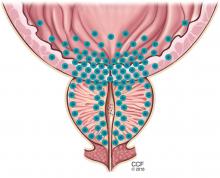
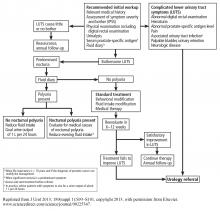
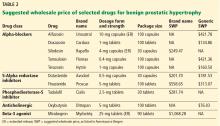
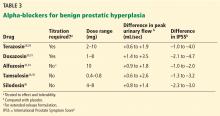
User login
Finding the Sweet Spot: The Diabetic Kidney
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Serum magnesium level reflects risk of death, irrespective of CKD
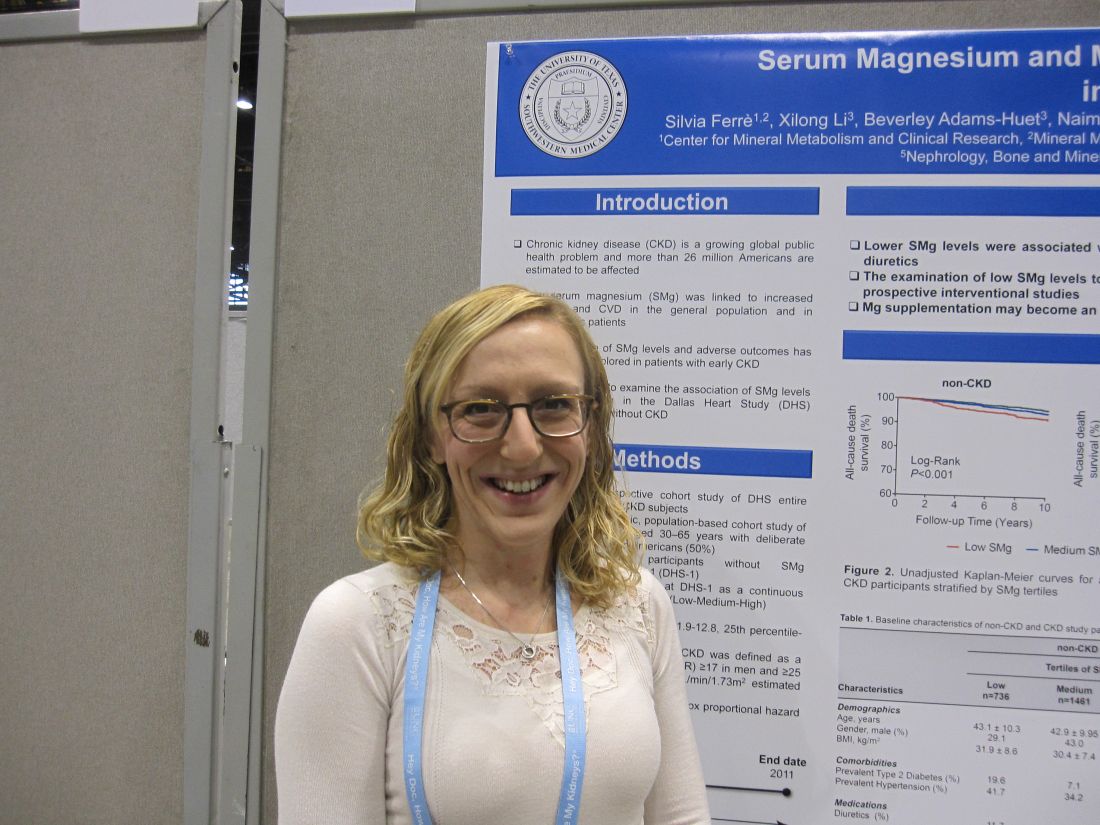
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
CHICAGO – Low levels of serum magnesium were associated with increased all-cause mortality, whether or not patients had chronic kidney disease, in a single center, retrospective study of 3,551 people.
The association was independent of sociodemographic factors, comorbidities, and use of diuretics.
If causality is shown, “magnesium supplementation could be a simple therapy to lessen the chance of death in CKD patients,” study investigator Silvia Ferrè, PhD, University of Texas Southwestern, Dallas, said in an interview regarding the results of the Dallas Heart Study, which was presented at the annual meeting of the American Society of Nephrology.
In both groups, the subjects with low serum magnesium were younger, more likely to be female, had a higher body mass index, and were more burdened by comorbidities including type 2 diabetes mellitus and hypertension. Subjects without CKD and low serum magnesium were significantly more likely to use diuretics. Diuretic use was comparable in subjects with CKD regardless of serum magnesium level.
Irrespective of CKD status, survival was significantly lower in subjects with low serum magnesium in the median 12.3-year follow-up compared to the other two serum magnesium tertiles (P less than 0.001 and P equal to 0.03, respectively). Following adjustment for age, gender, race/ethnicity, body mass index, phosphorus, calcium, bicarbonate, albumin, intact parathyroid hormone, total cholesterol, high-density lipoprotein, and use of diuretics and supplements, low serum magnesium was independently associated with all-cause death in subjects with CKD (Hazard Ratio, 1.92; 9%% Confidence Interval, 1.03 to 3.59; P equal to 0.04) and those without CKD (HR, 1.43; 1.43; 95% CI, 0.95 to 2.15; P equal to 0.09), when compared to high serum magnesium as the referent.
Dr. Ferrè said that screening for serum magnesium and supplementation with magnesium as part of routine blood testing might improve survival. Low magnesium level alsonhas been linked with osteoporosis, diabetes, and cardiovascular disease.
The Dallas Heart Study was a multiethnic, population-based study involving 6,101 adults residing in Dallas County. The study, which ran from 2000 to the end of 2011, was designed to explore the early detection of cardiovascular disease and the social, behavioral, and environmental factors associated with risk, with the goal of interventions that can be provided at the community level.
The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: If causality is shown, magnesium supplementation could be a simple therapy to reduce mortality in patients with chronic kidney disease.
Major finding: Low serum magnesium was significantly associated with risk of death in patients without CKD (P less than 0.01) and patients with CKD (P equal to 0.03).
Data source: A single-center, retrospective cohort of 3,551 patients in the Dallas Heart Study.
Disclosures: The study sponsor was University of Texas Southwestern Medical Center. The study was funded by the National Institutes of Health and the Donald W. Reynolds Foundation. Dr. Ferrè reported having no financial disclosures.
Constipation severity linked with chronic kidney disease and decline in kidney function
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
CHICAGO – Constipation was associated with poor kidney health in a large nationwide cohort of 3.5 million United States veterans, and researchers are considering whether effectively treating constipation could help prevent or treat kidney disease.
“In this large nationwide cohort ... patients with constipation had higher risks of developing chronic kidney disease and end-stage renal disease, and were more likely to experience rapid decline in kidney function, even after adjusting for various known risk factors. We also found that more severe constipation was associated with an incrementally higher risk for both incident CKD (chronic kidney disease) and ESRD (end-stage renal disease),” said Keiichi Sumida, MD, a visiting scholar at the University of Tennessee Health Science Center in Memphis.
In a multivariable analysis, those with constipation had a 13% higher likelihood of developing CKD (Hazard Ratio, 1.13; 95% Confidence Interval, 1.11 to 1.14) and a 9% higher likelihood of developing ESRD (HR, 1.09; 95% CI, 1.01 to 1.18) compared to those without constipation. As well, those with constipation experienced a faster decline in estimated glomerular filtration ratio (eGFR).
Scrutiny of US Veterans Administration databases identified nearly 4.5 million patients with serum creatinine measurements obtained between October 2004 and September 2006. Of these, 3,504,732 patients had an eGFR greater than or equal to 60 ml/min/1.73 m2 but no other symptoms of CKD. All were followed through 2013.
Constipation was defined as at least two ICD-9-CM diagnoses for constipation made at least 60 days apart or two or more prescriptions for laxatives separated by 60 days for up to a year. The severity of constipation was based on the number of different type of laxatives prescribed, with no laxative use being considered as absence of constipation, one laxative type being indicative of mild constipation, and two or more types of laxatives being indicative of severe constipation.
Co-primary outcomes were incident CKD, incident ESRD, and change in eGFR from baseline. As expected in the propensity-matched cohort, baseline demographic and clinical characteristics were comparable for the 3,251,291 individuals who experienced constipation and the 253,441 individuals who did not.
“Our findings highlight the plausible link between the gut and the kidneys, and provide additional insights into the pathogenesis of kidney disease progression. Our results suggest the need for careful observation of kidney function in patients with constipation, particularly among those with more severe constipation,” Dr. Sumida concluded.
Dr. Sumida hypothesized that altered gut microflora in constipation may result in inflammation, changes in metabolites, or accumulation of toxins. Alternative explanations increased serotonin related to laxative use, nephrotoxicity, dehydration, or electrolyte imbalance.
These possibilities need to be examined, as does the idea that relieving constipation could prevent renal decline. “Given the high prevalence of constipation in the general population and the simplicity of its assessment in primary care settings, the management of constipation through lifestyle modifications and/or use of probiotics rather than laxatives could become a useful tool in preventing the development of CKD, or in retarding the progression of existing CKD,” Dr. Sumida said.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: Presence and severity of constipation increases the risks of developing chronic kidney disease and end stage renal disease, and accelerates the decline in kidney function.
Major finding: Individuals with constipation were 13% more likely to develop chronic kidney disease and 9% more likely to develop end stage renal disease compared to those without constipation.
Data source: Retrospective analysis of Veteran’s Administration databases. The study included 3,504,732 subjects.
Disclosures: The study sponsor was the University of Tennessee Health Science Center. Funding was provided by the United States Department of Veterans Affairs. Dr. Sumida reported having no financial disclosures.
Cell ratios predict short-term possibility of death in patients beginning hemodialysis
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
CHICAGO – Two simple-to-calculate ratios – neutrophil lymphocyte ratio and platelet lymphocyte ratio – may be able to predict impending death in patients who have recently begun hemodialysis, based on data from 108,548 incident hemodialysis patients in the database of DaVita HealthCare Partners from 2007 to 2011.
“Neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR), and inflammatory and nutritional indices, which are calculated from complete blood count, were identified as strong predictors of impending death ... and thus are inexpensive and immediately available markers for predicting short-term mortality,” said Yoshitsugu Obi, MD, PhD, a visiting scholar at the Harold Simmons Center for Kidney Disease Research & Epidemiology, University of California Irvine School of Medicine, Irvine, California.
The data were obtained from the database of a large dialysis organization; 108,548 patients who began hemodialysis from 2007 to 2011 were included. The range of NLR values were divided into 12 categories with ratios of less than 1.5 and greater than or equal to 6.5 as the bracketing ratios. The 10 other intervening ratios differed incrementally by 0.5. Eight SLR categories were created with the bracketing ratios being less than 5 and greater than or equal to 35. The intervening six ratios differed incrementally by 5.
The mean age of the cohort was 63 ± 15 years. Males predominated (56%), 59% of the subjects were diabetic, and 31% were African American. At baseline the median NLR and PLR were 3.64 and 13.12, respectively.
In an unadjusted regression analysis, the categories of NLR and PLR had a strong and linear relationship with all-cause mortality. In an analysis that adjusted for covariates, including demographics and comorbidities, as well as markers of malnutrition and inflammation, the association of the two ratios with all-cause mortality persisted.
Unlike previous small and inconclusive studies, the size of the present study makes robust the connection between these cell ratios and death in dialysis patients, he said. The plan now is to compare the mortality predictability of NLR and PLR with other established risk factors including albumin, phosphorus, and alkaline phosphatase.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR NEPHROLOGY
Key clinical point: The neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio (PLR) are strongly associated with imminent death in patients who have recently started hemodialysis.
Major finding: Increasing NLR and PLR were linearly associated with death in 108,548 hemodialysis patients.
Data source: Database of DaVita HealthCare Partners from 2007 to 2011.
Disclosures: The study was sponsored by University of Irvine School of Medicine. The study was funded by the National Institutes of Health. Dr. Obi had no disclosures.
CKD death rate highest in Mexico
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.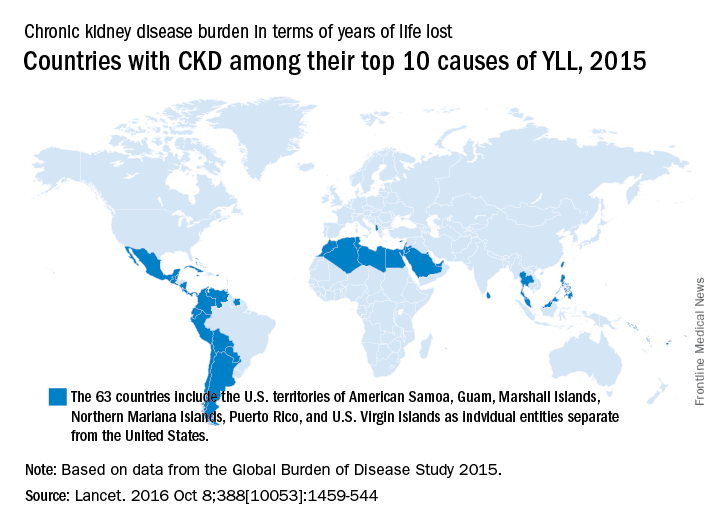
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
FROM THE LANCET
Kidney Disease Progression: How to Attenuate Risk
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
Q)I overheard a conversation at the hospital in which one of the nephrologists told an internist that allopurinol is better than other medications for treating gout because it slows the progression of chronic kidney disease (CKD). What does the data say?
CKD is a growing problem in America; the number of adults with CKD doubled from 2000 to 2008.1 Gout is considered an independent risk factor for CKD progression.2 Some randomized contr
A recent large retrospective review of Medicare charts assessed the correlation between use and dose of allopurinol and incidence of renal failure in patients older than 65.1 The researchers found that, compared with lower doses, allopurinol doses of 200 to 299 mg/d and > 300 mg/d were associated with a significantly lower hazard ratio for kidney failure, in a multivariate-adjusted model. The findings therefore suggest that doses > 199 mg may slow progression to kidney failure in the elderly.
Despite the strengths of this study, it is worth noting that it did not consider stage of kidney disease, nor did it distinguish comorbidities of the patients. The retrospective chart review format did not allow for identification of concurrent medication use (including OTC and herbal products).
The National Institute of Diabetes and Digestive and Kidney Diseases is currently conducting an RCT to investigate the renoprotective effects of allopurinol versus placebo in diabetic patients. (Clinical Trials.gov identifier: NCT02017171). Enrollment was completed in 2014, and results are expected in June 2019.
One important proviso about allopurinol: While it is inexpensive and generally well tolerated, prescribers should be aware of rare sensitivity reactions, particularly Stevens-Johnson syndrome. —MRS
Mary Rogers Sorey, MSN
Division of Nephrology and Hypertension at Vanderbilt University Medical Center, Nashville
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
1. Singh JA, Yu S. Are allopurinol dose and duration of use nephroprotective in the elderly? A Medicare claims study of allopurinol use and incident renal failure. Ann Rheum Dis. 2016 Jun 13. [Epub ahead of print]
2. Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
3. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887-1894.
Kidney Disease Progression: How to Calculate Risk
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
Q)When I diagnose patients with minor kidney disease, they often ask if they will require dialysis. I know it is unlikely, but I wish I could give them a better answer. Can you help me?
The diagnosis of chronic kidney disease (CKD) is understandably concerning for many patients. Being able to estimate CKD progression helps patients gain a better understanding of their condition while allowing clinicians to develop more personalized care plans. Tangri and colleagues developed a model that can be used to predict risk for kidney failure requiring dialysis or transplantation in patients with stage III to V CKD. This model has been validated in multiple diverse populations in North America and worldwide.1
The Kidney Failure Risk Equation (found at www.kidneyfailurerisk.com) uses four variables—age, gender, glomerular filtration rate (GFR), and urine albumin-to-creatinine ratio (ACR)—to assess two- and five-year risk for kidney failure.1,2 For example
- A 63-year-old woman with a GFR of 45 mL/min and an ACR of 30 mg/g has a 0.4% two-year risk and a 1.3% five-year risk for progression to kidney failure requiring dialysis or transplant.1
- Alternatively, a 55-year-old man with a GFR of 38 mL/min and an ACR of 150 mg/g has a 2.9% two-year risk and a 9% five-year risk for progression to end-stage renal disease (ESRD).1
Per proposed thresholds, patients with a score < 5% would be deemed “low risk”; with scores of 5% to 15%, “intermediate risk”; and with scores > 15%, “high risk.”1,2
The Kidney Failure Risk Equation can be incorporated into clinic visits to provide context for lab results. For patients with low risk for progression, optimal care and lifestyle measures can be reinforced. For those with intermediate or high risk, more intensive treatments and appropriate referrals can be initiated. (The National Kidney Foundation advises referral when a patient’s estimated GFR is 20 mL/min or the urine ACR is ≥ 300 mg/g.3) Providing a numeric risk for progression can help alleviate the patient’s uncertainty surrounding the diagnosis of CKD. —NDM
Nicole D. McCormick, MS, MBA, NP-C, CCTC
University of Colorado Renal Transplant Clinic, Aurora, Colorado
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
1. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164-174.
2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559.
3. National Kidney Foundation. Renal Replacement Therapy: What the PCP Needs to Know. www.kidney.org/sites/default/files/PCP%20in%20a%20Box%20-%20Module%203.pptx. Accessed December 5, 2016.
A patient with altered mental status and an acid-base disturbance
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
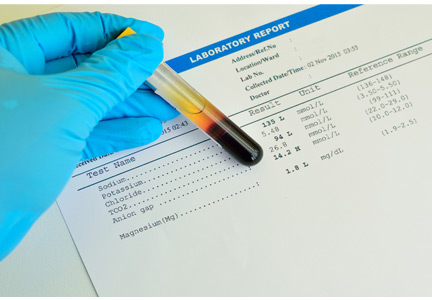
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
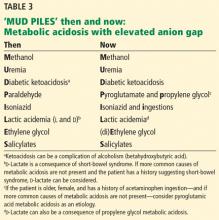
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
Benign prostatic hyperplasia: Evaluation and medical management in primary care
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
KEY POINTS
- Watchful waiting is appropriate for patients with mild to moderate symptoms that cause minimal bother.
- Patients with severe or bothersome symptoms should be offered pharmacotherapy, not only to improve symptoms but also to reduce the risk of disease progression.
- Several effective, minimally invasive surgical options are available for patients whose symptoms do not respond to medical therapy. These patients and those with abnormal findings on diagnostic evaluation warrant referral to a urologist for further evaluation.
Ferric citrate effective for anemia in non–dialysis-dependent CKD
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
CHICAGO – Ferric citrate was safe and effective for treatment of iron-deficiency anemia in patients who had non–dialysis-dependent chronic kidney disease (NDD-CKD), based on data from a phase III, randomized, double-blind study.
The responses were durable, and none of the patients received erythropoiesis-stimulating agents (ESAs), presenter Pablo Pergola, MD, PhD, of Renal Associates, San Antonio, said in an interview at a meeting sponsored by the American Society of Nephrology.
The trial involved 234 anemic adults who had NDD-CKD and had not responded to oral iron supplements. The subjects were randomized to receive oral ferric citrate (n = 117) or placebo (n = 115) with meals (one patient did not receive placebo and laboratory data were lacking for one patient). The mean dose in the treatment arm was 5 pills per day.
The primary endpoint was the proportion of patients with hemoglobin (Hgb) greater than or equal to 1.0 g/dL anytime from baseline through week 16. Secondary endpoints included mean changes from baseline in Hgb, transferrin saturation, ferritin, and serum phosphate and evidence of sustained treatment effect based on target changes in Hgb with time.
Both arms were comparable at baseline for demographic and clinical characteristics, including phosphorus and hemoglobin levels and estimated glomerular filtration rate.
The primary endpoint was met by 51.2% of patients receiving ferric citrate and 19.1% of patients receiving placebo (P less than .001). All secondary efficacy endpoints were met, with statistically significant differences between the treatment and placebo arms, Dr. Pergola reported.
Serum phosphate level was significantly reduced from baseline at week 16 (–0.21 mg/dL; 95% confidence interval, –0.39 to –0.03 mg/dL; P equal to .02) in the active treatment group, and the levels remained in the normal range, he said.
During the 16-week treatment period and subsequent 8-week, open-label safety extension period, ferric citrate was well tolerated. Treatment-emergent adverse events (AEs), most commonly diarrhea, occurred in 93 (79.5%) and 75 (64.7%) patients in the treatment and placebo arms, respectively. Serious AEs developed in 14 (12.0%) and 13 (11.2%) of patients in the same respective order. Two deaths occurred, both in the treatment group. The deaths and serious AEs were not considered drug related.
Ferric citrate binds with dietary phosphate in the gastrointestinal tract. The resulting ferric phosphate is insoluble and is excreted. The remaining unbound ferric citrate increases serum iron parameters, including ferritin and transferrin saturation.
The findings potentially extend the therapeutic reach of the drug beyond its Food and Drug Administration–approved use for control of phosphorus levels in CKD patients on dialysis, Dr. Pergola said. The trial data will be used to seek approval for the oral iron medication as a treatment for iron-deficiency anemia in adults with NDD-CKD.
The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.
AT KIDNEY WEEK 2016
Key clinical point: Ferric citrate appears to be safe and effective for treating anemia in non–dialysis-dependent CKD patients.
Major finding: Prevalence of increased hemoglobin was 52.1% in patients receiving the active drug and 19.1% in those given placebo.
Data source: Randomized, double-blind, placebo-controlled, phase III trial with 234 patients.
Disclosures: The study was sponsored by Keryx Biopharmaceuticals. Dr. Pergola is supported by honoraria and lecture fees from Akebia Therapeutics, Keryx, Relypsa, Vifor/Fresenius Pharma, and ZS Pharma.