User login
IV fluid and narcotics for renal colic
A 40-year-old man presents with severe right flank pain for 1 hour. He has had this in the past when he passed a kidney stone. Urinalysis shows greater than 100 red blood cells per high power field (HPF). CT shows a 6-mm stone in the left ureter.
What do you recommend for therapy?
A. IV ketorolac and IV fluids.
B. IV morphine and IV fluids.
C. IV morphine.
D. IV ketorolac.
This is a common scenario, especially in emergency department settings and acute care clinics. Patients arrive in severe pain because of renal colic from kidney stones. Standard teaching that I received many years ago was that this patient should receive IV fluid to “help float the stone out” and narcotic pain medications to treat the severe pain the patient was in.
Is there good evidence that this is the best therapy?
There are scant data on the practice of IV fluid for treatment of renal stone passage. W. Patrick Springhart, MD, and his colleagues studied 43 patients who presented to the ED for treatment of renal colic.1 All patients had CT evaluation for stones and received intravenous analgesia. Twenty patients were randomized to receive 2 L of normal saline over 2 hours, and 23 patients received minimal IV saline (20 mL/hour). There were no differences between the two groups in pain scores, narcotic requirements, or stone passage rates.
In an older study, Tom-Harald Edna, PhD, and colleagues studied 60 patients with ureteral colic, randomizing them to receive either no fluid or 3 L of IV fluid over 6 hours.2 There was no significant difference in pain between treatment groups.
A Cochrane analysis in 2012 concluded that there was no reliable evidence to support the use of high-volume fluid therapy in the treatment of acute ureteral colic.3
Standard treatment of pain for renal colic has been to use narcotics. In a randomized, double-blind trial comparing ketorolac and meperidine, William Cordell, MD, and his colleagues found that pain relief was superior in ketorolac-treated patients. Seventy-five percent of ketorolac patients had a 50% reduction in pain scores versus only 23% of the patients who received meperidine (P less than .001).4
Anna Holdgate and Tamara Pollock reviewed 20 studies that evaluated NSAIDs and narcotics for acute renal colic. They concluded that patients treated with NSAIDs had greater pain relief with less vomiting than did patients treated with narcotics.5
In the past decade, tamsulosin has been frequently used in patients with renal stones to possibly help with pain and promote more rapid stone passage. A recent randomized, controlled trial with 512 patients, authored by Andrew Meltzer, MD, and his colleagues, showed no improvement in stone passage rate in patients taking tamsulosin, compared with the rate seen with placebo.6
Previously published meta-analyses of multiple studies have shown a benefit to the use of alpha-blockers. Thijs Campschroer and colleagues included 67 studies that altogether included 10,509 participants.7 They found that the use of alpha-blockers led to possibly shorter stone expulsion times (3.4 days), less NSAID use, and fewer hospitalizations, with the evidence graded as low to moderate quality. Stone size seems to matter because use of alpha-blockers does not seem to make a difference for stones larger than 5 mm.
I think IV ketorolac would be the best of the options presented here for this patient. If a patient can safely take NSAIDs, those are probably the best option. There does not appear to be any reason to bolus hydrate patients with acute renal colic.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. J Endourol. 2006 Oct;20(10):713-6.
2. Scand J Urol Nephrol. 1983;17(2):175-8.
3. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD004926.
4. Ann Emerg Med. 1996 Aug;28(2):151-8.
5. BMJ. 2004 Jun 12;328(7453):1401.
6. JAMA Intern Med. 2018 Aug 1;178(8):1051-7.
7. Cochrane Database Syst Rev. 2018 Apr 5;4:CD008509.
A 40-year-old man presents with severe right flank pain for 1 hour. He has had this in the past when he passed a kidney stone. Urinalysis shows greater than 100 red blood cells per high power field (HPF). CT shows a 6-mm stone in the left ureter.
What do you recommend for therapy?
A. IV ketorolac and IV fluids.
B. IV morphine and IV fluids.
C. IV morphine.
D. IV ketorolac.
This is a common scenario, especially in emergency department settings and acute care clinics. Patients arrive in severe pain because of renal colic from kidney stones. Standard teaching that I received many years ago was that this patient should receive IV fluid to “help float the stone out” and narcotic pain medications to treat the severe pain the patient was in.
Is there good evidence that this is the best therapy?
There are scant data on the practice of IV fluid for treatment of renal stone passage. W. Patrick Springhart, MD, and his colleagues studied 43 patients who presented to the ED for treatment of renal colic.1 All patients had CT evaluation for stones and received intravenous analgesia. Twenty patients were randomized to receive 2 L of normal saline over 2 hours, and 23 patients received minimal IV saline (20 mL/hour). There were no differences between the two groups in pain scores, narcotic requirements, or stone passage rates.
In an older study, Tom-Harald Edna, PhD, and colleagues studied 60 patients with ureteral colic, randomizing them to receive either no fluid or 3 L of IV fluid over 6 hours.2 There was no significant difference in pain between treatment groups.
A Cochrane analysis in 2012 concluded that there was no reliable evidence to support the use of high-volume fluid therapy in the treatment of acute ureteral colic.3
Standard treatment of pain for renal colic has been to use narcotics. In a randomized, double-blind trial comparing ketorolac and meperidine, William Cordell, MD, and his colleagues found that pain relief was superior in ketorolac-treated patients. Seventy-five percent of ketorolac patients had a 50% reduction in pain scores versus only 23% of the patients who received meperidine (P less than .001).4
Anna Holdgate and Tamara Pollock reviewed 20 studies that evaluated NSAIDs and narcotics for acute renal colic. They concluded that patients treated with NSAIDs had greater pain relief with less vomiting than did patients treated with narcotics.5
In the past decade, tamsulosin has been frequently used in patients with renal stones to possibly help with pain and promote more rapid stone passage. A recent randomized, controlled trial with 512 patients, authored by Andrew Meltzer, MD, and his colleagues, showed no improvement in stone passage rate in patients taking tamsulosin, compared with the rate seen with placebo.6
Previously published meta-analyses of multiple studies have shown a benefit to the use of alpha-blockers. Thijs Campschroer and colleagues included 67 studies that altogether included 10,509 participants.7 They found that the use of alpha-blockers led to possibly shorter stone expulsion times (3.4 days), less NSAID use, and fewer hospitalizations, with the evidence graded as low to moderate quality. Stone size seems to matter because use of alpha-blockers does not seem to make a difference for stones larger than 5 mm.
I think IV ketorolac would be the best of the options presented here for this patient. If a patient can safely take NSAIDs, those are probably the best option. There does not appear to be any reason to bolus hydrate patients with acute renal colic.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. J Endourol. 2006 Oct;20(10):713-6.
2. Scand J Urol Nephrol. 1983;17(2):175-8.
3. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD004926.
4. Ann Emerg Med. 1996 Aug;28(2):151-8.
5. BMJ. 2004 Jun 12;328(7453):1401.
6. JAMA Intern Med. 2018 Aug 1;178(8):1051-7.
7. Cochrane Database Syst Rev. 2018 Apr 5;4:CD008509.
A 40-year-old man presents with severe right flank pain for 1 hour. He has had this in the past when he passed a kidney stone. Urinalysis shows greater than 100 red blood cells per high power field (HPF). CT shows a 6-mm stone in the left ureter.
What do you recommend for therapy?
A. IV ketorolac and IV fluids.
B. IV morphine and IV fluids.
C. IV morphine.
D. IV ketorolac.
This is a common scenario, especially in emergency department settings and acute care clinics. Patients arrive in severe pain because of renal colic from kidney stones. Standard teaching that I received many years ago was that this patient should receive IV fluid to “help float the stone out” and narcotic pain medications to treat the severe pain the patient was in.
Is there good evidence that this is the best therapy?
There are scant data on the practice of IV fluid for treatment of renal stone passage. W. Patrick Springhart, MD, and his colleagues studied 43 patients who presented to the ED for treatment of renal colic.1 All patients had CT evaluation for stones and received intravenous analgesia. Twenty patients were randomized to receive 2 L of normal saline over 2 hours, and 23 patients received minimal IV saline (20 mL/hour). There were no differences between the two groups in pain scores, narcotic requirements, or stone passage rates.
In an older study, Tom-Harald Edna, PhD, and colleagues studied 60 patients with ureteral colic, randomizing them to receive either no fluid or 3 L of IV fluid over 6 hours.2 There was no significant difference in pain between treatment groups.
A Cochrane analysis in 2012 concluded that there was no reliable evidence to support the use of high-volume fluid therapy in the treatment of acute ureteral colic.3
Standard treatment of pain for renal colic has been to use narcotics. In a randomized, double-blind trial comparing ketorolac and meperidine, William Cordell, MD, and his colleagues found that pain relief was superior in ketorolac-treated patients. Seventy-five percent of ketorolac patients had a 50% reduction in pain scores versus only 23% of the patients who received meperidine (P less than .001).4
Anna Holdgate and Tamara Pollock reviewed 20 studies that evaluated NSAIDs and narcotics for acute renal colic. They concluded that patients treated with NSAIDs had greater pain relief with less vomiting than did patients treated with narcotics.5
In the past decade, tamsulosin has been frequently used in patients with renal stones to possibly help with pain and promote more rapid stone passage. A recent randomized, controlled trial with 512 patients, authored by Andrew Meltzer, MD, and his colleagues, showed no improvement in stone passage rate in patients taking tamsulosin, compared with the rate seen with placebo.6
Previously published meta-analyses of multiple studies have shown a benefit to the use of alpha-blockers. Thijs Campschroer and colleagues included 67 studies that altogether included 10,509 participants.7 They found that the use of alpha-blockers led to possibly shorter stone expulsion times (3.4 days), less NSAID use, and fewer hospitalizations, with the evidence graded as low to moderate quality. Stone size seems to matter because use of alpha-blockers does not seem to make a difference for stones larger than 5 mm.
I think IV ketorolac would be the best of the options presented here for this patient. If a patient can safely take NSAIDs, those are probably the best option. There does not appear to be any reason to bolus hydrate patients with acute renal colic.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. J Endourol. 2006 Oct;20(10):713-6.
2. Scand J Urol Nephrol. 1983;17(2):175-8.
3. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD004926.
4. Ann Emerg Med. 1996 Aug;28(2):151-8.
5. BMJ. 2004 Jun 12;328(7453):1401.
6. JAMA Intern Med. 2018 Aug 1;178(8):1051-7.
7. Cochrane Database Syst Rev. 2018 Apr 5;4:CD008509.
Short-term NSAIDs appear safe for high-risk patients
in a retrospective, observational study.
The findings of the study challenge the Choosing Wisely campaign of the American Society of Nephrology, which recommends against NSAIDs for high-risk patients, according to lead author Zachary Bouck, MPH, of the department of medicine at Sunnybrook Health Sciences Centre in Toronto, and his coauthors.
“While these recommendations offer basic analgesics and nonpharmacological treatments as preferable alternatives, it is both possible and disconcerting that some physicians might instead prescribe opioids, which typically pose elevated risk of adverse events and dependence vs. NSAIDs,” the investigators wrote. The report is in JAMA Internal Medicine.
They sought to estimate the frequency and characteristics of NSAID prescriptions while also looking for associations with acute renal and cardiovascular complications. The retrospective, observational study involved 814,049 adults with musculoskeletal disease and 7,365 primary care physicians in Ontario, Canada. All patients were aged 65 years and older, and had been diagnosed with hypertension, chronic kidney disease, or heart failure in the past year. Instances in which a patient was prescribed an NSAID within 7 days of presentation were included.
To assess for associations between prescription NSAIDs and negative outcomes, the investigators searched for renal or cardiovascular complications within 37 days of presentation. Over-the-counter NSAID usage was not evaluated.
There were 224,825 visits. An NSAID was prescribed after 9.3% of these visits.
Renal and cardiovascular outcomes were similar between high-risk patients who received a prescription NSAID and those who did not (absolute risk reduction, .0003; P = .74).
“The similarity in risk between users and nonusers, each group primarily consisting of patients with hypertension, suggests that the short-term association of NSAIDs in high-risk patients with musculoskeletal pain may not be as dangerous as initially thought,” the authors concluded.
The investigators found that prescribing rates varied widely, ranging from 6.7% to 14.4% of different health regions, and from 0.9% to 60.3% among 688 primary care practices, with “substantial variation in use” among primary care physicians.
The authors acknowledged limitations, including the use of administrative data, but noted that their study, showing substantial variations in NSAID prescribing, “along with the identification of patient and physician characteristics associated with NSAID use, presents an opportunity for quality improvement, with some potential targets for any resulting interventions,” they wrote.
The Institute for Clinical Evaluative Sciences funded the study. The authors reported compensation from the Canadian Institute of Health Research, the department of family and community medicine at the University of Toronto, the Heart and Stroke Foundation of Canada, and Women’s College Hospital.
SOURCE: Bouck et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4273.
The study by Bouck and colleagues found that short-term prescription NSAIDs were safe for high-risk patients; however, physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings challenge the American Society of Nephrology, which recommends against NSAIDs in patients with chronic kidney disease (CKD), heart failure, or hypertension.
Among the advantages of observational studies over randomized trials is that they often include patients not eligible for randomized controlled trials, “and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” they wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials, with nonrandomized allocation, which “raises the possibilities of selection bias and confounding by indication,” they wrote. Furthermore, “readers are left questioning whether patients who were not prescribed NSAIDs were simply taking over-the-counter medications (including NSAIDs),” they wrote.
“Although we rely on observational studies to answer questions poorly suited to clinical trials, we have to interpret these findings with caution,” they added.
Jonathan Zipursky, MD, and Dr. Juurlink are affiliated with the department of medicine at Sunnybrook Health Sciences Centre in Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med 2018 Oct 8. doi: 10.1136/ebmed-2016-110401).
The study by Bouck and colleagues found that short-term prescription NSAIDs were safe for high-risk patients; however, physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings challenge the American Society of Nephrology, which recommends against NSAIDs in patients with chronic kidney disease (CKD), heart failure, or hypertension.
Among the advantages of observational studies over randomized trials is that they often include patients not eligible for randomized controlled trials, “and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” they wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials, with nonrandomized allocation, which “raises the possibilities of selection bias and confounding by indication,” they wrote. Furthermore, “readers are left questioning whether patients who were not prescribed NSAIDs were simply taking over-the-counter medications (including NSAIDs),” they wrote.
“Although we rely on observational studies to answer questions poorly suited to clinical trials, we have to interpret these findings with caution,” they added.
Jonathan Zipursky, MD, and Dr. Juurlink are affiliated with the department of medicine at Sunnybrook Health Sciences Centre in Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med 2018 Oct 8. doi: 10.1136/ebmed-2016-110401).
The study by Bouck and colleagues found that short-term prescription NSAIDs were safe for high-risk patients; however, physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings challenge the American Society of Nephrology, which recommends against NSAIDs in patients with chronic kidney disease (CKD), heart failure, or hypertension.
Among the advantages of observational studies over randomized trials is that they often include patients not eligible for randomized controlled trials, “and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” they wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials, with nonrandomized allocation, which “raises the possibilities of selection bias and confounding by indication,” they wrote. Furthermore, “readers are left questioning whether patients who were not prescribed NSAIDs were simply taking over-the-counter medications (including NSAIDs),” they wrote.
“Although we rely on observational studies to answer questions poorly suited to clinical trials, we have to interpret these findings with caution,” they added.
Jonathan Zipursky, MD, and Dr. Juurlink are affiliated with the department of medicine at Sunnybrook Health Sciences Centre in Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med 2018 Oct 8. doi: 10.1136/ebmed-2016-110401).
in a retrospective, observational study.
The findings of the study challenge the Choosing Wisely campaign of the American Society of Nephrology, which recommends against NSAIDs for high-risk patients, according to lead author Zachary Bouck, MPH, of the department of medicine at Sunnybrook Health Sciences Centre in Toronto, and his coauthors.
“While these recommendations offer basic analgesics and nonpharmacological treatments as preferable alternatives, it is both possible and disconcerting that some physicians might instead prescribe opioids, which typically pose elevated risk of adverse events and dependence vs. NSAIDs,” the investigators wrote. The report is in JAMA Internal Medicine.
They sought to estimate the frequency and characteristics of NSAID prescriptions while also looking for associations with acute renal and cardiovascular complications. The retrospective, observational study involved 814,049 adults with musculoskeletal disease and 7,365 primary care physicians in Ontario, Canada. All patients were aged 65 years and older, and had been diagnosed with hypertension, chronic kidney disease, or heart failure in the past year. Instances in which a patient was prescribed an NSAID within 7 days of presentation were included.
To assess for associations between prescription NSAIDs and negative outcomes, the investigators searched for renal or cardiovascular complications within 37 days of presentation. Over-the-counter NSAID usage was not evaluated.
There were 224,825 visits. An NSAID was prescribed after 9.3% of these visits.
Renal and cardiovascular outcomes were similar between high-risk patients who received a prescription NSAID and those who did not (absolute risk reduction, .0003; P = .74).
“The similarity in risk between users and nonusers, each group primarily consisting of patients with hypertension, suggests that the short-term association of NSAIDs in high-risk patients with musculoskeletal pain may not be as dangerous as initially thought,” the authors concluded.
The investigators found that prescribing rates varied widely, ranging from 6.7% to 14.4% of different health regions, and from 0.9% to 60.3% among 688 primary care practices, with “substantial variation in use” among primary care physicians.
The authors acknowledged limitations, including the use of administrative data, but noted that their study, showing substantial variations in NSAID prescribing, “along with the identification of patient and physician characteristics associated with NSAID use, presents an opportunity for quality improvement, with some potential targets for any resulting interventions,” they wrote.
The Institute for Clinical Evaluative Sciences funded the study. The authors reported compensation from the Canadian Institute of Health Research, the department of family and community medicine at the University of Toronto, the Heart and Stroke Foundation of Canada, and Women’s College Hospital.
SOURCE: Bouck et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4273.
in a retrospective, observational study.
The findings of the study challenge the Choosing Wisely campaign of the American Society of Nephrology, which recommends against NSAIDs for high-risk patients, according to lead author Zachary Bouck, MPH, of the department of medicine at Sunnybrook Health Sciences Centre in Toronto, and his coauthors.
“While these recommendations offer basic analgesics and nonpharmacological treatments as preferable alternatives, it is both possible and disconcerting that some physicians might instead prescribe opioids, which typically pose elevated risk of adverse events and dependence vs. NSAIDs,” the investigators wrote. The report is in JAMA Internal Medicine.
They sought to estimate the frequency and characteristics of NSAID prescriptions while also looking for associations with acute renal and cardiovascular complications. The retrospective, observational study involved 814,049 adults with musculoskeletal disease and 7,365 primary care physicians in Ontario, Canada. All patients were aged 65 years and older, and had been diagnosed with hypertension, chronic kidney disease, or heart failure in the past year. Instances in which a patient was prescribed an NSAID within 7 days of presentation were included.
To assess for associations between prescription NSAIDs and negative outcomes, the investigators searched for renal or cardiovascular complications within 37 days of presentation. Over-the-counter NSAID usage was not evaluated.
There were 224,825 visits. An NSAID was prescribed after 9.3% of these visits.
Renal and cardiovascular outcomes were similar between high-risk patients who received a prescription NSAID and those who did not (absolute risk reduction, .0003; P = .74).
“The similarity in risk between users and nonusers, each group primarily consisting of patients with hypertension, suggests that the short-term association of NSAIDs in high-risk patients with musculoskeletal pain may not be as dangerous as initially thought,” the authors concluded.
The investigators found that prescribing rates varied widely, ranging from 6.7% to 14.4% of different health regions, and from 0.9% to 60.3% among 688 primary care practices, with “substantial variation in use” among primary care physicians.
The authors acknowledged limitations, including the use of administrative data, but noted that their study, showing substantial variations in NSAID prescribing, “along with the identification of patient and physician characteristics associated with NSAID use, presents an opportunity for quality improvement, with some potential targets for any resulting interventions,” they wrote.
The Institute for Clinical Evaluative Sciences funded the study. The authors reported compensation from the Canadian Institute of Health Research, the department of family and community medicine at the University of Toronto, the Heart and Stroke Foundation of Canada, and Women’s College Hospital.
SOURCE: Bouck et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4273.
FROM JAMA INTERNAL MEDICINE
Key clinical point: In patients with musculoskeletal disease and hypertension, chronic kidney disease, or heart failure, short-term prescription NSAIDs may be safer than once thought.
Major finding: Renal and cardiovascular outcomes were similar between high-risk patients who received a prescription NSAID and those who did not (absolute risk reduction, .0003; P = .74).
Study details: A retrospective, observational study involving 814,049 adults with musculoskeletal disease and 7,365 primary care physicians in Ontario, Canada.
Disclosures: The Institute for Clinical Evaluative Sciences funded the study. The authors reported compensation from the Canadian Institute of Health Research, the Heart and Stroke Foundation of Canada, and Women’s College Hospital.
Source: Bouck et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4273.
Allopurinol reduces risk of renal decline in gout patients
In patients with gout, at least 300 mg of allopurinol daily may reduce the risk of renal function decline, according to a new study.
Since no evidence supports allopurinol nephrotoxicity and usage does not appear to worsen chronic kidney disease (CKD), clinicians should consider other causes of declining renal function, according to lead author Ana Beatriz Vargas-Santos, MD, of the rheumatology unit at the State University of Rio de Janeiro and her colleagues.
These findings reinforce the American College of Rheumatology’s 2012 treatment recommendation that the dose of allopurinol, a urate-lowering therapy (ULT), “can be raised above 300 mg daily, even with renal impairment, as long as it is accompanied by adequate patient education and monitoring for drug toxicity may worsen renal function.”*
“Renal-dosing of allopurinol compounds the poor management of gout and adds to the perception that allopurinol may be detrimental for renal function,” the investigators wrote in JAMA Internal Medicine. “In contrast, recent studies provide support for starting allopurinol at a low dose with gradual dose escalation to serum urate target with close monitoring, even among patients with renal insufficiency, without increased risk of allopurinol hypersensitivity syndrome (AHS). Further, there is emerging evidence that ULT may be beneficial for kidney dysfunction.”
Building upon these developments, the investigators “aimed to assess the relation of allopurinol initiation to the risk of developing CKD stage 3 or higher among people with newly diagnosed gout.”
Patients for the cohort study were drawn from the Health Improvement Network (THIN), a database of records from general practitioners in the United Kingdom. Included patients were recently diagnosed with gout but did not have stage 3 or higher chronic kidney disease or ULT usage within a year prior to diagnosis. After screening, 4,760 allopurinol users were matched with 4,760 allopurinol nonusers. Overall, 71% of patients had CKD stage 2, while the remaining 29% had CKD stage 1 or normal kidney function.
The primary outcome of CKD stage 3 or higher was defined as glomerular filtration rate below 60 mL/min (recorded at least twice in 1 year with a 3-month interval between readings and GFR never exceeding 75 mL/min during the intervening period), kidney transplant, or dialysis. The mean follow-up time was 5 years for allopurinol users and 4 years for nonusers.
The investigators found that 579 allopurinol users developed CKD stage 3 or higher, compared with 623 nonusers, suggesting that allopurinol reduced risk of CKD stage 3 or higher by 13%. Allopurinol doses of at least 300 mg/day were associated with a hazard ratio of 0.87, but lower doses did not share this association (HR = 1.02).
In defense of their findings, Dr. Vargas-Santos and her associates evaluated the relevance of their study, compared with previous allopurinol studies.
“This study is one of few that have evaluated the relation of allopurinol to renal function among patients with gout and normal or near-normal kidney function at baseline,” the authors wrote, noting that most gout patients do not have severe kidney disease.
Previous studies have suggested that allopurinol worsens kidney function, but these studies were often conducted in nongout populations, with patients exhibiting CKD stage 3 or higher, they noted. Instead of allopurinol-induced kidney damage, renal decline in gout patients is likely multifactorial.
“Because people with gout have intrinsic differences compared with those with asymptomatic hyperuricemia, including higher mortality, more comorbidities, and more NSAID use, these studies’ results are not directly applicable to gout patients,” the investigators wrote.
“At minimum, allopurinol does not seem to have a detrimental effect on renal function in individuals with gout,” Dr. Vargas-Santos and her associates concluded. “Clinicians should consider evaluating other factors when faced with renal function decline in their patients with gout rather than lowering the dose of or discontinuing allopurinol, a strategy that has contributed to the ongoing suboptimal treatment of gout.”
The authors reported funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Ministry of Science, Technology and Innovation of Brazil; and National Institutes of Health. Dr Vargas-Santos has received speaking fees and support for international medical events from Grünenthal. No other disclosures were reported.
SOURCE: Vargas-Santos AB et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4463
*Correction, 11/5/2018: An earlier version of this story incorrectly stated the American College of Rheumatology’s 2012 gout treatment recommendation for using allopurinol in patients with renal impairment.
Physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings in this paper challenge the American College of Rheumatology, which recommends lower allopurinol doses in patients with chronic kidney disease (CKD).
On one hand, they noted, observational studies have some advantages over randomized trials.
“Observational studies frequently include patients who are ineligible for RCTs, and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” Dr. Zipursky and Dr. Juurlink wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials.
As “treatment allocation is nonrandom, it raises the possibilities of selection bias and confounding by indication,” Dr. Zipursky and Dr. Juurlink wrote. “Perhaps the drugs were preferentially prescribed to patients destined to tolerate them, or fare better in some other way apparent to prescribers but beyond the resolution of large databases. For example, of the nearly 43,000 patients in the study by Vargos-Santos et al, only 10% were started on 300 mg or more per day of allopurinol. This leaves readers to wonder what motivated practitioners to start such doses, or, conversely, what it was about the remaining 90% of patients that led them to receive lower doses of allopurinol or none at all.”
Along with these unanswered questions, the study compared treated patients to untreated ones, but “it is generally desirable to compare 1 drug with another used for the same indication, which can help mitigate the effect of unmeasured factors that might have influenced the decision to treat in the first place.”
Familiarity with observational studies is essential for clinicians, as Dr. Zipursky and Dr. Juurlink expect such trials will become more common in the future, and they provide useful insight if clinicians maintain an appropriate viewpoint.
“The findings will need to be contextualized and viewed with more skepticism than RCTs,” they wrote, “but in some instances, they can be thoughtfully integrated into our treatment decisions.”
Dr. Zipursky and Dr. Juurlink are with the department of medicine at Sunnybrook Health Sciences Centre, Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.5766).
Physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings in this paper challenge the American College of Rheumatology, which recommends lower allopurinol doses in patients with chronic kidney disease (CKD).
On one hand, they noted, observational studies have some advantages over randomized trials.
“Observational studies frequently include patients who are ineligible for RCTs, and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” Dr. Zipursky and Dr. Juurlink wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials.
As “treatment allocation is nonrandom, it raises the possibilities of selection bias and confounding by indication,” Dr. Zipursky and Dr. Juurlink wrote. “Perhaps the drugs were preferentially prescribed to patients destined to tolerate them, or fare better in some other way apparent to prescribers but beyond the resolution of large databases. For example, of the nearly 43,000 patients in the study by Vargos-Santos et al, only 10% were started on 300 mg or more per day of allopurinol. This leaves readers to wonder what motivated practitioners to start such doses, or, conversely, what it was about the remaining 90% of patients that led them to receive lower doses of allopurinol or none at all.”
Along with these unanswered questions, the study compared treated patients to untreated ones, but “it is generally desirable to compare 1 drug with another used for the same indication, which can help mitigate the effect of unmeasured factors that might have influenced the decision to treat in the first place.”
Familiarity with observational studies is essential for clinicians, as Dr. Zipursky and Dr. Juurlink expect such trials will become more common in the future, and they provide useful insight if clinicians maintain an appropriate viewpoint.
“The findings will need to be contextualized and viewed with more skepticism than RCTs,” they wrote, “but in some instances, they can be thoughtfully integrated into our treatment decisions.”
Dr. Zipursky and Dr. Juurlink are with the department of medicine at Sunnybrook Health Sciences Centre, Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.5766).
Physicians should consider the inherent limitations of observational studies before altering clinical decisions, according to Jonathan Zipursky, MD, and David N. Juurlink, MD, PhD. This is particularly important since the findings in this paper challenge the American College of Rheumatology, which recommends lower allopurinol doses in patients with chronic kidney disease (CKD).
On one hand, they noted, observational studies have some advantages over randomized trials.
“Observational studies frequently include patients who are ineligible for RCTs, and extended follow-up enables examination of outcomes that might not have arisen earlier in treatment,” Dr. Zipursky and Dr. Juurlink wrote in an editorial. “Moreover, sample sizes often greatly exceed those of RCTs, facilitating detection of less common adverse events. Consequently, population-based observational studies are critical to postmarketing surveillance and, increasingly, evidence-based prescribing recommendations.”
On the other hand, observational studies are less tightly controlled than randomized trials.
As “treatment allocation is nonrandom, it raises the possibilities of selection bias and confounding by indication,” Dr. Zipursky and Dr. Juurlink wrote. “Perhaps the drugs were preferentially prescribed to patients destined to tolerate them, or fare better in some other way apparent to prescribers but beyond the resolution of large databases. For example, of the nearly 43,000 patients in the study by Vargos-Santos et al, only 10% were started on 300 mg or more per day of allopurinol. This leaves readers to wonder what motivated practitioners to start such doses, or, conversely, what it was about the remaining 90% of patients that led them to receive lower doses of allopurinol or none at all.”
Along with these unanswered questions, the study compared treated patients to untreated ones, but “it is generally desirable to compare 1 drug with another used for the same indication, which can help mitigate the effect of unmeasured factors that might have influenced the decision to treat in the first place.”
Familiarity with observational studies is essential for clinicians, as Dr. Zipursky and Dr. Juurlink expect such trials will become more common in the future, and they provide useful insight if clinicians maintain an appropriate viewpoint.
“The findings will need to be contextualized and viewed with more skepticism than RCTs,” they wrote, “but in some instances, they can be thoughtfully integrated into our treatment decisions.”
Dr. Zipursky and Dr. Juurlink are with the department of medicine at Sunnybrook Health Sciences Centre, Toronto. These comments are adapted from their accompanying editorial (JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.5766).
In patients with gout, at least 300 mg of allopurinol daily may reduce the risk of renal function decline, according to a new study.
Since no evidence supports allopurinol nephrotoxicity and usage does not appear to worsen chronic kidney disease (CKD), clinicians should consider other causes of declining renal function, according to lead author Ana Beatriz Vargas-Santos, MD, of the rheumatology unit at the State University of Rio de Janeiro and her colleagues.
These findings reinforce the American College of Rheumatology’s 2012 treatment recommendation that the dose of allopurinol, a urate-lowering therapy (ULT), “can be raised above 300 mg daily, even with renal impairment, as long as it is accompanied by adequate patient education and monitoring for drug toxicity may worsen renal function.”*
“Renal-dosing of allopurinol compounds the poor management of gout and adds to the perception that allopurinol may be detrimental for renal function,” the investigators wrote in JAMA Internal Medicine. “In contrast, recent studies provide support for starting allopurinol at a low dose with gradual dose escalation to serum urate target with close monitoring, even among patients with renal insufficiency, without increased risk of allopurinol hypersensitivity syndrome (AHS). Further, there is emerging evidence that ULT may be beneficial for kidney dysfunction.”
Building upon these developments, the investigators “aimed to assess the relation of allopurinol initiation to the risk of developing CKD stage 3 or higher among people with newly diagnosed gout.”
Patients for the cohort study were drawn from the Health Improvement Network (THIN), a database of records from general practitioners in the United Kingdom. Included patients were recently diagnosed with gout but did not have stage 3 or higher chronic kidney disease or ULT usage within a year prior to diagnosis. After screening, 4,760 allopurinol users were matched with 4,760 allopurinol nonusers. Overall, 71% of patients had CKD stage 2, while the remaining 29% had CKD stage 1 or normal kidney function.
The primary outcome of CKD stage 3 or higher was defined as glomerular filtration rate below 60 mL/min (recorded at least twice in 1 year with a 3-month interval between readings and GFR never exceeding 75 mL/min during the intervening period), kidney transplant, or dialysis. The mean follow-up time was 5 years for allopurinol users and 4 years for nonusers.
The investigators found that 579 allopurinol users developed CKD stage 3 or higher, compared with 623 nonusers, suggesting that allopurinol reduced risk of CKD stage 3 or higher by 13%. Allopurinol doses of at least 300 mg/day were associated with a hazard ratio of 0.87, but lower doses did not share this association (HR = 1.02).
In defense of their findings, Dr. Vargas-Santos and her associates evaluated the relevance of their study, compared with previous allopurinol studies.
“This study is one of few that have evaluated the relation of allopurinol to renal function among patients with gout and normal or near-normal kidney function at baseline,” the authors wrote, noting that most gout patients do not have severe kidney disease.
Previous studies have suggested that allopurinol worsens kidney function, but these studies were often conducted in nongout populations, with patients exhibiting CKD stage 3 or higher, they noted. Instead of allopurinol-induced kidney damage, renal decline in gout patients is likely multifactorial.
“Because people with gout have intrinsic differences compared with those with asymptomatic hyperuricemia, including higher mortality, more comorbidities, and more NSAID use, these studies’ results are not directly applicable to gout patients,” the investigators wrote.
“At minimum, allopurinol does not seem to have a detrimental effect on renal function in individuals with gout,” Dr. Vargas-Santos and her associates concluded. “Clinicians should consider evaluating other factors when faced with renal function decline in their patients with gout rather than lowering the dose of or discontinuing allopurinol, a strategy that has contributed to the ongoing suboptimal treatment of gout.”
The authors reported funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Ministry of Science, Technology and Innovation of Brazil; and National Institutes of Health. Dr Vargas-Santos has received speaking fees and support for international medical events from Grünenthal. No other disclosures were reported.
SOURCE: Vargas-Santos AB et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4463
*Correction, 11/5/2018: An earlier version of this story incorrectly stated the American College of Rheumatology’s 2012 gout treatment recommendation for using allopurinol in patients with renal impairment.
In patients with gout, at least 300 mg of allopurinol daily may reduce the risk of renal function decline, according to a new study.
Since no evidence supports allopurinol nephrotoxicity and usage does not appear to worsen chronic kidney disease (CKD), clinicians should consider other causes of declining renal function, according to lead author Ana Beatriz Vargas-Santos, MD, of the rheumatology unit at the State University of Rio de Janeiro and her colleagues.
These findings reinforce the American College of Rheumatology’s 2012 treatment recommendation that the dose of allopurinol, a urate-lowering therapy (ULT), “can be raised above 300 mg daily, even with renal impairment, as long as it is accompanied by adequate patient education and monitoring for drug toxicity may worsen renal function.”*
“Renal-dosing of allopurinol compounds the poor management of gout and adds to the perception that allopurinol may be detrimental for renal function,” the investigators wrote in JAMA Internal Medicine. “In contrast, recent studies provide support for starting allopurinol at a low dose with gradual dose escalation to serum urate target with close monitoring, even among patients with renal insufficiency, without increased risk of allopurinol hypersensitivity syndrome (AHS). Further, there is emerging evidence that ULT may be beneficial for kidney dysfunction.”
Building upon these developments, the investigators “aimed to assess the relation of allopurinol initiation to the risk of developing CKD stage 3 or higher among people with newly diagnosed gout.”
Patients for the cohort study were drawn from the Health Improvement Network (THIN), a database of records from general practitioners in the United Kingdom. Included patients were recently diagnosed with gout but did not have stage 3 or higher chronic kidney disease or ULT usage within a year prior to diagnosis. After screening, 4,760 allopurinol users were matched with 4,760 allopurinol nonusers. Overall, 71% of patients had CKD stage 2, while the remaining 29% had CKD stage 1 or normal kidney function.
The primary outcome of CKD stage 3 or higher was defined as glomerular filtration rate below 60 mL/min (recorded at least twice in 1 year with a 3-month interval between readings and GFR never exceeding 75 mL/min during the intervening period), kidney transplant, or dialysis. The mean follow-up time was 5 years for allopurinol users and 4 years for nonusers.
The investigators found that 579 allopurinol users developed CKD stage 3 or higher, compared with 623 nonusers, suggesting that allopurinol reduced risk of CKD stage 3 or higher by 13%. Allopurinol doses of at least 300 mg/day were associated with a hazard ratio of 0.87, but lower doses did not share this association (HR = 1.02).
In defense of their findings, Dr. Vargas-Santos and her associates evaluated the relevance of their study, compared with previous allopurinol studies.
“This study is one of few that have evaluated the relation of allopurinol to renal function among patients with gout and normal or near-normal kidney function at baseline,” the authors wrote, noting that most gout patients do not have severe kidney disease.
Previous studies have suggested that allopurinol worsens kidney function, but these studies were often conducted in nongout populations, with patients exhibiting CKD stage 3 or higher, they noted. Instead of allopurinol-induced kidney damage, renal decline in gout patients is likely multifactorial.
“Because people with gout have intrinsic differences compared with those with asymptomatic hyperuricemia, including higher mortality, more comorbidities, and more NSAID use, these studies’ results are not directly applicable to gout patients,” the investigators wrote.
“At minimum, allopurinol does not seem to have a detrimental effect on renal function in individuals with gout,” Dr. Vargas-Santos and her associates concluded. “Clinicians should consider evaluating other factors when faced with renal function decline in their patients with gout rather than lowering the dose of or discontinuing allopurinol, a strategy that has contributed to the ongoing suboptimal treatment of gout.”
The authors reported funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Ministry of Science, Technology and Innovation of Brazil; and National Institutes of Health. Dr Vargas-Santos has received speaking fees and support for international medical events from Grünenthal. No other disclosures were reported.
SOURCE: Vargas-Santos AB et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4463
*Correction, 11/5/2018: An earlier version of this story incorrectly stated the American College of Rheumatology’s 2012 gout treatment recommendation for using allopurinol in patients with renal impairment.
FROM JAMA INTERNAL MEDICINE
Key clinical point: In patients with gout, allopurinol was associated with a reduced risk of renal function decline.
Major finding: Allopurinol doses of at least 300 mg/day reduced risk of stage-3 or higher chronic kidney disease by 13%.
Study details: A retrospective, observational study involving newly diagnosed gout patients who either started allopurinol or did not (n = 4,760 in each group).
Disclosures: The authors reported funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Ministry of Science, Technology and Innovation of Brazil; and National Institutes of Health. Dr Vargas-Santos has received speaking fees and support for international medical events from Grünenthal. No other disclosures were reported.
Source: Vargas-Santos AB et al. JAMA Intern Med. 2018 Oct 8. doi: 10.1001/jamainternmed.2018.4463
The Unsaid Dangers of NSAIDs
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.
CKD children need office blood pressures below the 75th percentile
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
CHICAGO – according to a review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Hypertensive children with CKD were less likely to progress to dialysis or kidney transplant or have a 30% decline in estimated glomerular filtration rate (eGFR) when kept in that range, compared with children above or below it. “Achieved office [blood pressure] between the 50th and 75th percentiles appeared to offer the greatest protection against CKD progression in this cohort,” said investigators led by Joseph Flynn, MD, chief of the nephrology division at Seattle Children’s Hospital.
“We needed to have some [evidence] on what to do based on office blood pressure,” something that had been missing in the literature until now. “I think this is going to be very impactful on the care of children with CKD. Right now, the guidelines say to keep” pressures below the 90th percentile for age and height. “The guidelines [might] need to be changed,” said Dr. Flynn, also the lead author of the American Academy of Pediatrics 2017 blood pressure guidelines for children and adolescents (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
There was “no evidence to go below the 50th percentile; the 50th-75th seems to be the sweet spot for office blood pressure,” he said at the joint scientific sessions of the AHA Council on Hypertension, the AHA Council on Kidney in Cardiovascular Disease, and the American Society of Hypertension.
The 476 children with nonglomerular CKD actually did worse when their blood pressures were pushed below the 50th percentile, perhaps because of renal hypoperfusion. The 476 children with glomerular CKD did no better or worse below the 50th percentile than they did in the 50th-75th.
Children at or above the 90th percentile had the highest risk of progression, with about 80% needing renal replacement therapy or having a 30% drop in eGFR at 5-8 years of follow-up. Compared with children with glomerular CKD who were in the 90th percentile, the risk hazard (RH) for progression over 3 years was 0.10-0.30 (P less than 0.001) among those children with glomerular CKD who were kept in the 50th-75th percentile. Compared with children with nonglomerular CKD who were in the 90th percentile, the RH over 8 years among those in the 50th-75th percentile was 0.48 (P less than 0.001). Risk of progression in both glomerular and nonglomerular patients in the sweet spot was less than 50% at 5-8 years of follow-up.
When glomerular and nonglomerular patients were considered together, those with pressures below the 50th percentile were less likely to progress than children with pressures between the 75th and 90th, but they were more likely to progress than the 50th-75th percentile group.
Research nurses took three blood pressures by auscultation a minute apart after 5 minutes of rest. Most of the children were treated at first with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, per recommendations. Dihydropyridine calcium channel blockers (for example, amlodipine and nifedipine) were most likely to be used next.
It was a curious finding because dihydropyridines have been show to worsen proteinuria in adults with CKD. The team is investigating to see whether they have the same effect in children. If so, “we’ll be able to tell people to stop using them,” Dr. Flynn said.
The median age in the study was 11.3 years, and almost two-thirds of the subjects were boys. The median duration of disease was 8 years. Some children in the ongoing cohort have been followed for almost 10 years. The analysis is based on children who entered the cohort with hypertension or developed it after enrollment.
The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: It’s best to aim for an office blood pressure between the 50th and 75th percentiles in children with CKD.
Major finding: The risk of CKD progression was less than 50% in children kept in that range, which was better than children both above or below it.
Study details: Review of 690 pediatric patients in the Chronic Kidney Disease in Children Cohort Study.
Disclosures: The nationwide Chronic Kidney Disease in Children Cohort Study is funded by the National Institutes of Health. Dr. Joseph Flynn is an advisor for Ultragenyx and Silvergate Pharmaceuticals.
2017 ACC/AHA hypertension guidelines: Toward tighter control
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
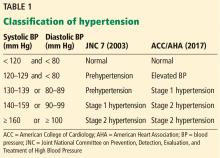
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
Decision aid aims to make lupus nephritis treatment decisions more understandable
A computer-based decision aid aims to facilitate the complex discussions and decisions that face patients with lupus nephritis.
Under development at the University of Alabama at Birmingham, the Shared Decision-Making in Lupus Electronic tool (SMILE) describes in easy-to-understand modules the pathology of the disease, and what can happen if it progresses untreated. The tool also identifies treatment options and discusses the potential benefits of each one, as well as the risks. It can be folded into office visits, accessed during waiting times, or viewed at the patient’s leisure. Its creators hope that patients will experience more fruitful discussions with their physicians by learning more about these complicated concepts as they co-navigate the difficult decisions both must make.
“Our intent was to figure out how best to offer the education about lupus and its treatments that women need when coming into a busy clinic,” said Jasvinder Singh, MD, who is leading the developmental team. “Everyone’s time is limited, and patients want information delivered quickly, but in a way they can understand.”
SMILE is the first tool of its kind for patients with lupus nephritis, said Alexa Meara, MD, one of the rheumatologists investigating it.
“There is just nothing out there like this for either lupus, or lupus nephritis,” said Dr. Meara, a rheumatologist at Ohio State University, Columbus. “Lupus nephritis is a heterogeneous relapsing-remitting disease with complex medical regimens. It can have cognitive impacts and affect fertility, but these are things that may happen over decades. Patients may not be ready to talk about them early on, but they must understand them. We would like to enable the conversation between physician and patient, so they’re on the same page with everyone’s values and concerns addressed on both sides.”
But getting everyone on the same page at the same time is challenging, said Dr. Singh, a rheumatologist and professor of medicine and epidemiology at the University of Alabama at Birmingham. “This is a bit of a dilemma and a challenge. What’s great about the lupus decision aid is that it is culturally and linguistically appropriate for patients of all backgrounds, races, ages, literacy levels, and socioeconomic statuses. This levels the playing field for patients who are trying to understand their diagnosis and options to the best of their abilities.”
Three years in the making, SMILE comprises several modules addressing different facets of lupus nephritis. In simple words and concepts, the tool informs patients about what lupus is and what it can do to the kidneys, the information gleaned from blood and urine tests and what it means, and the potential consequences of not treating lupus kidney disease. The heart of the program is its breakdown of treatment options.
“It helps patients understand why they may be getting these cancer medications, and what their side effects are,” Dr. Singh said. “The tool also walks them through a comparison of the medications, with one slide on each medication and its risks and benefits. We really focus on that – about 60%-70% of the tool is comparing the drugs.”
The decision aid also contains optional modules that patients can bypass or explore. “There are separate sections on the risks and benefits of corticosteroids, on pregnancy and lactation, and on preserving fertility.”
A complete click-through of the basic information takes about 20 minutes, Dr. Singh said. An ideal time for a patient to do so would be before the initial visit to discuss treatment options, which can be an overwhelming experience. The tool could be presented in several ways. In the office, preloaded iPads could be one vehicle. These could be standalone, or linked to the patient’s electronic health record, so that any entered information could be directly transferred. At home, patients could navigate to a web link for an online version.
The first visit is a crucial moment, he said, where patients can evolve into management partners, or leave even more confused than when they arrived.
“We aim to motivate and provide tools to the patient, so that the conversation isn’t just, ‘You have the disease and we can put you on this or that drug,’ ” Dr. Singh said. “You can lose the patient right away in this scenario, while you’re talking about so many things the patient isn’t even hearing you, because she’s thinking about other issues that are important in her life, completely overwhelmed with the shock of the diagnosis and with fear.”
The Patient-Centered Outcomes Research Institute (PCORI) has funded much of the work getting SMILE up and running, including a soon-to-be-published trial that favorably compared it against the educational standard: a paper pamphlet on lupus nephritis prepared by the American College of Rheumatology.
In this study, 301 high‐risk women with lupus kidney disease, including racial/ethnic minorities with low socioeconomic status, either received the pamphlet or the SMILE tool. The group was demographically diverse: 47% black, 26% Hispanic, 15% white, and 7% Asian. Other groups made up the remainder.
More patients rated the information in the decision aid to be excellent for understanding the impact of lupus (49% vs. 33%), risk factors (43% vs. 27%), medication options (50% vs. 33%), and evidence about medications (47% vs. 24%). About half of the tool users rated the ease of use of materials as excellent (51% vs. 38%). Women who used the tool also reported much less decisional conflict for immunosuppressive drugs and were much more likely to choose the treatment option most consistent with their values, having viewed information that mattered the most for the treatment decision.
PCORI continues to support the project, too. Dr. Singh just received a $2.2 million grant to investigate the tool in 16 clinics. The 2-year observational study has three aims:
• Identify physician attitudes and patient barriers toward the tool, to guide decisions about how to best implement it in its final form.
• Track changes in subjective and objective measures of implementation effectiveness.
• Identify opportunities for disseminating the tool and develop a step‐by‐step implementation guide for incorporating the decision aid tool into regular lupus clinic visits and care pathways.
The study will also look at some patient‐centered outcomes, including decision conflict, emergency department and inpatient visits, patient‐physician communication, and implementation success. Some practice-level outcomes will be assessed, including potential barriers to implementation. “The team will focus on context and strategy as facilitators and barriers to effective implementation of the lupus decision aid in busy, clinical practices of various types – for example, private versus academic, urban versus suburban, large versus small group practice,” according to the PCORI study description.
“This study can’t solve the entire problem about patients choosing treatment or no treatment, but it can provide a start to the conversation,” Dr. Singh said. “And my hope is that once it’s available, people will modify it to fit their needs, make adaptations and modifications to keep it relevant and valuable.”
A computer-based decision aid aims to facilitate the complex discussions and decisions that face patients with lupus nephritis.
Under development at the University of Alabama at Birmingham, the Shared Decision-Making in Lupus Electronic tool (SMILE) describes in easy-to-understand modules the pathology of the disease, and what can happen if it progresses untreated. The tool also identifies treatment options and discusses the potential benefits of each one, as well as the risks. It can be folded into office visits, accessed during waiting times, or viewed at the patient’s leisure. Its creators hope that patients will experience more fruitful discussions with their physicians by learning more about these complicated concepts as they co-navigate the difficult decisions both must make.
“Our intent was to figure out how best to offer the education about lupus and its treatments that women need when coming into a busy clinic,” said Jasvinder Singh, MD, who is leading the developmental team. “Everyone’s time is limited, and patients want information delivered quickly, but in a way they can understand.”
SMILE is the first tool of its kind for patients with lupus nephritis, said Alexa Meara, MD, one of the rheumatologists investigating it.
“There is just nothing out there like this for either lupus, or lupus nephritis,” said Dr. Meara, a rheumatologist at Ohio State University, Columbus. “Lupus nephritis is a heterogeneous relapsing-remitting disease with complex medical regimens. It can have cognitive impacts and affect fertility, but these are things that may happen over decades. Patients may not be ready to talk about them early on, but they must understand them. We would like to enable the conversation between physician and patient, so they’re on the same page with everyone’s values and concerns addressed on both sides.”
But getting everyone on the same page at the same time is challenging, said Dr. Singh, a rheumatologist and professor of medicine and epidemiology at the University of Alabama at Birmingham. “This is a bit of a dilemma and a challenge. What’s great about the lupus decision aid is that it is culturally and linguistically appropriate for patients of all backgrounds, races, ages, literacy levels, and socioeconomic statuses. This levels the playing field for patients who are trying to understand their diagnosis and options to the best of their abilities.”
Three years in the making, SMILE comprises several modules addressing different facets of lupus nephritis. In simple words and concepts, the tool informs patients about what lupus is and what it can do to the kidneys, the information gleaned from blood and urine tests and what it means, and the potential consequences of not treating lupus kidney disease. The heart of the program is its breakdown of treatment options.
“It helps patients understand why they may be getting these cancer medications, and what their side effects are,” Dr. Singh said. “The tool also walks them through a comparison of the medications, with one slide on each medication and its risks and benefits. We really focus on that – about 60%-70% of the tool is comparing the drugs.”
The decision aid also contains optional modules that patients can bypass or explore. “There are separate sections on the risks and benefits of corticosteroids, on pregnancy and lactation, and on preserving fertility.”
A complete click-through of the basic information takes about 20 minutes, Dr. Singh said. An ideal time for a patient to do so would be before the initial visit to discuss treatment options, which can be an overwhelming experience. The tool could be presented in several ways. In the office, preloaded iPads could be one vehicle. These could be standalone, or linked to the patient’s electronic health record, so that any entered information could be directly transferred. At home, patients could navigate to a web link for an online version.
The first visit is a crucial moment, he said, where patients can evolve into management partners, or leave even more confused than when they arrived.
“We aim to motivate and provide tools to the patient, so that the conversation isn’t just, ‘You have the disease and we can put you on this or that drug,’ ” Dr. Singh said. “You can lose the patient right away in this scenario, while you’re talking about so many things the patient isn’t even hearing you, because she’s thinking about other issues that are important in her life, completely overwhelmed with the shock of the diagnosis and with fear.”
The Patient-Centered Outcomes Research Institute (PCORI) has funded much of the work getting SMILE up and running, including a soon-to-be-published trial that favorably compared it against the educational standard: a paper pamphlet on lupus nephritis prepared by the American College of Rheumatology.
In this study, 301 high‐risk women with lupus kidney disease, including racial/ethnic minorities with low socioeconomic status, either received the pamphlet or the SMILE tool. The group was demographically diverse: 47% black, 26% Hispanic, 15% white, and 7% Asian. Other groups made up the remainder.
More patients rated the information in the decision aid to be excellent for understanding the impact of lupus (49% vs. 33%), risk factors (43% vs. 27%), medication options (50% vs. 33%), and evidence about medications (47% vs. 24%). About half of the tool users rated the ease of use of materials as excellent (51% vs. 38%). Women who used the tool also reported much less decisional conflict for immunosuppressive drugs and were much more likely to choose the treatment option most consistent with their values, having viewed information that mattered the most for the treatment decision.
PCORI continues to support the project, too. Dr. Singh just received a $2.2 million grant to investigate the tool in 16 clinics. The 2-year observational study has three aims:
• Identify physician attitudes and patient barriers toward the tool, to guide decisions about how to best implement it in its final form.
• Track changes in subjective and objective measures of implementation effectiveness.
• Identify opportunities for disseminating the tool and develop a step‐by‐step implementation guide for incorporating the decision aid tool into regular lupus clinic visits and care pathways.
The study will also look at some patient‐centered outcomes, including decision conflict, emergency department and inpatient visits, patient‐physician communication, and implementation success. Some practice-level outcomes will be assessed, including potential barriers to implementation. “The team will focus on context and strategy as facilitators and barriers to effective implementation of the lupus decision aid in busy, clinical practices of various types – for example, private versus academic, urban versus suburban, large versus small group practice,” according to the PCORI study description.
“This study can’t solve the entire problem about patients choosing treatment or no treatment, but it can provide a start to the conversation,” Dr. Singh said. “And my hope is that once it’s available, people will modify it to fit their needs, make adaptations and modifications to keep it relevant and valuable.”
A computer-based decision aid aims to facilitate the complex discussions and decisions that face patients with lupus nephritis.
Under development at the University of Alabama at Birmingham, the Shared Decision-Making in Lupus Electronic tool (SMILE) describes in easy-to-understand modules the pathology of the disease, and what can happen if it progresses untreated. The tool also identifies treatment options and discusses the potential benefits of each one, as well as the risks. It can be folded into office visits, accessed during waiting times, or viewed at the patient’s leisure. Its creators hope that patients will experience more fruitful discussions with their physicians by learning more about these complicated concepts as they co-navigate the difficult decisions both must make.
“Our intent was to figure out how best to offer the education about lupus and its treatments that women need when coming into a busy clinic,” said Jasvinder Singh, MD, who is leading the developmental team. “Everyone’s time is limited, and patients want information delivered quickly, but in a way they can understand.”
SMILE is the first tool of its kind for patients with lupus nephritis, said Alexa Meara, MD, one of the rheumatologists investigating it.
“There is just nothing out there like this for either lupus, or lupus nephritis,” said Dr. Meara, a rheumatologist at Ohio State University, Columbus. “Lupus nephritis is a heterogeneous relapsing-remitting disease with complex medical regimens. It can have cognitive impacts and affect fertility, but these are things that may happen over decades. Patients may not be ready to talk about them early on, but they must understand them. We would like to enable the conversation between physician and patient, so they’re on the same page with everyone’s values and concerns addressed on both sides.”
But getting everyone on the same page at the same time is challenging, said Dr. Singh, a rheumatologist and professor of medicine and epidemiology at the University of Alabama at Birmingham. “This is a bit of a dilemma and a challenge. What’s great about the lupus decision aid is that it is culturally and linguistically appropriate for patients of all backgrounds, races, ages, literacy levels, and socioeconomic statuses. This levels the playing field for patients who are trying to understand their diagnosis and options to the best of their abilities.”
Three years in the making, SMILE comprises several modules addressing different facets of lupus nephritis. In simple words and concepts, the tool informs patients about what lupus is and what it can do to the kidneys, the information gleaned from blood and urine tests and what it means, and the potential consequences of not treating lupus kidney disease. The heart of the program is its breakdown of treatment options.
“It helps patients understand why they may be getting these cancer medications, and what their side effects are,” Dr. Singh said. “The tool also walks them through a comparison of the medications, with one slide on each medication and its risks and benefits. We really focus on that – about 60%-70% of the tool is comparing the drugs.”
The decision aid also contains optional modules that patients can bypass or explore. “There are separate sections on the risks and benefits of corticosteroids, on pregnancy and lactation, and on preserving fertility.”
A complete click-through of the basic information takes about 20 minutes, Dr. Singh said. An ideal time for a patient to do so would be before the initial visit to discuss treatment options, which can be an overwhelming experience. The tool could be presented in several ways. In the office, preloaded iPads could be one vehicle. These could be standalone, or linked to the patient’s electronic health record, so that any entered information could be directly transferred. At home, patients could navigate to a web link for an online version.
The first visit is a crucial moment, he said, where patients can evolve into management partners, or leave even more confused than when they arrived.
“We aim to motivate and provide tools to the patient, so that the conversation isn’t just, ‘You have the disease and we can put you on this or that drug,’ ” Dr. Singh said. “You can lose the patient right away in this scenario, while you’re talking about so many things the patient isn’t even hearing you, because she’s thinking about other issues that are important in her life, completely overwhelmed with the shock of the diagnosis and with fear.”
The Patient-Centered Outcomes Research Institute (PCORI) has funded much of the work getting SMILE up and running, including a soon-to-be-published trial that favorably compared it against the educational standard: a paper pamphlet on lupus nephritis prepared by the American College of Rheumatology.
In this study, 301 high‐risk women with lupus kidney disease, including racial/ethnic minorities with low socioeconomic status, either received the pamphlet or the SMILE tool. The group was demographically diverse: 47% black, 26% Hispanic, 15% white, and 7% Asian. Other groups made up the remainder.
More patients rated the information in the decision aid to be excellent for understanding the impact of lupus (49% vs. 33%), risk factors (43% vs. 27%), medication options (50% vs. 33%), and evidence about medications (47% vs. 24%). About half of the tool users rated the ease of use of materials as excellent (51% vs. 38%). Women who used the tool also reported much less decisional conflict for immunosuppressive drugs and were much more likely to choose the treatment option most consistent with their values, having viewed information that mattered the most for the treatment decision.
PCORI continues to support the project, too. Dr. Singh just received a $2.2 million grant to investigate the tool in 16 clinics. The 2-year observational study has three aims:
• Identify physician attitudes and patient barriers toward the tool, to guide decisions about how to best implement it in its final form.
• Track changes in subjective and objective measures of implementation effectiveness.
• Identify opportunities for disseminating the tool and develop a step‐by‐step implementation guide for incorporating the decision aid tool into regular lupus clinic visits and care pathways.
The study will also look at some patient‐centered outcomes, including decision conflict, emergency department and inpatient visits, patient‐physician communication, and implementation success. Some practice-level outcomes will be assessed, including potential barriers to implementation. “The team will focus on context and strategy as facilitators and barriers to effective implementation of the lupus decision aid in busy, clinical practices of various types – for example, private versus academic, urban versus suburban, large versus small group practice,” according to the PCORI study description.
“This study can’t solve the entire problem about patients choosing treatment or no treatment, but it can provide a start to the conversation,” Dr. Singh said. “And my hope is that once it’s available, people will modify it to fit their needs, make adaptations and modifications to keep it relevant and valuable.”
The VADT at 15 years: No legacy effect of intensive glucose control in T2DM
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
EXPERT ANALYSIS FROM ADA 2018
Phosphorus binders: The new and the old, and how to choose
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
The balance between dietary intake and excretion of phosphorus can be impaired in patients with decreased renal function, leading to hyperphosphatemia. Many patients with end-stage renal disease on dialysis require phosphorus-binding drugs to control their serum phosphorus levels.
See related editorial and article
In this review, we discuss the pathophysiology of hyperphosphatemia in kidney disease, its consequences, and how to control it, focusing on the different classes of phosphorus binders.
ROLE OF THE INTERNIST
With kidney disease common and on the increase,1 nephrologists and internists need to work together to provide optimal care.
Further, many internists in managed care plans and accountable care organizations now handle many tasks previously left to specialists—including prescribing and managing phosphorus binders in patients with kidney disease.
PATHOPHYSIOLOGY OF HYPERPHOSPHATEMIA
The pathophysiology of bone mineral disorders in kidney disease is complex. To simplify the discussion, we will address it in 3 parts:
- Phosphorus balance
- The interplay of hormones, including fibroblast growth factor 23 (FGF23)
- The mechanism of hyperphosphatemia in kidney disease.
Phosphorus balance
Phosphorus is a macronutrient essential for a range of cellular functions that include structure, energy production, metabolism, and cell signaling. It exists primarily in the form of inorganic phosphate.
An average Western diet provides 20 mg of phosphorus per kilogram of body weight per day. Of this, 13 mg/kg is absorbed, and the rest is excreted in the feces.2
Absorption of dietary phosphorus occurs mainly in the jejunum. It is mediated by both a paracellular sodium-independent pathway (driven by high intraluminal phosphorus content) and by active sodium-dependent cotransporters. It is also influenced by diet and promoted by active vitamin D (1,25 dihydroxyvitamin D3, also called calcitriol).3
Absorbed phosphorus enters the extracellular fluid and shifts in and out of the skeleton under the influence of parathyroid hormone.
Phosphorus excretion is handled almost entirely by the kidneys. Phosphorus is freely filtered at the glomerulus and reabsorbed mainly in the proximal tubule by sodium-phosphate cotransporters.
Normally, when phosphorus intake is adequate, most of the filtered phosphorus is reabsorbed and only 10% to 20% is excreted in the urine. However, the threshold for phosphorus reabsorption in the proximal tubule is influenced by parathyroid hormone, FGF23, and dietary phosphorus intake: low serum phosphate levels lead to an increase in the synthesis of sodium-phosphorus cotransporters, resulting in increased (nearly complete) proximal reabsorption and an increase in the serum phosphorus concentration.4 Conversely, both parathyroid hormone and FGF23 are phosphaturic and decrease the number of phosphorus transporters, which in turn leads to increased phosphorus excretion and a decrease in serum phosphorus concentration.5
Interplay of hormones
FGF23 is a phosphaturic glycoprotein secreted by osteoblasts and osteocytes. It acts by binding to fibroblastic growth receptor 1 in the presence of its coreceptor, the Klotho protein.6
FGF23 is regulated by serum phosphorus levels and plays a major role in the response to elevated serum phosphorus. It causes a direct increase in urinary phosphorus excretion, a decrease in intestinal phosphorus absorption (indirectly via inhibition of calcitriol), and decreased bone resorption via a decrease in parathyroid hormone production.7
Mechanism of hyperphosphatemia in kidney disease
In chronic kidney disease, phosphorus retention can trigger secondary hyperparathyroidism, as rising phosphorus levels stimulate FGF23. In the early stages of chronic kidney disease, this response can correct the phosphorus levels, but with several consequences:
- Decreased calcitriol due to its inhibition by FGF239
- Hypocalcemia due to decreased calcitriol (leading to decreased intestinal calcium absorption) and calcium binding of retained phosphorus
- Elevated parathyroid hormone due to low calcitriol levels (lack of inhibitory feedback by calcitriol), hyperphosphatemia, and hypocalcemia (direct parathyroid hormone stimulation).
As the elevated phosphorus level is likely to be the triggering event behind secondary renal hyperparathyroidism, it needs to be controlled. This is accomplished by restricting dietary phosphorus and using phosphorus binders.
HYPERPHOSPHATEMIA MAY LEAD TO VASCULAR CALCIFICATION
Elevated serum phosphorus levels (normal range 2.48–4.65 mg/dL in adults11) are associated with cardiovascular calcification and subsequent increases in mortality and morbidity rates. Elevations in serum phosphorus and calcium levels are associated with progression in vascular calcification12 and likely account for the accelerated vascular calcification that is seen in kidney disease.13
Hyperphosphatemia has been identified as an independent risk factor for death in patients with end-stage renal disease,14 but that relationship is less clear in patients with chronic kidney disease. A study in patients with chronic kidney disease and not on dialysis found a lower mortality rate in those who were prescribed phosphorus binders,15 but the study was criticized for limitations in its design.
Hyperphosphatemia can also lead to adverse effects on bone health due to complications such as renal osteodystrophy.
However, in its 2017 update, the Kidney Disease: Improving Global Outcomes (KDIGO) program only “suggests” lowering elevated phosphorus levels “toward” the normal range in patients with chronic kidney disease stages G3a through G5D, ie, those with glomerular filtration rates less than 60 mL/min/1.73 m2, including those on dialysis. The recommendation is graded 2C, ie, weak, based on low-quality evidence (https://kdigo.org/guidelines/ckd-mbd).
DIETARY RESTRICTION OF PHOSPHORUS
Diet is the major source of phosphorus intake. The average daily phosphorus consumption is 20 mg/kg, or 1,400 mg, and protein is the major source of dietary phosphorus.
In patients with stage 4 or 5 chronic kidney disease, the Kidney Disease Outcomes Quality Initiative recommends limiting protein intake to 0.6 mg/kg/day.16 However, in patients on hemodialysis, they recommend increasing protein intake to 1.1 mg/kg/day while limiting phosphorus intake to about 800 to 1,000 mg/day. This poses a challenge, as limiting phosphorus intake can reduce protein intake.
Sources of protein can be broadly classified as plant-based or animal-based. Animal protein contains organic phosphorus, which is easily absorbed.18 Plant protein may not be absorbed as easily.
Moe et al19 studied the importance of the protein source of phosphorus after 7 days of controlled diets. Despite equivalent protein and phosphorus concentrations in the vegetarian and meat-based diets, participants on the vegetarian diet had lower serum phosphorus levels, a trend toward lower 24-hour urinary phosphorus excretion, and significantly lower FGF23 levels than those on the meat-based diet. This suggests that a vegetarian diet may have advantages in terms of preventing hyperphosphatemia.
Another measure to reduce phosphorus absorption from meat is to boil it, which reduces the phosphorus content by 50%.20
Processed foods containing additives and preservatives are very high in phosphorus21 and should be avoided, particularly as there is no mandate to label phosphorus content in food.
PHOSPHORUS AND DIALYSIS
Although hemodialysis removes phosphorus, it does not remove enough to keep levels within normal limits. Indeed, even when patients adhere to a daily phosphorus limit of 1,000 mg, phosphorus accumulates. If 70% of the phosphorus in the diet is absorbed, this is 4,500 to 5,000 mg in a week. A 4-hour hemodialysis session will remove only 1,000 mg of phosphorus, which equals about 3,000 mg for patients undergoing dialysis 3 times a week,22 far less than phosphorus absorption.
In patients on continuous ambulatory peritoneal dialysis, a daily regimen of 4 exchanges of 2 L per exchange removes about 200 mg of phosphorus per day. In a 2012 study, patients on nocturnal dialysis or home dialysis involving longer session length had greater lowering of phosphorus levels than patients undergoing routine hemodialysis.23
Hence, phosphorus binders are often necessary in patients on routine hemodialysis or peritoneal dialysis.
PHOSPHORUS BINDERS
Phosphorus binders reduce serum phosphorus levels by binding with ingested phosphorus in the gastrointestinal tract and forming insoluble complexes that are not absorbed. For this reason they are much more effective when taken with meals. Phosphorus binders come in different formulations: pills, capsules, chewable tablets, liquids, and even powders that can be sprinkled on food.
The potency of each binder is quantified by its “phosphorus binder equivalent dose,” ie, its binding capacity compared with that of calcium carbonate as a reference.24
Phosphorus binders are broadly divided into those that contain calcium and those that do not.
Calcium-containing binders
The 2 most commonly used preparations are calcium carbonate (eg, Tums) and calcium acetate (eg, Phoslo). While these are relatively safe, some studies suggest that their use can lead to accelerated vascular calcification.25
According to KDIGO,26 calcium-containing binders should be avoided in hypercalcemia and adynamic bone disease. Additionally, the daily elemental calcium intake from binders should be limited to 1,500 mg, with a total daily intake that does not exceed 2,000 mg.
The elemental calcium content of calcium carbonate is about 40% of its weight (eg, 200 mg of elemental calcium in a 500-mg tablet of Tums), while the elemental calcium content of calcium acetate is about 25%. Therefore, a patient who needs 6 g of calcium carbonate for efficacy will be ingesting 2.4 g of elemental calcium per day, and that exceeds the recommended daily maximum. The main advantage of calcium carbonate is its low cost and easy availability. Commonly reported side effects include nausea and constipation.
A less commonly used calcium-based binder is calcium citrate (eg, Calcitrate). It should, however, be avoided in chronic kidney disease because of the risk of aluminum accumulation. Calcium citrate can enhance intestinal absorption of aluminum from dietary sources, as aluminum can form complexes with citrate.27
Calcium-free binders
There are several calcium-free binders. Some are based on metals such as aluminum, magnesium, iron, and lanthanum; others, such as sevelamer, are resin-based.
Aluminum- and magnesium-based binders are generally not used long-term in kidney disease because of the toxicity associated with aluminum and magnesium accumulation. However, aluminum hydroxide has an off-label use as a phosphorus binder in the acute setting, particularly when serum phosphorus levels are above 7 mg/dL.28 The dose is 300 to 600 mg 3 times daily with meals for a maximum of 4 weeks.
Sevelamer. Approved by the US Food and Drug Administration (FDA) in 1998, sevelamer acts by trapping phosphorus through ion exchange and hydrogen binding. It has the advantage of being calcium-free, which makes it particularly desirable in patients with hypercalcemia.
The Renagel in New Dialysis25 and Treat-To-Goal29 studies were randomized controlled trials that looked at the effects of sevelamer vs calcium-based binders on the risk of vascular calcification. The primary end points were serum phosphorus and calcium levels, while the secondary end points were coronary artery calcification on computed tomography and thoracic vertebral bone density. Both studies demonstrated a higher risk of vascular calcification with the calcium-based binders.
Another possible benefit of sevelamer is an improvement in lipid profile. Sevelamer lowers total cholesterol and low-density lipoprotein cholesterol levels without affecting high-density lipoprotein cholesterol or triglyceride levels.30 This is likely due to its bile acid-binding effect.31 Sevelamer has also been shown to lower C-reactive protein levels.32 While the cardiovascular profile appears to be improved with the treatment, there are no convincing data to confirm that those properties translate to a proven independent survival benefit.
The Calcium Acetate Renagel Evaluation33 was a randomized controlled study comparing sevelamer and calcium acetate. The authors attempted to control for the lipid-lowering effects of sevelamer by giving atorvastatin to all patients in both groups who had a low-density lipoprotein level greater than 70 mg/dL. The study found sevelamer to be not inferior to calcium acetate in terms of mortality and coronary calcification.
Further studies such as the Brazilian Renagel and Calcium trial34 and the Dialysis Clinical Outcomes Revisited trial failed to show a significant long-term benefit of sevelamer over calcium-based binders. However, a secondary statistical analysis of the latter study showed possible benefit of sevelamer over calcium acetate among those age 65 and older.35
To understand how sevelamer could affect vascular calcification, Yilmaz et al36 compared the effects of sevelamer vs calcium acetate on FGF23 and fetuin A levels. Fetuin A is an important inhibitor of vascular calcification and is progressively diminished in kidney disease, leading to accelerated calcification.37 Patients on sevelamer had higher levels of fetuin A than their counterparts on calcium acetate.37 The authors proposed increased fetuin A levels as a mechanism for decreased vascular calcification.
In summary, some studies suggest that sevelamer may offer the advantage of decreasing vascular calcification, but the data are mixed and do not provide a solid answer. The main disadvantages of sevelamer are a high pill burden and side effects of nausea and dyspepsia.
Lanthanum, a metallic element, was approved as a phosphorus binder by the FDA in 2008. It comes as a chewable tablet and offers the advantage of requiring the patient to take fewer pills than sevelamer and calcium-based binders.
Sucroferric oxyhydroxide comes as a chewable tablet. It was approved by the FDA in 2013. Although each tablet contains 500 mg of iron, it has not been shown to improve iron markers. In terms of phosphorus-lowering ability, it has been shown to be noninferior to sevelamer.39 Advantages include a significantly lower pill burden. Disadvantages include gastrointestinal side effects such as diarrhea and nausea and the drug’s high cost.
Ferric citrate was approved by the FDA in 2014, and 1 g delivers 210 mg of elemental iron. The main advantage of ferric citrate is its ability to increase iron markers. The phase 3 trial that demonstrated its efficacy as a binder showed an increase in ferritin compared with the active control.40 The study also showed a decrease in the need to use intravenous iron and erythropoesis-stimulating agents. This was thought to be due to improved iron stores, leading to decreased erythropoietin resistance.41
The mean number of ferric citrate tablets needed to achieve the desired phosphorus-lowering effect was 8 per day, containing 1,680 mg of iron. In comparison, oral ferrous sulfate typically provides 210 mg of iron per day.42
Disadvantages of ferric citrate include high pill burden, high cost, and gastrointestinal side effects such as nausea and constipation.
Chitosan binds salivary phosphorus. It can potentially be used, but it is not approved, and its efficacy in lowering serum phosphorus remains unclear.43
CHOOSING THE APPROPRIATE PHOSPHORUS BINDER
The choice of phosphorus binder is based on the patient’s serum calcium level and iron stores and on the drug’s side effect profile, iron pill burden, and cost. Involving patients in the choice after discussing potential side effects, pill burden, and cost is important for shared decision-making and could play a role in improving adherence.
Phosphorus binders are a major portion of the pill burden in patients with end-stage renal disease, possibly affecting patient adherence. The cost of phosphorus binders is estimated at half a billion dollars annually, underlining the significant economic impact of phosphorus control.11
Calcium-based binders should be the first choice when there is secondary hyperparathyroidism without hypercalcemia. There is no clear evidence regarding the benefit of correcting hypocalcemia, but KDIGO recommends keeping the serum calcium level within the reference range. KDIGO also recommends restricting calcium-based binders in persistent hypercalcemia, arterial calcification, and adynamic bone disease. This recommendation is largely based on expert opinion.
Noncalcium-based binders, which in theory might prevent vascular calcification, should be considered for patients with at least 1 of the following44:
- Complicated diabetes mellitus
- Vascular or valvular calcification
- Persistent inflammation.
Noncalcium-based binders are also preferred in low bone-turnover states such as adynamic bone disease, as elevated calcium can inhibit parathyroid hormone.
However, the advantage of noncalcium-based binders regarding vascular calcification is largely theoretical and has not been proven clinically. Indeed, there are data comparing long-term outcomes of the different classes of phosphorus binders, but studies were limited by short follow-up, and individual studies have lacked power to detect statistical significance between two classes of binders on long-term outcomes. Meta-analyses have provided conflicting data, with some suggesting better outcomes with sevelamer than with calcium-based binders, and with others failing to show any difference.45
Because iron deficiency is common in kidney disease, ferric citrate, which can improve iron markers, may be a suitable option, provided its cost is covered by insurance.
SPECIAL CIRCUMSTANCES FOR THE USE OF PHOSPHORUS BINDERS
Tumor lysis syndrome
Tumor lysis syndrome occurs when tumor cells release their contents into the bloodstream, either spontaneously or in response to therapy, leading to the characteristic findings of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.46 Phosphorus binders in conjunction with intravenous hydration are used to treat hyperphosphatemia, but evidence about their efficacy in this setting is limited.
Hypocalcemia in tumor lysis syndrome is usually not treated unless symptomatic, as the calcium-phosphorus product can increase, leading to calcium phosphate crystallization. When the calcium-phosphorus product is greater than 60, there is a higher risk of calcium phosphate deposition in the renal tubules that can lead to acute renal failure in tumor lysis syndrome.47 To lower the risk of calcium phosphate crystallization, calcium-based binders should be avoided in tumor lysis syndrome.
Total parenteral nutrition
Since patients on total parenteral nutrition do not eat, phosphorus binders are considered ineffective; there are no concrete data showing that phosphorus binders are effective in these patients.48 In patients with kidney disease, the phosphorus content in the parenteral nutrition formulation must be reduced.
Pregnancy
Data on phosphorus binders in pregnancy are limited. Calcium can cross the placenta. Calcium carbonate can be used in pregnancy, and fetal harm is not expected if calcium concentrations are within normal limits.49 Calcium acetate, sevelamer, and lanthanum are considered pregnancy category C drugs. Patients with advanced chronic kidney disease and end-stage renal disease who become pregnant must receive specialized obstetric care for high-risk pregnancy.
FUTURE DIRECTIONS
Future therapies may target FGF23 and other inflammatory markers that are up-regulated in renal hyperparathyroidism. However, trials studying these markers are needed to provide a better understanding of their role in bone mineral and cardiovascular health and in overall long-term outcomes. Additionally, randomized controlled trials are needed to study long-term nonsurrogate outcomes such as reduction in cardiovascular disease and rates of overall mortality.
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis 2013; 61(1 suppl 1):A7,e1–476. doi:10.1053/j.ajkd.2012.11.031
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. doi:10.1146/annurev.nutr.25.050304.092642
- Lederer E. Regulation of serum phosphate. J Physiol 2014; 592(18):3985–3995. doi:10.1113/jphysiol.2014.273979
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5):502–506. doi:10.1097/MNH.0000000000000053
- Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 2012; 303(3):F321–F327. doi:10.1152/ajprenal.00093.2012
- Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 2013; 62(3):457–473. doi:10.1053/j.ajkd.2013.03.042
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92(1):131–155. doi:10.1152/physrev.00002.2011
- Nissenson RA, Juppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed. Ames, IA: Wiley-Blackwell; 2013:208–214.
- Chauhan V, Kelepouris E, Chauhan N, Vaid M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. South Med J 2012; 105(9):479–485. doi:10.1097/SMJ.0b013e318261f7fe
- Slatopolsky E, Robson AM, Elkan I, Bricker NS. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47(8):1865–1874. doi:10.1172/JCI105877
- Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol 2016; 11(6):1088–1100. doi:10.2215/CJN.11901115
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15(8):2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31(4):607–617. pmid:9531176
- Bhandari SK, Liu IA, Kujubu DA, et al. Use of phosphorus binders among non-dialysis chronic kidney disease patients and mortality outcomes. Am J Nephrol 2017; 45(5):431–441. doi:10.1159/000474959
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 suppl 2):S1–S140. pmid:10895784
- Streja E, Lau WL, Goldstein L, et al. Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 2013; 3(5):462–468. doi:10.1038/kisup.2013.96
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5(3):519–530. doi:10.2215/CJN.06080809
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Cupisti A, Comar F, Benini O, et al. Effect of boiling on dietary phosphate and nitrogen intake. J Ren Nutr 2006; 16(1):36–40. doi:10.1053/j.jrn.2005.10.005
- Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003; 16(3):186–188. pmid:12753675
- Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18(2):217–224. pmid:1867178
- Daugirdas JT, Chertow GM, Larive B, et al; Frequent Hemodialysis Network (FHN) Trial Group. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 2012; 23(4):727–738. doi:10.1681/ASN.2011070688
- Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial 2011; 24(1):41–49. doi:10.1111/j.1525-139X.2011.00849.x
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005; 68(4):1815–1824. doi:10.1111/j.1523-1755.2005.00600.x
- National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–S201. pmid:14520607
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int 1990; 38(5):937–941. pmid:2266679
- Schucker JJ, Ward KE. Hyperphosphatemia and phosphate binders. Am J Health Syst Pharm 2005; 62(22):2355–2361. doi:10.2146/ajhp050198
- Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62(1):245–252. doi:10.1046/j.1523-1755.2002.00434.x
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant 1999; 14(12):2907–2914. pmid:10570096
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int 2002; 62(2):611–619. doi:10.1046/j.1523-1755.2002.00459.x
- Yamada K, Fujimoto S, Tokura T, et al. Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 2005; 27(4):361–365. pmid:16060120
- Qunibi W, Moustafa M, Muenz LR, et al; CARE-2 Investigators. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51(6):952–965. doi:10.1053/j.ajkd.2008.02.298
- Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRIC study. Nephron Clin Pract 2008; 110(4):c273–c283. doi:10.1159/000170783
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 2011; 26(2):402–407. doi:10.1093/ndt/gfq691
- Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59(2):177–185. doi:10.1053/j.ajkd.2011.11.007
- Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010; 21(1):103–112. doi:10.1681/ASN.2009060640
- Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology (Carlton) 2016; 21(12):987–994. doi:10.1111/nep.12864
- Floege J, Covic AC, Ketteler M, et al; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86(3):638–647. doi:10.1038/ki.2014.58
- Lewis JB, Sika M, Koury MJ, et al; Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26(2):493–503. doi:10.1681/ASN.2014020212
- Liu K, Kaffes AJ. Iron deficiency anemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol 2012; 24(2):109–116. doi:10.1097/MEG.0b013e32834f3140
- Shah HH, Hazzan AD, Fishbane S. Novel iron-based phosphate binders in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24(4):330–335. doi:10.1097/MNH.0000000000000128
- Eknoyan G. Salivary phosphorus binding: a novel approach to control hyperphosphatemia. J Am Soc Nephrol 2009; 20(3):460–462. doi:10.1681/ASN.2009010067
- Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 2010; 5(suppl 1):S31–S40. doi:10.2215/CJN.05880809
- Airy M, Winkelmayer WC, Navaneethan SD. Phosphate binders: the evidence gap persists. Am J Kidney Dis 2016; 68(5):667–670. doi:10.1053/j.ajkd.2016.08.008
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364(19):1844–1854. doi:10.1056/NEJMra0904569
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol 2004; 15(1):175–176. pmid:14679140
- Suzuki NT. Hyperphosphatemia in nondialyzed TPN patients. JPEN J Parenter Enteral Nutr 1987; 11(5):512. doi:10.1177/0148607187011005512
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
KEY POINTS
- Serum phosphorus is maintained within normal levels in a tightly regulated system involving interplay between organs, hormones, diet, and other factors.
- Dietary phosphorus comes mainly from protein, so restricting phosphorus without introducing protein deficiency is difficult. Food with a low phosphorus-to-protein ratio and plant-based sources of protein may be preferable.
- Although dialysis removes phosphorus, it usually does not remove enough, and many patients require phosphorus-binding drugs.
- Selection of an appropriate binder should consider serum calcium levels, pill burden, serum iron stores, and cost.
Phosphorus in kidney disease: Culprit or bystander?
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317