy at once (oral prednisone 1 mg/kg per day or intravenous methylprednisolone 125 mg twice daily) and withdraw it after several days if it is determined that it is not needed. Steroids for suspected thrombotic thrombocytopenic purpura can be justified for several reasons:
- The results of the ADAMTS13 assay are usually delayed, so steroids provide coverage for other diagnoses.
- They are helpful if thrombotic thrombocytopenic purpura is idiopathic (which is true for most cases) and if the patient has a poor response to initial therapy with plasma exchange.
- They are indicated for patients whose platelet counts do not increase with several days of plasma exchange or whose thrombocytopenia recurs as plasma exchange is decreased.
Rituximab improves survival
Rituximab, a chimeric (half murine) monoclonal antibody against CD19 and CD20 B cells, suppresses antibody production by knocking out the precursors of antibody-producing cells.
Anecdotal reports and small studies involving a total of 42 patients have been published on the use of rituximab for thrombotic thrombocytopenic purpura. Courses of rituximab varied greatly, from 1 to 13 weekly doses at 375 mg/m2, with 4 doses being the most common. Complete remission occurred in 90% of cases.5,6 A typical study from 2014 involved 48 patients (30 of whom received rituximab) followed by severe ADAMTS13 deficiency during remission.7 Despite the small study size, the investigators found significantly improved relapse-free survival rates with rituximab treatment.
But rituximab can cost $25,000 for 2 doses of 1,000 mg, and this will most likely prohibit its routine use. The cost and insurance coverage vary with location and policies.
Based on such studies, a reasonable strategy is to treat thrombotic thrombocytopenic purpura with:
- Daily plasma exchange
- Steroids, at least until the diagnosis is certain
- Rituximab if warranted.
New targeted therapies
Caplacizumab, a humanized immunoglobulin that inhibits the interaction between ultralarge von Willebrand factor multimers and platelets, has the potential to change this strategy when it receives US Food and Drug Administration approval, which is expected soon.
Peyvandi et al8 randomized 75 patients with acquired thrombotic thrombocytopenic purpura to either subcutaneous caplacizumab 10 mg daily for 30 days or placebo. Both groups had daily plasma exchange. The treatment group had a 39% reduction in median time to normalization of platelets vs the placebo group, and 3 of 36 patients had exacerbations, compared with 11 of 39 patients in the placebo group. Although 8 patients relapsed within the first month after stopping caplacizumab, their cases were brought under control. There were also more bleeding episodes with caplacizumab (54% vs 38%), most being mild to moderate. Two patients in the placebo group died, but none in the treatment group.
The fact that platelet normalization occurred significantly faster with caplacizumab, even in some patients who had not yet had plasma exchange therapy initiated, has enormous clinical significance. The low platelet count in thrombotic thrombocytopenic purpura is a marker of susceptibility to rapid damage to the brain and kidneys, so correcting it quickly is critical.
Other strategies for new drug development include replacing the deficient ADAMTS13 with a recombinant molecule and blocking antibody production (the same mode of action as rituximab and glucocorticoids).9 Using all 3 strategies to treat thrombotic thrombocytopenic purpura may be the future standard of care.
HEMOLYTIC UREMIC SYNDROME
A child with sudden onset of bloody diarrhea and kidney failure
A 4-year-old girl plays with baby animals at a petting zoo and does not wash her hands immediately afterwards. Three days later, she develops fever, abdominal cramps, nausea, vomiting, and bloody diarrhea. Her pediatrician gives her antibiotics. On day 6, she develops ecchymoses on the extremities and lips, thrombocytopenia, low urine output, and seizures. Her stool tests positive for Escherichia coli O157:H7
Classic presentation: Young patient with bloody diarrhea
The classic presentation of hemolytic uremic syndrome is of a young patient with bloody diarrhea typically lasting 5 to 10 days. Kidney failure may follow, requiring dialysis in about 60% of patients for a mean of 10 days. About one-fourth of patients develop neurologic symptoms, and about the same fraction are left with long-term morbidity, eg, hypertension, proteinuria, and reduced glomerular filtration rate. The mortality rate is typically 4%10,11 but varies with the outbreak.
Histologically, the kidneys look identical to those in thrombotic thrombocytopenic purpura, with thrombi in glomeruli and small vessels.
E coli is the most common culprit, but other bacteria, including Shigella dysenteriae, and viruses are sometimes the cause. Fewer than 10% of children infected with Shiga toxin-positive E coli, also known as enterohemorrhagic E coli (O157:H7, O104:H4), develop hemolytic uremic syndrome.
Lessons from outbreaks
Petting zoos are a common source of transmission of pathogenic bacteria. Disease can be extremely serious: in 15 cases linked to a Florida petting zoo, 3 children died.
Other outbreaks involving pathogenic E coli have been tied to fresh vegetables and to undercooked hamburger at fast-food chains.
In Germany in 2011, more than 3,000 people acquired Shiga toxin nonhemolytic uremic syndrome due to E coli, and 16 of them died. In addition, 845 acquired hemolytic uremic syndrome, and 36 died. This outbreak was associated with the more virulent and less common O104:H4 strain, which has acquired a Shiga toxin-encoding phage. Patients were treated with quinolone antibiotics, which actually increase toxin production in this strain.12
Unusual in the German epidemic was that more adults were affected (88%), especially women (68% of cases).13 The source of infection was eventually found to be alfalfa sprouts, the seeds of which had been contaminated by E coli. Women did not harbor any intrinsic factor making them more susceptible; rather, they were more likely to eat salads.13
Supportive management
Supportive care is most important. Transfusion with packed red blood cells is indicated for hemoglobin below 6 g/dL. Hypertension should be controlled and dialysis provided. For central nervous system involvement or severe disease, plasma exchange is sometimes used.
Eculizumab was tried for a time as therapy but did not prove to be of benefit. Shiga toxin-binding agents have been developed, but by the time they are given it is too late in the disease process to help.
Antibiotics may harm; it is possible that they kill beneficial bacteria, allowing the Shiga toxin-producing E coli to better proliferate. Antimotility agents also are contraindicated. Other agents not recommended include urokinase, heparin, dipyridamole, and vincristine. Splenectomy is not advised.
The most important way to control hemolytic uremic syndrome is to prevent it by thoroughly cooking meat, cleaning fresh produce, and having children wash their hands after petting animals.
ATYPICAL HEMOLYTIC UREMIC SYNDROME
A young man in renal failure
A 28-year-old man has a history of “thrombotic thrombocytopenic purpura-hemolytic uremic syndrome” at age 12. He slowly progresses to end-stage renal disease and receives a renal transplant from his mother at age 20 that fails after 3 months. The renal transplant biopsy report at the time reads “thrombotic microangiopathy.” The patient’s brother also requires dialysis.
The patient’s complement values are low, especially C3. His father is offering him a kidney at this time, and the patient wants to know whether to proceed.
Normal ADAMTS13, no diarrhea
Hemolytic uremic syndrome without diarrhea is now called atypical hemolytic uremic syndrome. Patients have normal levels of ADAMTS13, do not have diarrhea, and have no evidence of Shiga toxin-producing E coli.
Continuous complement pathway activation
The complement system is part of the innate immune system, which provides immediate defense against infection and does not evolve as does the adaptive immune system. The classic complement pathway is activated by the C1 antibody-antigen complex. The alternative complement pathway leads to the same pathway via C3.14 Both pathways lead to the formation of C5 through C9 membrane attack complexes, which form channels across the membranes of target cells, leading to cell lysis and death.
The alternate pathway does not require an antibody trigger so is always active at a low level. Inhibitory factors (factor H, factor I, membrane cofactor protein, factor H-related proteins) are naturally present and slow it down at various steps. People who are born with an abnormal factor or, more commonly, develop antibodies against one of the factors, have uninhibited complement activation. If this happens in the blood vessels, massive coagulation and atypical hemolytic uremic syndrome ensues. The endothelial damage and clotting in the brain, kidney, and other organs are identical to that of hemolytic uremic syndrome caused by Shiga toxin.
Treat with eculizumab
Historically, atypical hemolytic uremic syndrome was treated with plasma exchange, which replaces defective complement regulatory proteins and removes inhibitory antibodies.
Understanding the complement pathways is key to developing drugs that target atypical hemolytic uremic syndrome, and about 60 are in the pipeline. The only one currently approved in the United States for atypical hemolytic uremic syndrome is eculizumab, a humanized monoclonal antibody that binds with high affinity to C5, blocking the end of the complement cascade and preventing formation of the membrane attack complex.15–18
The effects of eculizumab on atypical hemolytic uremic syndrome were studied in 2 prospective trials.19 Platelet counts rose rapidly within weeks of starting treatment, and kidney function improved. Benefits continued throughout the 64 weeks studied. There were no deaths among the 37 patients enrolled, and although these were single-arm trials, they provide evidence of dramatic benefit considering the high mortality risk of this disease.
Eculizumab is now considered the treatment of choice. It may be used empirically for patients with hemolytic uremic syndrome who test negative for Shiga toxin and antiphospholipid antibody, and who do not have a very low level of ADAMTS13. The big drawback of eculizumab is its high price,20–22 which varies by amount used, location, and pharmacy negotiation, but can be in the hundreds of thousands of dollars.
For a patient with atypical hemolytic uremic syndrome on dialysis, treatment with eculizumab should continue for 4 to 6 months if there are no extrarenal manifestations. But many patients continue to have the defect in the complement system, so the problem may recur.
Case revisited
For our patient considering a kidney transplant, many experts feel that a transplant can be done as long as platelet counts are monitored and treatment with eculizumab is restarted if needed. One can also make the case for waiting a few years for new oral drugs to become available before offering transplant.
ANTIPHOSPHOLIPID SYNDROME
A young woman with a history of thrombosis and miscarriages
A 27-year-old woman presents with arthralgias, low-grade fever, and malaise. She has a history of 3 spontaneous abortions and Raynaud phenomenon. Two years ago, she had deep vein thrombosis of the right calf after a long automobile trip.
She now has swollen metacarpophalangeal and proximal interphalangeal joints, livedo reticularis (a mottled venous pattern of the skin best seen under fluorescent light) of the legs and arms, and ankle edema (2-cm indentation).
Her blood pressure is 152/92 mm Hg. Laboratory values:
- White blood cell count 3.6 × 109/L (reference range 4.5–11.0)
- Hematocrit 24% (36%–47%)
- Platelet count 89 × 109/L (150–450)
- Urinalysis: protein 4+, heme 3+, red blood cells 8–15 per high-power field (< 3), red blood cell casts present
- Blood urea nitrogen 43 mg/dL (10–20)
- Creatinine 2.6 mg/dL (0.5–1.1).
- Prothrombin time 14.6 s (10–14)
- Partial thromboplastin time 85 s (25–35)
- Antinuclear antibody positive at 1:160
- Anti-double-stranded DNA and serum complement normal
- Syphilis serologic screening (VDRL) positive.
The patient has leukopenia, anemia, thrombocytopenia, hematuria, proteinuria, high blood urea nitrogen, and markedly elevated partial thromboplastin time. Although she has a positive antinuclear antibody test and renal dysfunction, her anti-dsDNA and serum complement tests are normal, making the diagnosis of systemic lupus erythematosus unlikely.
Consider antiphospholipid syndrome
In any patient with multiple pregnancy losses, lupus, or a history of thrombosis, antiphospholipid syndrome should be considered.
In a series of patients with antiphospholipid antibody who underwent kidney biopsy, more than half were men, indicating that, unlike lupus, this is not primarily a disease of young women.
Diagnosis based on specific criteria
Clinical criteria require at least one of the following in the patient’s history23:
- One or more episodes of arterial, venous, or small-vessel thrombosis
- Unexplained pregnancy morbidity (death of a fetus or neonate with normal morphology or 3 or more spontaneous abortions).
Serologic criteria for any of the following antiphospholipid antibodies require that at least one of the following tests be positive at least twice and at least 12 weeks apart:
- Anticardiolipin antibodies—high-titer immunoglobulin (Ig) G or IgM
- Autoantibodies for beta 2-glycoprotein
- Lupus anticoagulant—autoantibodies that increase clotting time in vitro and target beta 2-glycoprotein I and prothrombin (despite its name and actions in vitro, lupus anticoagulant functions as a coagulant).
As with the other thrombotic microangiopathies, patients with anticardiolipin syndrome have microthrombi in the glomeruli and blood vessels that are evident on kidney biopsy.
Suspect condition in likely groups
Antiphospholipid syndrome is surprisingly common.24 In a case-control study, de Groot et al25 found that 3.1% of patients under age 70 with a first episode of venous thrombosis and no known cancer were positive for lupus anticoagulant vs 0.9% of controls. In another case-control study, Urbanus et al26 found that 17% of women under age 50 with a stroke tested positive for lupus anticoagulant compared with less than 1% of controls. Because of such studies, it has become routine to test for anticardiolipin and lupus anticoagulant in young patients presenting with a stroke.
About 1% of women trying to have children have recurrent miscarriages, and of these, 10% to 15% have antiphospholipid antibody present.27–30
Pathogenesis
Patients with antiphospholipid syndrome have a much higher proportion of plasma beta 2-glycoprotein in the oxidized form than do healthy controls. The level is also higher than in patients with a different autoimmune disease whether or not they have antibodies against beta 2-glycoprotein 1. Although about 40% of patients with lupus have an anticardiolipin antibody, only a small percentage develop antiphospholipid syndrome with clotting.
It is thought that antiphospholipid syndrome involves initial injury to the endothelium, then potentiation of thrombus formation. Oxidized beta 2-glycoprotein complexes may bind to the endothelial cell surface, causing it to become the target of antibodies. The exact relationships between the factors are not yet understood.
The risk of a thrombotic event in an asymptomatic patient positive for all 3 factors—lupus anticoagulant, anticardiolipin antibody, and anti-beta 2-glycoprotein I antibody—is more than 5% per year.31
Manage thrombosis with anticoagulation
Khamashta et al,32 in a 1995 study, retrospectively studied patients with antiphospholipid antibodies and a history of thrombosis. Of 147 patients, 66 had idiopathic primary disease, 62 had systemic lupus, and 19 had “lupus-like” disease. Almost 70% (101 patients) had a recurrence of thrombosis, totaling 186 events. The mean time to recurrence was 12 months (range 2 weeks to 12 years). Recurrence rates were 0.01 events per patient per year with high-dose warfarin, 0.23 with low-dose warfarin, and 0.18 with aspirin. But the highest bleeding rates were in the 6 months after warfarin withdrawal; 29 patients had bleeding events, one-fourth of which were severe.
Standard therapy has become anticoagulation, starting with heparin or enoxaparin, then warfarin. There is inadequate evidence for the role of newer oral anticoagulant therapy.
A very high INR is not generally better than a moderately elevated level
For a time, it was thought that the international normalized ratio (INR) should be kept on the very high side to prevent thrombosis.
Crowther et al33 conducted a randomized, double-blind trial comparing moderate warfarin therapy (INR 2.0–3.0) and high-intensity warfarin therapy (INR 3.1–4.0) in antiphospholipid syndrome. Thrombosis actually recurred more frequently in the high-intensity therapy group (10.7% vs 3.4%), with no significant difference in major bleeding events.
A reasonable strategy is to keep the INR between 2.5 and 3.0, keeping in mind that values fluctuate in any individual patient. A higher goal often leads to excessive anticoagulation and bleeding. If the goal is too low, recurrent thrombosis becomes more likely. There are fewer data on the newer oral anticoagulants, but their role is likely to increase as reversal agents are developed.
Recommendations published in 2003 for treating antiphospholipid syndrome include34:
- Warfarin (INR 2.0–3.0) after the first thrombotic event
- Warfarin (INR 3.0–4.0) if a clot develops despite warfarin
- Warfarin (INR > 3.0) for an arterial event.
For the rare but catastrophic antiphospholipid syndrome in which thrombosis occurs in multiple organs, recommendations are for heparin plus steroids, with or without intravenous immunoglobulin and plasmapheresis. This approach has not always been successful, and the mortality rate is high.
Treatment of asymptomatic carriers is uncertain
Treatment of asymptomatic carriers of the antiphospholipid antibody is controversial. Evidence for management is scarce; some experts recommend aspirin therapy, but benefit has yet to be proven in clinical trials.
Canaud et al35 documented the role of activation of the kinase mammalian target of rapamycin (mTOR) in the vascular changes characteristic of antiphospholipid nephropathy. Postkidney transplant surveillance biopsies of patients with antiphospholipid antibodies showed vascular damage occurring over time (despite patients being asymptomatic) compared with other renal transplant patients. Patients with antiphospholipid antibodies who were treated with the immunosuppressive drug sirolimus were protected from developing these changes. Twelve years after transplant, 70% of patients with antiphospholipid antibodies taking sirolimus still had a functioning graft compared with 11% of untreated patients.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am Soc Nephrol 2003; 14:1072–1081.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Brunskill SJ, Tusold A, Benjamin S, Stanworth SJ, Murphy MF. A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus Med 2007; 17:17–35.
- Jasti S, Coyle T, Gentile T, Rosales L, Poiesz B. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher 2008; 23:151–156.
- Ling HT, Field JJ, Blinder MA. Sustained response with rituximab in patients with thrombotic thrombocytopenic purpura: a report of 13 cases and review of the literature. Am J Hematol 2009; 84:418–421.
- Hie M, Gay J, Galicier L, et al; French Thrombotic Microangiopathies Reference Centre. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood 2014; 124:204–210.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016; 374:511–522.
- Veyradier A. Von Willebrand factor—a new target for TTP treatment? N Engl J Med 2016; 374:583–585.
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med 1995; 333:364–368.
- Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J Infect Dis 2002; 186:493–500.
- Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 2011; 365:709–717.
- Frank C, Werber D, Cramer JP, et al; HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365:1771–1780.
- Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol 2012; 8:634–642.
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359–395.
- Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344:1058–1066.
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007; 25:1256–1264.
- Soliris (eculizumab). Prescribing information. Alexion Pharmaceuticals, Inc.
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med 2013; 368:2169–2181.
- Kim JJ, Waller SC, Reid CJ. Eculizumab in atypical haemolytic-uraemic syndrome allows cessation of plasma exchange and dialysis. Clin Kidney J 2012; 5:34–36.
- Povey H, Vundru R, Junglee N, Jibani M. Renal recovery with eculizumab in atypical hemolytic uremic syndrome following prolonged dialysis. Clin Nephrol 2014; 82:326–331.
- Gargau M, Azancot M, Ramos R, Sanchez-Corral P, Montero MA, Seron D. Early treatment with eculizumab may be beneficial in atypical haemolytic uraemic syndrome. Clin Kidney J 2012; 5:1–3.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044.
- de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost 2005; 3:1993–1997.
- Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 2009; 8:998–1005.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Stirrat GM. Recurrent miscarriage I: definition and epidemiology. Lancet 1990; 336:673–675.
- Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod 1995; 10:2001–2005.
- Yetman DL, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertil Steril 1996; 66:540–546.
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–4718.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995; 332:993–997.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Lockshin M, Tenedios F, Petri M, et al. Cardiac disease in the antiphospholipid syndrome: recommendations for treatment. Committee consensus report. Lupus 2003; 12:518–523.
- Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371:303–312.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am Soc Nephrol 2003; 14:1072–1081.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991; 325:393–397.
- Brunskill SJ, Tusold A, Benjamin S, Stanworth SJ, Murphy MF. A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus Med 2007; 17:17–35.
- Jasti S, Coyle T, Gentile T, Rosales L, Poiesz B. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher 2008; 23:151–156.
- Ling HT, Field JJ, Blinder MA. Sustained response with rituximab in patients with thrombotic thrombocytopenic purpura: a report of 13 cases and review of the literature. Am J Hematol 2009; 84:418–421.
- Hie M, Gay J, Galicier L, et al; French Thrombotic Microangiopathies Reference Centre. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood 2014; 124:204–210.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016; 374:511–522.
- Veyradier A. Von Willebrand factor—a new target for TTP treatment? N Engl J Med 2016; 374:583–585.
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med 1995; 333:364–368.
- Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J Infect Dis 2002; 186:493–500.
- Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 2011; 365:709–717.
- Frank C, Werber D, Cramer JP, et al; HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365:1771–1780.
- Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol 2012; 8:634–642.
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359–395.
- Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344:1058–1066.
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007; 25:1256–1264.
- Soliris (eculizumab). Prescribing information. Alexion Pharmaceuticals, Inc.
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med 2013; 368:2169–2181.
- Kim JJ, Waller SC, Reid CJ. Eculizumab in atypical haemolytic-uraemic syndrome allows cessation of plasma exchange and dialysis. Clin Kidney J 2012; 5:34–36.
- Povey H, Vundru R, Junglee N, Jibani M. Renal recovery with eculizumab in atypical hemolytic uremic syndrome following prolonged dialysis. Clin Nephrol 2014; 82:326–331.
- Gargau M, Azancot M, Ramos R, Sanchez-Corral P, Montero MA, Seron D. Early treatment with eculizumab may be beneficial in atypical haemolytic uraemic syndrome. Clin Kidney J 2012; 5:1–3.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044.
- de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost 2005; 3:1993–1997.
- Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 2009; 8:998–1005.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Stirrat GM. Recurrent miscarriage I: definition and epidemiology. Lancet 1990; 336:673–675.
- Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod 1995; 10:2001–2005.
- Yetman DL, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertil Steril 1996; 66:540–546.
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–4718.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995; 332:993–997.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Lockshin M, Tenedios F, Petri M, et al. Cardiac disease in the antiphospholipid syndrome: recommendations for treatment. Committee consensus report. Lupus 2003; 12:518–523.
- Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371:303–312.
KEY POINTS
- Thrombotic thrombocytopenic purpura is diagnosed with the ADAMTS13 assay. As soon as it is suspected, it should be treated with daily plasma exchange, steroids (at least until the diagnosis is certain), and, if additional treatment is needed, rituximab.
- Hemolytic uremic syndrome is seen in children who handle farm animals and in children and adults in food outbreaks. It is managed supportively with transfusion of packed red blood cells and dialysis.
- Atypical hemolytic uremic syndrome should be suspected in patients with normal ADAMTS13 and without diarrhea or evidence of Shiga toxin-producing Escherichia coli. It often responds well to eculizumab, a blocker of C5 (the fifth component of complement).
- Antiphospholipid syndrome should be investigated in women who have multiple miscarriages or thrombotic events. Symptomatic disease requires long-term anticoagulation therapy.
Hemodialysis patient with finger ulcerations
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.
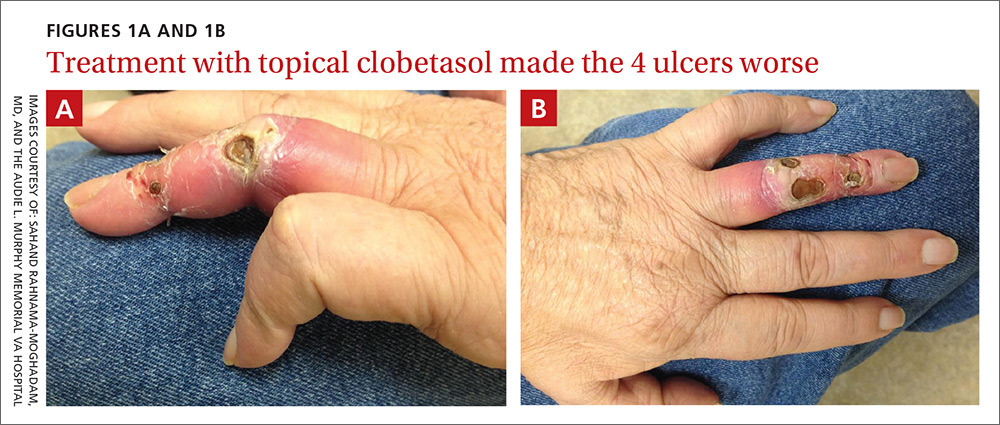
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.
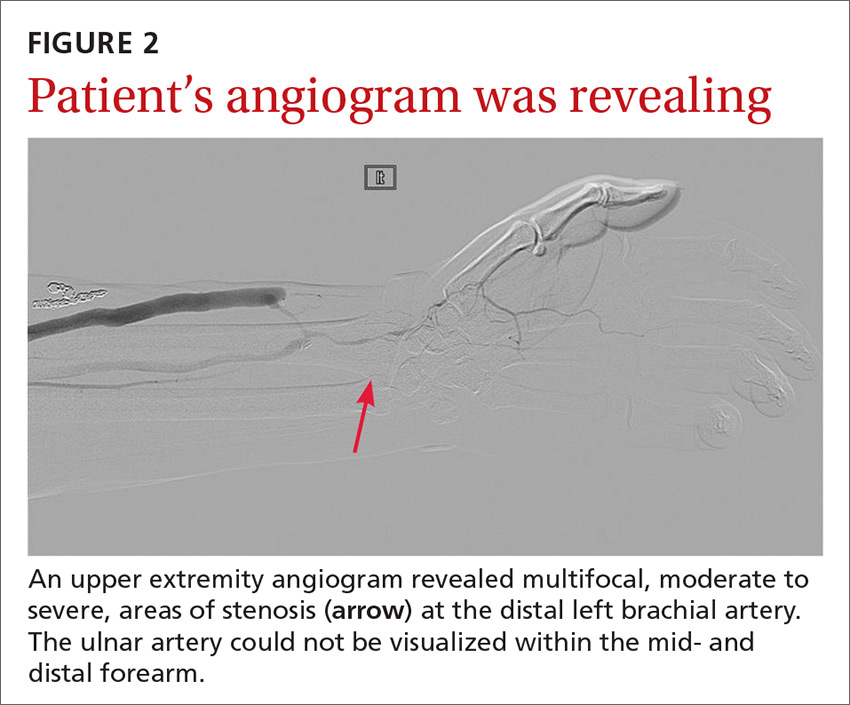
Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.
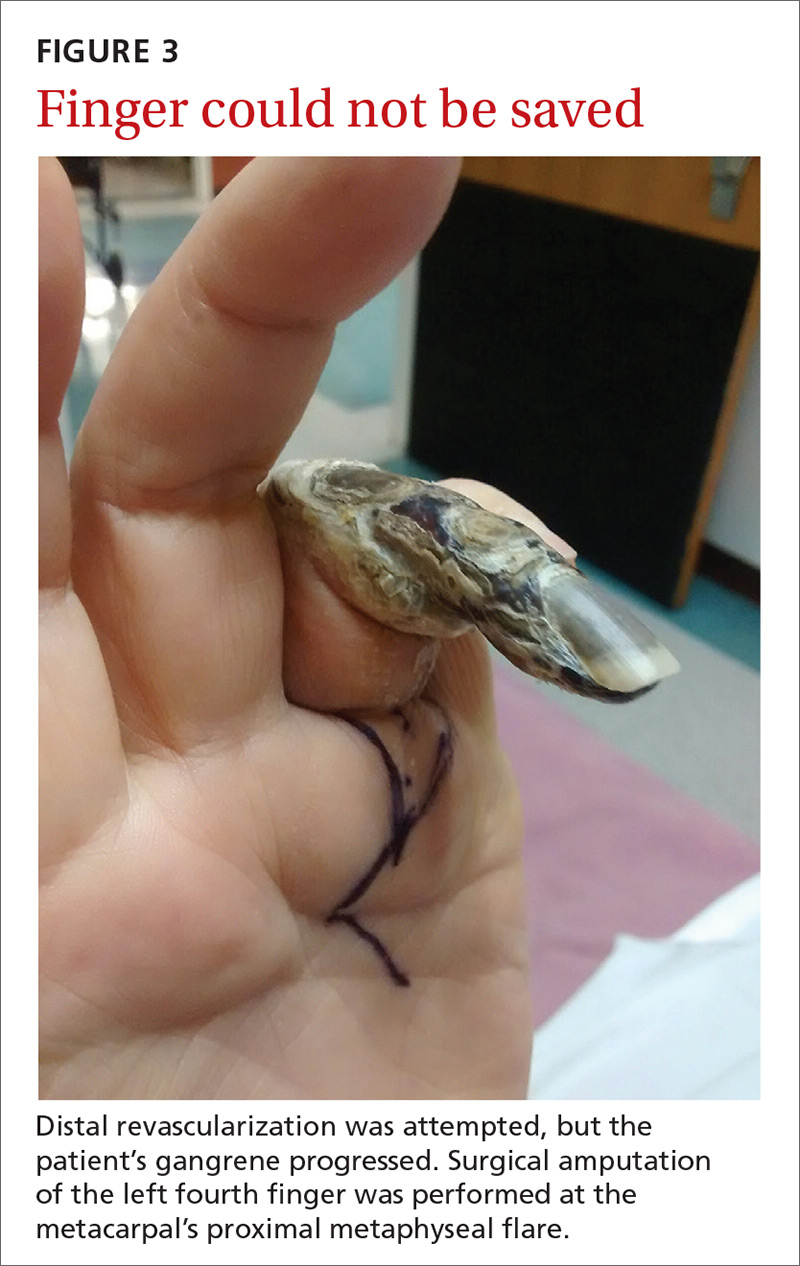
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; srahnama@iupui.edu.
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.

Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.

CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; srahnama@iupui.edu.
A 62-year-old man with end-stage renal disease presented to our dermatology clinic with 2-month-old ulcerations on his distal left ring finger. He was on hemodialysis and had a radiocephalic arteriovenous fistula (AVF) on his left arm. He had been empirically treated elsewhere with oral trimethoprim-sulfamethoxazole for a presumed bacterial infection, without improvement. He was then treated for contact dermatitis with topical clobetasol, which led to ulcer expansion and worsening pain.
At our clinic, the patient reported intermittent pain in his finger and paresthesias during activity and dialysis, but no tenderness of the ulcers. He had atrophy of the intrinsic left hand muscles (his non-dominant hand) with associated weakness. Three weeks earlier, he’d received a blood transfusion for anemia. Afterward, the pain in his hand improved and the ulcers decreased in size.
On exam, the AVF had a palpable thrill over the left forearm. The radial pulses were palpable bilaterally (2+) and the left ulnar artery was palpable, but diminished (1+). The patient’s left hand was cooler than the right (with a slight cyanotic hue and visible intrinsic muscle atrophy) and had decreased sensation to pain and temperature. Four ulcers with dry yellow eschar were located over the dorsal interphalangeal joints (FIGURES 1A AND 1B). They were essentially non-tender, but there was tenderness in the adjacent intact skin. There was violaceous blue edematous congestion noted on the fourth finger, and the distal phalange was constricted, giving it a “pseudoainhum” appearance.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Dialysis access steal syndrome
We suspected dialysis access steal syndrome (also known as AVF steal syndrome), so a duplex ultrasound was performed. The ultrasound was inconclusive. (We couldn’t confirm a limitation in blood flow, nor delineate anatomy.) So, we referred the patient for a thoracic and upper extremity angiogram.
The angiogram demonstrated multifocal, moderate to severe, areas of stenosis at the distal left brachial artery. The radial artery was patent at the level of the wrist, but showed diffuse narrowing beyond the level of the arteriovenous (AV) anastomosis. There was only a faint palmar arch identified on the radial aspect of the hand with digital branches feeding the radial portion of the hand. In contrast, the ulnar artery was not seen within the mid- and distal forearm (FIGURE 2). Palmar branches to the ulnar half of the hand were not identified. The fistula itself didn’t show any stenosis.

Based on these findings, our suspicions of dialysis access steal syndrome were confirmed.
Dialysis access steal syndrome is caused by a significant decrease or reversal of blood flow in the arterial segment distal to the AVF or graft, which is induced by the low resistance of the fistula outflow. Patients with adequate collateral vasculature are able to compensate for the steal effect; however, patients with end-stage renal disease typically have preexisting vascular disease that increases the risk for vascular steal and, ultimately, demand ischemia after placement of an AVF.1 Interestingly, a steal effect occurs in 73% of patients after AVF construction, yet it is estimated that only 10% of patients demonstrating a steal phenomenon become symptomatic.2
In our patient’s case, the vaso-occlusive properties of topical steroids explain why the superpotent steroid (clobetasol) he was prescribed increased his pain and worsened the underlying problem.
A broad differential; a useful exam maneuver
The differential diagnosis of ulcers includes infection (mainly from bacterial or mycobacterial sources), trauma facilitated by neuropathy (neuropathic ulceration), vasculitis, and ischemia. When the history and physical exam suggest ischemic ulceration, then thromboembolism, thoracic outlet syndrome, vasculitis, atherosclerosis, and steal syndrome become more likely causes.
Signs and symptoms of ischemic steal syndrome are initially subtle and include extremity coolness, neurosensory changes, intrinsic muscle weakness, ulceration, and ultimately, gangrene of the affected extremity.3,4 A cold, numb, and/or painful hand during dialysis is another clue.3 Factors that increase the likelihood of the syndrome include age >60 years, female sex, and the presence of diabetes or peripheral artery disease.2,3,5,6
One physical exam maneuver that can help make the diagnosis of steal syndrome is manual occlusion of the AVF. If palpable distal pulses disappear when the AVF is patent and reappear when the fistula is occluded with downward pressure, then AVF steal syndrome is likely.4 Pain at rest, sensory loss, loss of pulse, and digital gangrene are emergency symptoms that warrant immediate surgical evaluation.3
Tests will confirm suspicions. Doppler ultrasound can be used to assess changes in the blood flow rate of the affected vessels when the AVF is patent vs when it is occluded. Similarly, pulse oximetry can be used with and without AVF occlusion to compare changes in oxygen saturation. The confirmatory diagnosis, however, is made via a fistulogram (angiography) with and without manual compression.6 Images taken after dye injection into the AVF show dramatic improvement of distal blood flow with AVF compression.
Treatment requires surgery
Severe steal-related ischemic manifestations that threaten the function and viability of digits require surgical treatment that is primarily directed toward improving distal blood flow and secondarily toward preserving hemodialysis access. Several surgical treatments are commonly used, including access ligation, banding, elongation, distal arterial ligation, and distal revascularization and interval ligation.2-5,7
Our patient. Distal revascularization was attempted, but unfortunately, the patient’s gangrene was progressive (FIGURE 3) and surgical amputation of the left fourth finger was performed at the metacarpal’s proximal metaphyseal flare. The patient was transitioned to peritoneal dialysis to avoid further ischemia.

CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, Department of Dermatology, Indiana University, 545 Barnhill Drive, Indianapolis, IN 46202; srahnama@iupui.edu.
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.
1. Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
2. Puryear A, Villarreal S, Wells MJ, et al. JAAD grand rounds quiz. Hand ischemia in a hemodialysis patient. J Am Acad Dermatol. 2014;70:393-395.
3. Pelle MT, Miller OF 3rd. Dermatologic manifestations and management of vascular steal syndrome in hemodialysis patients with arteriovenous fistulas. Arch Dermatol. 2002;138:1296-1298.
4. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg. 2000;191:301-310.
5. Gupta N, Yuo TH, Konig G 4th, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162-167.
6. Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14:371-376.
7. Leake AE, Winger DG, Leers SA, et al. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61:754-760.