User login
For interstitial cystitis, restrictive diet pays off
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Reproductive planning for women after solid-organ transplant
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
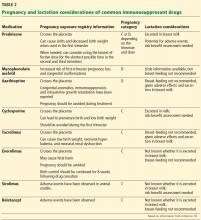
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
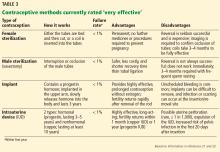
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
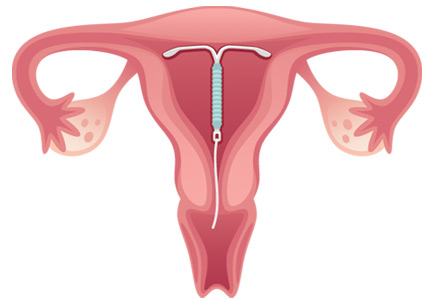
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
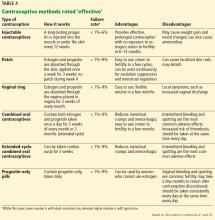
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
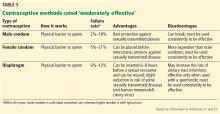
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.
Metastatic pulmonary calcification and end-stage renal disease
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
Renal denervation: What happened, and why?
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
KEY POINTS
- Renal denervation consists of passing a catheter into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, it should lower blood pressure and be an attractive option for treating resistant hypertension.
- SYMPLICITY HTN-3 was a blinded trial in which patients with resistant hypertension were randomized to undergo real or sham renal denervation.
- At 6 months, office systolic blood pressure had failed to fall more in the renal denervation group than in the sham denervation group by a margin of at least 5 mm Hg, the primary efficacy end point of the trial.
- Methodologic and technical shortcomings may explain the negative results of the SYMPLICITY HTN-3 trial, but most device manufacturers have put the brakes on future research into this novel therapy.
- Today, renal denervation is not available in the United States but is available for routine care in Europe and Australia.
Renal denervation: Are we on the right path?
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
FDA approves Vabomere for complicated UTI in adults
The Food and Drug Administration has approved Vabomere (meropenem and vaborbactam) for adults with complicated urinary tract infection (cUTI), including pyelonephritis caused by susceptible Enterobacteriaceae, the agency has announced.
The approval was based on results of the TANGO 1 trial, a phase 3, multicenter, double-blind study of 545 adult patients with cUTI. Overall, 98.4% of patients treated with Vabomere saw improvement in symptoms and negative urine culture tests by the end of intravenous treatment, compared with 94.3% of those treated with piperacillin/tazobactam (95% confidence interval, 0.3%-8.8%). Improvement continued in about 77% of patients treated with Vabomere who had resolved symptoms 7 days after completing treatment, compared with about 73% of those who were treated with piperacillin/tazobactam, the FDA said Aug. 29 in a press release.
Headache, infusion site reactions, and diarrhea were common adverse effects of Vabomere. The drug also has been associated with allergic reactions and seizures, so it should not be administered to patients with a history of anaphylaxis.
“Vabomere represents a significant new advancement in addressing [Klebsiella pneumoniae carbapenemase]-producing Enterobacteriaceae, for which there are currently limited treatment options,” Clive A. Meanwell, MD, PhD, chief executive officer of The Medicines Company, said in the statement.
Rempex Pharmaceuticals, a Medicines Company unit, received the approval. The Medicines Company said the drug is expected to be available before the end of the year.
The Food and Drug Administration has approved Vabomere (meropenem and vaborbactam) for adults with complicated urinary tract infection (cUTI), including pyelonephritis caused by susceptible Enterobacteriaceae, the agency has announced.
The approval was based on results of the TANGO 1 trial, a phase 3, multicenter, double-blind study of 545 adult patients with cUTI. Overall, 98.4% of patients treated with Vabomere saw improvement in symptoms and negative urine culture tests by the end of intravenous treatment, compared with 94.3% of those treated with piperacillin/tazobactam (95% confidence interval, 0.3%-8.8%). Improvement continued in about 77% of patients treated with Vabomere who had resolved symptoms 7 days after completing treatment, compared with about 73% of those who were treated with piperacillin/tazobactam, the FDA said Aug. 29 in a press release.
Headache, infusion site reactions, and diarrhea were common adverse effects of Vabomere. The drug also has been associated with allergic reactions and seizures, so it should not be administered to patients with a history of anaphylaxis.
“Vabomere represents a significant new advancement in addressing [Klebsiella pneumoniae carbapenemase]-producing Enterobacteriaceae, for which there are currently limited treatment options,” Clive A. Meanwell, MD, PhD, chief executive officer of The Medicines Company, said in the statement.
Rempex Pharmaceuticals, a Medicines Company unit, received the approval. The Medicines Company said the drug is expected to be available before the end of the year.
The Food and Drug Administration has approved Vabomere (meropenem and vaborbactam) for adults with complicated urinary tract infection (cUTI), including pyelonephritis caused by susceptible Enterobacteriaceae, the agency has announced.
The approval was based on results of the TANGO 1 trial, a phase 3, multicenter, double-blind study of 545 adult patients with cUTI. Overall, 98.4% of patients treated with Vabomere saw improvement in symptoms and negative urine culture tests by the end of intravenous treatment, compared with 94.3% of those treated with piperacillin/tazobactam (95% confidence interval, 0.3%-8.8%). Improvement continued in about 77% of patients treated with Vabomere who had resolved symptoms 7 days after completing treatment, compared with about 73% of those who were treated with piperacillin/tazobactam, the FDA said Aug. 29 in a press release.
Headache, infusion site reactions, and diarrhea were common adverse effects of Vabomere. The drug also has been associated with allergic reactions and seizures, so it should not be administered to patients with a history of anaphylaxis.
“Vabomere represents a significant new advancement in addressing [Klebsiella pneumoniae carbapenemase]-producing Enterobacteriaceae, for which there are currently limited treatment options,” Clive A. Meanwell, MD, PhD, chief executive officer of The Medicines Company, said in the statement.
Rempex Pharmaceuticals, a Medicines Company unit, received the approval. The Medicines Company said the drug is expected to be available before the end of the year.
Acute lobar nephronia often has misleading presentation
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
AT ESPID 2017
Key clinical point:
Major finding: Urine dipstick results were negative in 5 instances, and ultrasound was negative in 16 cases.
Data source: This was a single-center, retrospective, descriptive study of 18 episodes of acute lobar nephronia in 16 children.
Disclosures: Dr. Sanchez-Marcos reported having no financial conflicts of interest.
Award for best hospital goes to … the Mayo Clinic
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
For the second consecutive year, the Mayo Clinic was named the top hospital in the country by U.S. News & World Report.
Also for the second consecutive year, the Cleveland Clinic is ranked second, while Johns Hopkins Hospital in Baltimore and Massachusetts General Hospital in Boston finished third and fourth – switching their places from last year’s ranking – and UCSF Medical Center in San Francisco is fifth after ranking seventh last year, according to the 2017-2018 Best Hospitals ranking.
The Mayo Clinic is nationally ranked in 15 of the 16 specialties included in the overall process, which started with 4,658 community inpatient hospitals and finished with 152 ranking nationally in at least one specialty and 20 earning Honor Roll status with high rankings in multiple specialties. The specialties used in the ranking process include 12 that are data driven – cancer; cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; orthopedics; pulmonology; and urology – and four rated by reputation only – ophthalmology; psychiatry; rehabilitation; and rheumatology.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News. The launch of this year’s edition of Best Hospitals is sponsored by Fidelity Investments.
Weight loss, fatigue, and renal failure
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
Transplant safety has improved for patients with diabetes
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS