User login
Skip Potassium After Cardiac Surgery
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
LONDON —
“The widespread practice of giving patients potassium after bypass heart surgery even though their blood levels are within the normal range can be abandoned,” said Benjamin O’Brien, MD, PhD, director of the Clinic for Cardioanesthesiology and Intensive Care Medicine at Charité Hospital in Berlin, Germany.
Results from the randomized TIGHT-K trial that assessed two levels of potassium supplementation were presented at the annual congress of the European Society of Cardiology.
In the tight-control group, supplementation was provided to maintain high-normal levels of potassium (> 4.5 mEq/L). In the relaxed-control group, supplementation was provided only when potassium levels fell below the low-normal threshold (< 3.6 mEq/L).
Trial Upending Popular Practice
The multinational trial involved 23 centers in Germany and the United Kingdom. All 1690 participants enrolled were scheduled to undergo a coronary artery bypass graft procedure, but Dr. O’Brien said he considers the results of TIGHT-K to be broadly applicable.
“There is no physiological basis to expect a different result in patients undergoing different types of cardiac surgery,” he said.
The primary endpoint was clinically and electrocardiography confirmed new-onset atrial fibrillation that occurred in the 5 days after the bypass procedure.
For the primary atrial fibrillation endpoint, event rates were similar in the tight-control and the relaxed-control groups (26.2% vs 27.8%); the 1.7% difference did not approach statistical significance (P = .44). The difference in dysrhythmias other than atrial fibrillation, although numerically lower in the tight-control group, was also not significant (19.1% vs 21.1%; P = .26).
There were no significant differences in several secondary endpoints, including length of hospital stay and in-patient mortality, but cost, a prespecified secondary endpoint, was approximately $120 lower per patient in the relaxed-control group than in the tight-control group (P < .001).
Lowering Cost Across Cardiac Surgeries
During the 5-day follow-up, median potassium levels were higher in the tight-control group. Levels in both groups fell gradually, but essentially in parallel, over the study period, so median potassium levels were always higher in the tight-control group than in the relaxed-control group. At the end of the observation period, mean potassium levels were 4.34 mEq/L in the tight-control group and 4.08 mEq/L in the relaxed-control group.
Prior to the development of atrial fibrillation, participants in the tight-control group received a medium of seven potassium administrations (range, 4-12), whereas those in the relaxed-control group received a medium of zero.
There were no significant differences in episodes in any subgroup evaluated, including those divided by age, sex, baseline left ventricular ejection fraction, and the absence or presence of beta blockers or loop diuretics. A per-protocol analysis also failed to show any advantage for tight potassium control.
Atrial fibrillation occurs in about one third of patients after bypass surgery, as it does after many types of cardiac surgery. Institutions often have strategies in place to reduce the risk after cardiac surgery, and potassium supplementation is one of the most common, despite the lack of supportive evidence, Dr. O’Brien said.
Narrow Window for Optimal Potassium Levels
The difference in potassium levels between the tight-control group and the relaxed-control group were modest in this study, said Subodh Verma, MD, a cardiac surgeon at St Michael’s Hospital and professor at the University of Toronto, Ontario, Canada.
However, this is unavoidable and central to the question being posed, Dr. O’Brien pointed out. Because of the risks for both hypokalemia and hyperkalemia, the window for safe supplementation is short. Current practice is to achieve high-normal levels to reduce atrial fibrillation, but TIGHT-K demonstrates this has no benefit.
The conclusion of TIGHT-K is appropriate, said Faiez Zannad, MD, PhD, professor of therapeutics in the Division of Cardiology at the University of Lorraine in Nancy, France, who praised the design and conduct of the study.
He acknowledged an unmet need for effective methods to reduce the risk for atrial fibrillation after cardiac surgery, but the ESC invited discussant said it is now necessary to look at other strategies. Several are under current evaluation, such as supplementary magnesium and the use of sodium-glucose transporter-2 inhibitors.
Although Dr. Zannad encouraged more studies of methods to reduce atrial fibrillation risk after cardiac surgery, he said that TIGHT-K has answered the question of whether potassium supplementation is beneficial.
Potassium supplementation should no longer be offered, he said, which will “reduce healthcare costs and decrease patient risk from an unnecessary intervention.”
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2024
FDA ‘Recalls’ Often Leave Targeted Medical Devices in Use
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Less Invasive, Overlooked Option in Cardiac Surgery May Offer Benefit
Compared with traditional replacement valves, sutureless valves placed through minimally invasive cardiac surgery have less data supporting their use but offer unique features that might make them the preferred option for certain patients, reported specialists.
The sutureless device known as Perceval (Corcym) and a rapidly deployed device called Intuity (Edwards Lifesciences) are used as an alternative to surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR). But despite being commercially available since 2016, the devices are still not being used much.
The devices are not discussed in substantial detail in either the joint guidelines from the American College of Cardiology and American Heart Association issued in 2020 or guidelines from the European Society of Cardiology issued in 2022.
Cristiano Spadaccio, MD, PhD, a cardiothoracic surgeon associated with Lancashire Cardiac Centre in Blackpool, England, and his colleagues reviewed the small number of studies evaluating the alternate approach to “make the cardiology world aware” of alternatives “that can relieve the surgical burden by minimizing the implantation time and length of the operation,” he said.
The comprehensive review is published in the Journal of the American College of Cardiology.
A Neglected Alternative
The sutureless Perceval device is held in place by a stent frame that self-expands. The Intuity device also relies primarily on its framework to anchor the valve in place but does involve three sutures. Both devices are still referred to as sutureless in the new review of them.
Only a small number of centers perform minimally invasive cardiac surgeries, and the main advantage of the devices — rapid deployment — has been eroded with the advent of automated knotting which has significantly reduced the time to implant and sutured valve.
The underuse of these devices is largely caused by the limited amount of comparative and prospective data, said Dr. Spadaccio. “The entire literature on sutureless aortic valve replacement with the exception of one randomized controlled trial is observational.”
That trial, PERSIST-AVR, found that the sutureless valves were just as good as conventional ones when it comes to major adverse cardiovascular events including all-cause death, myocardial infarction, stroke, or valve reintervention at 1 year.
In a subanalysis limited to patients who had isolated aortic valve replacement, the sutureless procedure was associated with lower adverse events (5.2% vs 10.8%) at the cost of a higher rate of pacemaker implantation (11% vs 1.6%).
There are also multiple retrospective studies and registries that have generated observational data comparing sutureless aortic valve replacement with SAVR and TAVR in various patient populations, said Dr. Spadaccio, and the review was based on more than a dozen studies published since 2015. Long-term follow-up data for sutureless aortic valve replacements, which now exceeds 10 years, suggest rates of structural valve deterioration and reintervention have been acceptably low.
The minimally invasive procedures have other advantages too. For example, relative to the greater trauma associated with open heart surgery, minimally invasive surgeries typically involve faster recovery, an advantage likely to appeal to many patients who are candidates for either.
Quicker Recovery
Collectively, these data suggest that sutureless aortic valve replacement might be a reasonable or even a more appropriate alternative to either SAVR or TAVR when considering specific patient characteristics and goals, according to the review, which included an algorithm identifying specifically where sutureless aortic valve replacement fits with SAVR and TAVR.
“The algorithm is based on different clinical scenarios and reflects current guidelines for SAVR,” said Dr. Spadaccio. For example, current guidelines identify SAVR as preferred in patients younger than 65 years and in older patients with a low Society of Thoracic Surgeons (STS) score, but there are many instances in which sutureless aortic valve replacement might be more attractive, such as in those also undergoing mitral valve repair, coronary artery bypass grafting, or another surgical procedure.
Dr. Spadaccio said that the STS score should not be considered in isolation when evaluating a patient for SAVR or TAVR. Other features such as mobility, frailty score, and comorbid liver or renal disease should also be considered when discussing the three options with patients. As a result, the algorithm emphasizes a detailed evaluation of patient characteristics in selecting one procedure over another.
“The treatment should be really tailored on the individual patient basis,” said Dr. Spadaccio.
Dr. Spadaccio acknowledged that there is a need for more comparative trials, particularly in regard to sutureless aortic valve replacement as an alternative to TAVR. “I really think that a 1:1 RCT on sutureless aortic valve replacement vs TAVR could give better answers to all of these interrogatives.”
But despite the limitations outlined in this review, Dr. Spadaccio and colleagues challenged the perception that current data are not sufficient to allow clinicians to consider sutureless aortic valve replacement in the mix of options.
A Viable Option
This comprehensive summary of what is known about sutureless aortic valve replacement compared with the other options addresses an important knowledge gap, said S. Chris Malaisrie, MD, a cardiac surgeon at Northwestern University Feinberg School of Medicine, Chicago, Illinois.
He said he agrees this option has unique qualities. “Minimally invasive surgery has been largely ignored by guideline writers, but patients certainly demand options that are less invasive than standard open heart surgery. Sutureless and rapid deployment valves facilitate minimally invasive surgery and offer an advantageous option for younger patients.”
Dr. Malaisrie said the review is generating discussion about a potentially valuable option within the cardiology community. And that is exactly what Dr. Spadaccio was hoping for. “This paper was meant to educate as much as possible on these details to assist and inform decision-making,” he said.
A version of this article first appeared on Medscape.com.
Compared with traditional replacement valves, sutureless valves placed through minimally invasive cardiac surgery have less data supporting their use but offer unique features that might make them the preferred option for certain patients, reported specialists.
The sutureless device known as Perceval (Corcym) and a rapidly deployed device called Intuity (Edwards Lifesciences) are used as an alternative to surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR). But despite being commercially available since 2016, the devices are still not being used much.
The devices are not discussed in substantial detail in either the joint guidelines from the American College of Cardiology and American Heart Association issued in 2020 or guidelines from the European Society of Cardiology issued in 2022.
Cristiano Spadaccio, MD, PhD, a cardiothoracic surgeon associated with Lancashire Cardiac Centre in Blackpool, England, and his colleagues reviewed the small number of studies evaluating the alternate approach to “make the cardiology world aware” of alternatives “that can relieve the surgical burden by minimizing the implantation time and length of the operation,” he said.
The comprehensive review is published in the Journal of the American College of Cardiology.
A Neglected Alternative
The sutureless Perceval device is held in place by a stent frame that self-expands. The Intuity device also relies primarily on its framework to anchor the valve in place but does involve three sutures. Both devices are still referred to as sutureless in the new review of them.
Only a small number of centers perform minimally invasive cardiac surgeries, and the main advantage of the devices — rapid deployment — has been eroded with the advent of automated knotting which has significantly reduced the time to implant and sutured valve.
The underuse of these devices is largely caused by the limited amount of comparative and prospective data, said Dr. Spadaccio. “The entire literature on sutureless aortic valve replacement with the exception of one randomized controlled trial is observational.”
That trial, PERSIST-AVR, found that the sutureless valves were just as good as conventional ones when it comes to major adverse cardiovascular events including all-cause death, myocardial infarction, stroke, or valve reintervention at 1 year.
In a subanalysis limited to patients who had isolated aortic valve replacement, the sutureless procedure was associated with lower adverse events (5.2% vs 10.8%) at the cost of a higher rate of pacemaker implantation (11% vs 1.6%).
There are also multiple retrospective studies and registries that have generated observational data comparing sutureless aortic valve replacement with SAVR and TAVR in various patient populations, said Dr. Spadaccio, and the review was based on more than a dozen studies published since 2015. Long-term follow-up data for sutureless aortic valve replacements, which now exceeds 10 years, suggest rates of structural valve deterioration and reintervention have been acceptably low.
The minimally invasive procedures have other advantages too. For example, relative to the greater trauma associated with open heart surgery, minimally invasive surgeries typically involve faster recovery, an advantage likely to appeal to many patients who are candidates for either.
Quicker Recovery
Collectively, these data suggest that sutureless aortic valve replacement might be a reasonable or even a more appropriate alternative to either SAVR or TAVR when considering specific patient characteristics and goals, according to the review, which included an algorithm identifying specifically where sutureless aortic valve replacement fits with SAVR and TAVR.
“The algorithm is based on different clinical scenarios and reflects current guidelines for SAVR,” said Dr. Spadaccio. For example, current guidelines identify SAVR as preferred in patients younger than 65 years and in older patients with a low Society of Thoracic Surgeons (STS) score, but there are many instances in which sutureless aortic valve replacement might be more attractive, such as in those also undergoing mitral valve repair, coronary artery bypass grafting, or another surgical procedure.
Dr. Spadaccio said that the STS score should not be considered in isolation when evaluating a patient for SAVR or TAVR. Other features such as mobility, frailty score, and comorbid liver or renal disease should also be considered when discussing the three options with patients. As a result, the algorithm emphasizes a detailed evaluation of patient characteristics in selecting one procedure over another.
“The treatment should be really tailored on the individual patient basis,” said Dr. Spadaccio.
Dr. Spadaccio acknowledged that there is a need for more comparative trials, particularly in regard to sutureless aortic valve replacement as an alternative to TAVR. “I really think that a 1:1 RCT on sutureless aortic valve replacement vs TAVR could give better answers to all of these interrogatives.”
But despite the limitations outlined in this review, Dr. Spadaccio and colleagues challenged the perception that current data are not sufficient to allow clinicians to consider sutureless aortic valve replacement in the mix of options.
A Viable Option
This comprehensive summary of what is known about sutureless aortic valve replacement compared with the other options addresses an important knowledge gap, said S. Chris Malaisrie, MD, a cardiac surgeon at Northwestern University Feinberg School of Medicine, Chicago, Illinois.
He said he agrees this option has unique qualities. “Minimally invasive surgery has been largely ignored by guideline writers, but patients certainly demand options that are less invasive than standard open heart surgery. Sutureless and rapid deployment valves facilitate minimally invasive surgery and offer an advantageous option for younger patients.”
Dr. Malaisrie said the review is generating discussion about a potentially valuable option within the cardiology community. And that is exactly what Dr. Spadaccio was hoping for. “This paper was meant to educate as much as possible on these details to assist and inform decision-making,” he said.
A version of this article first appeared on Medscape.com.
Compared with traditional replacement valves, sutureless valves placed through minimally invasive cardiac surgery have less data supporting their use but offer unique features that might make them the preferred option for certain patients, reported specialists.
The sutureless device known as Perceval (Corcym) and a rapidly deployed device called Intuity (Edwards Lifesciences) are used as an alternative to surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR). But despite being commercially available since 2016, the devices are still not being used much.
The devices are not discussed in substantial detail in either the joint guidelines from the American College of Cardiology and American Heart Association issued in 2020 or guidelines from the European Society of Cardiology issued in 2022.
Cristiano Spadaccio, MD, PhD, a cardiothoracic surgeon associated with Lancashire Cardiac Centre in Blackpool, England, and his colleagues reviewed the small number of studies evaluating the alternate approach to “make the cardiology world aware” of alternatives “that can relieve the surgical burden by minimizing the implantation time and length of the operation,” he said.
The comprehensive review is published in the Journal of the American College of Cardiology.
A Neglected Alternative
The sutureless Perceval device is held in place by a stent frame that self-expands. The Intuity device also relies primarily on its framework to anchor the valve in place but does involve three sutures. Both devices are still referred to as sutureless in the new review of them.
Only a small number of centers perform minimally invasive cardiac surgeries, and the main advantage of the devices — rapid deployment — has been eroded with the advent of automated knotting which has significantly reduced the time to implant and sutured valve.
The underuse of these devices is largely caused by the limited amount of comparative and prospective data, said Dr. Spadaccio. “The entire literature on sutureless aortic valve replacement with the exception of one randomized controlled trial is observational.”
That trial, PERSIST-AVR, found that the sutureless valves were just as good as conventional ones when it comes to major adverse cardiovascular events including all-cause death, myocardial infarction, stroke, or valve reintervention at 1 year.
In a subanalysis limited to patients who had isolated aortic valve replacement, the sutureless procedure was associated with lower adverse events (5.2% vs 10.8%) at the cost of a higher rate of pacemaker implantation (11% vs 1.6%).
There are also multiple retrospective studies and registries that have generated observational data comparing sutureless aortic valve replacement with SAVR and TAVR in various patient populations, said Dr. Spadaccio, and the review was based on more than a dozen studies published since 2015. Long-term follow-up data for sutureless aortic valve replacements, which now exceeds 10 years, suggest rates of structural valve deterioration and reintervention have been acceptably low.
The minimally invasive procedures have other advantages too. For example, relative to the greater trauma associated with open heart surgery, minimally invasive surgeries typically involve faster recovery, an advantage likely to appeal to many patients who are candidates for either.
Quicker Recovery
Collectively, these data suggest that sutureless aortic valve replacement might be a reasonable or even a more appropriate alternative to either SAVR or TAVR when considering specific patient characteristics and goals, according to the review, which included an algorithm identifying specifically where sutureless aortic valve replacement fits with SAVR and TAVR.
“The algorithm is based on different clinical scenarios and reflects current guidelines for SAVR,” said Dr. Spadaccio. For example, current guidelines identify SAVR as preferred in patients younger than 65 years and in older patients with a low Society of Thoracic Surgeons (STS) score, but there are many instances in which sutureless aortic valve replacement might be more attractive, such as in those also undergoing mitral valve repair, coronary artery bypass grafting, or another surgical procedure.
Dr. Spadaccio said that the STS score should not be considered in isolation when evaluating a patient for SAVR or TAVR. Other features such as mobility, frailty score, and comorbid liver or renal disease should also be considered when discussing the three options with patients. As a result, the algorithm emphasizes a detailed evaluation of patient characteristics in selecting one procedure over another.
“The treatment should be really tailored on the individual patient basis,” said Dr. Spadaccio.
Dr. Spadaccio acknowledged that there is a need for more comparative trials, particularly in regard to sutureless aortic valve replacement as an alternative to TAVR. “I really think that a 1:1 RCT on sutureless aortic valve replacement vs TAVR could give better answers to all of these interrogatives.”
But despite the limitations outlined in this review, Dr. Spadaccio and colleagues challenged the perception that current data are not sufficient to allow clinicians to consider sutureless aortic valve replacement in the mix of options.
A Viable Option
This comprehensive summary of what is known about sutureless aortic valve replacement compared with the other options addresses an important knowledge gap, said S. Chris Malaisrie, MD, a cardiac surgeon at Northwestern University Feinberg School of Medicine, Chicago, Illinois.
He said he agrees this option has unique qualities. “Minimally invasive surgery has been largely ignored by guideline writers, but patients certainly demand options that are less invasive than standard open heart surgery. Sutureless and rapid deployment valves facilitate minimally invasive surgery and offer an advantageous option for younger patients.”
Dr. Malaisrie said the review is generating discussion about a potentially valuable option within the cardiology community. And that is exactly what Dr. Spadaccio was hoping for. “This paper was meant to educate as much as possible on these details to assist and inform decision-making,” he said.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Trial of Impella Heart Pump Stopped
An international trial of the Impella heart pump in patients with ST elevation myocardial infarction (STEMI) and cardiogenic shock has been stopped by the sponsor, Abiomed Inc. The termination followed news that another international trial, DanGer Shock, found that the pump improved survival in these patients.
“I was convinced that the study could not continue,” one of the principal investigators William O’Neill, MD, an interventional cardiologist with the Henry Ford Health in Detroit, said in an interview. After 3.5 years of work and thousands of person-hours, he added, “It’s not a decision that people took lightly.”
The trial already had three sites in Europe and one in the United States up and running, with two more US sites slated to join the trial. It had started enrolling patients, although few to date.
DanGer Shock trial results were expected to have a serious effect on how RECOVER IV would unfold. It was previously uncertain whether the Impella heart pump would save lives vs existing approaches, said O’Neill and co-principal investigator Navin Kapur, MD, an interventional cardiologist at Tufts Medical Center in Boston. Once the DanGer Shock trial showed the benefits of using the heart pump, that equipoise vanished.
Loss of Clinical Equipoise
“The clinicians were challenged in getting consent from patients where they had to say, ‘If you are randomized to the control arm, we are not able to use an Impella,’ ” said Dr. Kapur. He pointed out that patients would be unlikely to agree to participate in a trial where they might not get the treatment already shown to improve survival.
Dr. Kapur and Dr. O’Neill said the clinicians participating in the trial expressed discomfort at continuing. The RECOVER IV trial was expected to take many years to enroll the targeted number of patients. To participate, hospitals had to have the equipment and expertise to use the Impella heart pump, as well as the control treatments — balloon-pump support and extracorporeal membrane oxygenation (ECMO), Dr. Kapur explained. He said most patients with STEMI and cardiogenic shock would present to their nearest community hospitals, many of which would not have these treatments and would be unable to participate in the study.
Patients with STEMI and cardiogenic shock are uncommon. About 80,000 patients in the United States each year present with cardiogenic shock, of whom about 40% are not experiencing a STEMI, said Dr. O’Neill.
But those who do fit into the population of both STEMI and cardiogenic shock are at very high risk, said Dr. Kapur. “One in three or one in two patients with STEMI and cardiogenic shock will die in hospital.”
Getting Hearts Pumping
The Impella heart pump was originally developed by Impella Cardiosystems in Aachen, Germany, which was acquired by Abiomed in 2005, according to the Abiomed website. And Abiomed was acquired by Johnson & Johnson MedTech in 2022. The company has developed a series of models over the years and said that Impella CP — the model used in DanGer Shock and RECOVER IV trials — is the world’s smallest heart pump.
“Impella is the only heart pump that can be introduced percutaneously through the leg,” said Dr. O’Neill, whereas other pumps available are used only in open-heart surgery. While Impella is the first pump to be used this way, he said it won’t be the last. Other, more powerful pumps are being developed.
DanGer Shock: A Leap Forward
Despite leading to the halt of another trial, the DanGer Shock results are a good news story, said the RECOVER IV investigators.
“The DanGer trial is a huge advance,” said Dr. O’Neill. “It’s the first study this century that shows something that improves survival in cardiogenic shock. You treat eight patients, and you save one life.” Dr. O’Neill said this is one of the best outcomes he has seen during his long career.
Dr. Kapur said the DanGer trial is also a leap forward in designing trials for cardiogenic shock. He said previous trials of mechanical support in cardiogenic shock had neutral results, probably due to broad inclusion criteria for patients.
“The DanGer trial was selective in its inclusion and exclusion criteria. That made it more difficult to enroll the population, so it took a lot longer. But it used the right device at the right time in the right patient, and it was successful,” he said.
“The DanGer investigators need to be applauded,” he added. “The lesson is, we have to design the right trials.”
New Cardiogenic Shock Trials
Dr. O’Neill and Dr. Kapur said the groundwork they laid for RECOVER IV can be used for new trials.
“We have 50 sites in the US, Germany, and Denmark. They’re interested, and they’re waiting,” said Dr. O’Neill. The researchers are poised to begin new trials once protocols are developed.
What will the next trials investigate?
DanGer Shock results showed higher rates of adverse events following Impella use than after standard care. “We need to come up with strategies to decrease bleeding problems and renal failure,” said Dr. O’Neill, and these could be tested in trials.
Other questions he would like to see investigated are using the Impella heart pump before or after angioplasty, and multi-vessel vs culprit-vessel percutaneous coronary intervention in cardiogenic shock with Impella support.
Dr. Kapur mentioned studying patients excluded from the DanGer Shock trial — such as those needing right ventricular support — because DanGer Shock covered only left ventricular support and those suffering cardiac arrest outside hospital. He said trials could compare differences between models of Impella and investigate the role of ECMO.
“I’m optimistic that we can design more randomized controlled trials with the right patient population and right treatment algorithm,” Dr. Kapur said. This is a critical step toward better outcomes for patients, he added. Another step is optimizing the design of heart pumps, which should decrease the rates of adverse events, he said. “I have a lot of optimism for the future of device design.”
A version of this article first appeared on Medscape.com.
An international trial of the Impella heart pump in patients with ST elevation myocardial infarction (STEMI) and cardiogenic shock has been stopped by the sponsor, Abiomed Inc. The termination followed news that another international trial, DanGer Shock, found that the pump improved survival in these patients.
“I was convinced that the study could not continue,” one of the principal investigators William O’Neill, MD, an interventional cardiologist with the Henry Ford Health in Detroit, said in an interview. After 3.5 years of work and thousands of person-hours, he added, “It’s not a decision that people took lightly.”
The trial already had three sites in Europe and one in the United States up and running, with two more US sites slated to join the trial. It had started enrolling patients, although few to date.
DanGer Shock trial results were expected to have a serious effect on how RECOVER IV would unfold. It was previously uncertain whether the Impella heart pump would save lives vs existing approaches, said O’Neill and co-principal investigator Navin Kapur, MD, an interventional cardiologist at Tufts Medical Center in Boston. Once the DanGer Shock trial showed the benefits of using the heart pump, that equipoise vanished.
Loss of Clinical Equipoise
“The clinicians were challenged in getting consent from patients where they had to say, ‘If you are randomized to the control arm, we are not able to use an Impella,’ ” said Dr. Kapur. He pointed out that patients would be unlikely to agree to participate in a trial where they might not get the treatment already shown to improve survival.
Dr. Kapur and Dr. O’Neill said the clinicians participating in the trial expressed discomfort at continuing. The RECOVER IV trial was expected to take many years to enroll the targeted number of patients. To participate, hospitals had to have the equipment and expertise to use the Impella heart pump, as well as the control treatments — balloon-pump support and extracorporeal membrane oxygenation (ECMO), Dr. Kapur explained. He said most patients with STEMI and cardiogenic shock would present to their nearest community hospitals, many of which would not have these treatments and would be unable to participate in the study.
Patients with STEMI and cardiogenic shock are uncommon. About 80,000 patients in the United States each year present with cardiogenic shock, of whom about 40% are not experiencing a STEMI, said Dr. O’Neill.
But those who do fit into the population of both STEMI and cardiogenic shock are at very high risk, said Dr. Kapur. “One in three or one in two patients with STEMI and cardiogenic shock will die in hospital.”
Getting Hearts Pumping
The Impella heart pump was originally developed by Impella Cardiosystems in Aachen, Germany, which was acquired by Abiomed in 2005, according to the Abiomed website. And Abiomed was acquired by Johnson & Johnson MedTech in 2022. The company has developed a series of models over the years and said that Impella CP — the model used in DanGer Shock and RECOVER IV trials — is the world’s smallest heart pump.
“Impella is the only heart pump that can be introduced percutaneously through the leg,” said Dr. O’Neill, whereas other pumps available are used only in open-heart surgery. While Impella is the first pump to be used this way, he said it won’t be the last. Other, more powerful pumps are being developed.
DanGer Shock: A Leap Forward
Despite leading to the halt of another trial, the DanGer Shock results are a good news story, said the RECOVER IV investigators.
“The DanGer trial is a huge advance,” said Dr. O’Neill. “It’s the first study this century that shows something that improves survival in cardiogenic shock. You treat eight patients, and you save one life.” Dr. O’Neill said this is one of the best outcomes he has seen during his long career.
Dr. Kapur said the DanGer trial is also a leap forward in designing trials for cardiogenic shock. He said previous trials of mechanical support in cardiogenic shock had neutral results, probably due to broad inclusion criteria for patients.
“The DanGer trial was selective in its inclusion and exclusion criteria. That made it more difficult to enroll the population, so it took a lot longer. But it used the right device at the right time in the right patient, and it was successful,” he said.
“The DanGer investigators need to be applauded,” he added. “The lesson is, we have to design the right trials.”
New Cardiogenic Shock Trials
Dr. O’Neill and Dr. Kapur said the groundwork they laid for RECOVER IV can be used for new trials.
“We have 50 sites in the US, Germany, and Denmark. They’re interested, and they’re waiting,” said Dr. O’Neill. The researchers are poised to begin new trials once protocols are developed.
What will the next trials investigate?
DanGer Shock results showed higher rates of adverse events following Impella use than after standard care. “We need to come up with strategies to decrease bleeding problems and renal failure,” said Dr. O’Neill, and these could be tested in trials.
Other questions he would like to see investigated are using the Impella heart pump before or after angioplasty, and multi-vessel vs culprit-vessel percutaneous coronary intervention in cardiogenic shock with Impella support.
Dr. Kapur mentioned studying patients excluded from the DanGer Shock trial — such as those needing right ventricular support — because DanGer Shock covered only left ventricular support and those suffering cardiac arrest outside hospital. He said trials could compare differences between models of Impella and investigate the role of ECMO.
“I’m optimistic that we can design more randomized controlled trials with the right patient population and right treatment algorithm,” Dr. Kapur said. This is a critical step toward better outcomes for patients, he added. Another step is optimizing the design of heart pumps, which should decrease the rates of adverse events, he said. “I have a lot of optimism for the future of device design.”
A version of this article first appeared on Medscape.com.
An international trial of the Impella heart pump in patients with ST elevation myocardial infarction (STEMI) and cardiogenic shock has been stopped by the sponsor, Abiomed Inc. The termination followed news that another international trial, DanGer Shock, found that the pump improved survival in these patients.
“I was convinced that the study could not continue,” one of the principal investigators William O’Neill, MD, an interventional cardiologist with the Henry Ford Health in Detroit, said in an interview. After 3.5 years of work and thousands of person-hours, he added, “It’s not a decision that people took lightly.”
The trial already had three sites in Europe and one in the United States up and running, with two more US sites slated to join the trial. It had started enrolling patients, although few to date.
DanGer Shock trial results were expected to have a serious effect on how RECOVER IV would unfold. It was previously uncertain whether the Impella heart pump would save lives vs existing approaches, said O’Neill and co-principal investigator Navin Kapur, MD, an interventional cardiologist at Tufts Medical Center in Boston. Once the DanGer Shock trial showed the benefits of using the heart pump, that equipoise vanished.
Loss of Clinical Equipoise
“The clinicians were challenged in getting consent from patients where they had to say, ‘If you are randomized to the control arm, we are not able to use an Impella,’ ” said Dr. Kapur. He pointed out that patients would be unlikely to agree to participate in a trial where they might not get the treatment already shown to improve survival.
Dr. Kapur and Dr. O’Neill said the clinicians participating in the trial expressed discomfort at continuing. The RECOVER IV trial was expected to take many years to enroll the targeted number of patients. To participate, hospitals had to have the equipment and expertise to use the Impella heart pump, as well as the control treatments — balloon-pump support and extracorporeal membrane oxygenation (ECMO), Dr. Kapur explained. He said most patients with STEMI and cardiogenic shock would present to their nearest community hospitals, many of which would not have these treatments and would be unable to participate in the study.
Patients with STEMI and cardiogenic shock are uncommon. About 80,000 patients in the United States each year present with cardiogenic shock, of whom about 40% are not experiencing a STEMI, said Dr. O’Neill.
But those who do fit into the population of both STEMI and cardiogenic shock are at very high risk, said Dr. Kapur. “One in three or one in two patients with STEMI and cardiogenic shock will die in hospital.”
Getting Hearts Pumping
The Impella heart pump was originally developed by Impella Cardiosystems in Aachen, Germany, which was acquired by Abiomed in 2005, according to the Abiomed website. And Abiomed was acquired by Johnson & Johnson MedTech in 2022. The company has developed a series of models over the years and said that Impella CP — the model used in DanGer Shock and RECOVER IV trials — is the world’s smallest heart pump.
“Impella is the only heart pump that can be introduced percutaneously through the leg,” said Dr. O’Neill, whereas other pumps available are used only in open-heart surgery. While Impella is the first pump to be used this way, he said it won’t be the last. Other, more powerful pumps are being developed.
DanGer Shock: A Leap Forward
Despite leading to the halt of another trial, the DanGer Shock results are a good news story, said the RECOVER IV investigators.
“The DanGer trial is a huge advance,” said Dr. O’Neill. “It’s the first study this century that shows something that improves survival in cardiogenic shock. You treat eight patients, and you save one life.” Dr. O’Neill said this is one of the best outcomes he has seen during his long career.
Dr. Kapur said the DanGer trial is also a leap forward in designing trials for cardiogenic shock. He said previous trials of mechanical support in cardiogenic shock had neutral results, probably due to broad inclusion criteria for patients.
“The DanGer trial was selective in its inclusion and exclusion criteria. That made it more difficult to enroll the population, so it took a lot longer. But it used the right device at the right time in the right patient, and it was successful,” he said.
“The DanGer investigators need to be applauded,” he added. “The lesson is, we have to design the right trials.”
New Cardiogenic Shock Trials
Dr. O’Neill and Dr. Kapur said the groundwork they laid for RECOVER IV can be used for new trials.
“We have 50 sites in the US, Germany, and Denmark. They’re interested, and they’re waiting,” said Dr. O’Neill. The researchers are poised to begin new trials once protocols are developed.
What will the next trials investigate?
DanGer Shock results showed higher rates of adverse events following Impella use than after standard care. “We need to come up with strategies to decrease bleeding problems and renal failure,” said Dr. O’Neill, and these could be tested in trials.
Other questions he would like to see investigated are using the Impella heart pump before or after angioplasty, and multi-vessel vs culprit-vessel percutaneous coronary intervention in cardiogenic shock with Impella support.
Dr. Kapur mentioned studying patients excluded from the DanGer Shock trial — such as those needing right ventricular support — because DanGer Shock covered only left ventricular support and those suffering cardiac arrest outside hospital. He said trials could compare differences between models of Impella and investigate the role of ECMO.
“I’m optimistic that we can design more randomized controlled trials with the right patient population and right treatment algorithm,” Dr. Kapur said. This is a critical step toward better outcomes for patients, he added. Another step is optimizing the design of heart pumps, which should decrease the rates of adverse events, he said. “I have a lot of optimism for the future of device design.”
A version of this article first appeared on Medscape.com.
Why Cardiac Biomarkers Don’t Help Predict Heart Disease
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.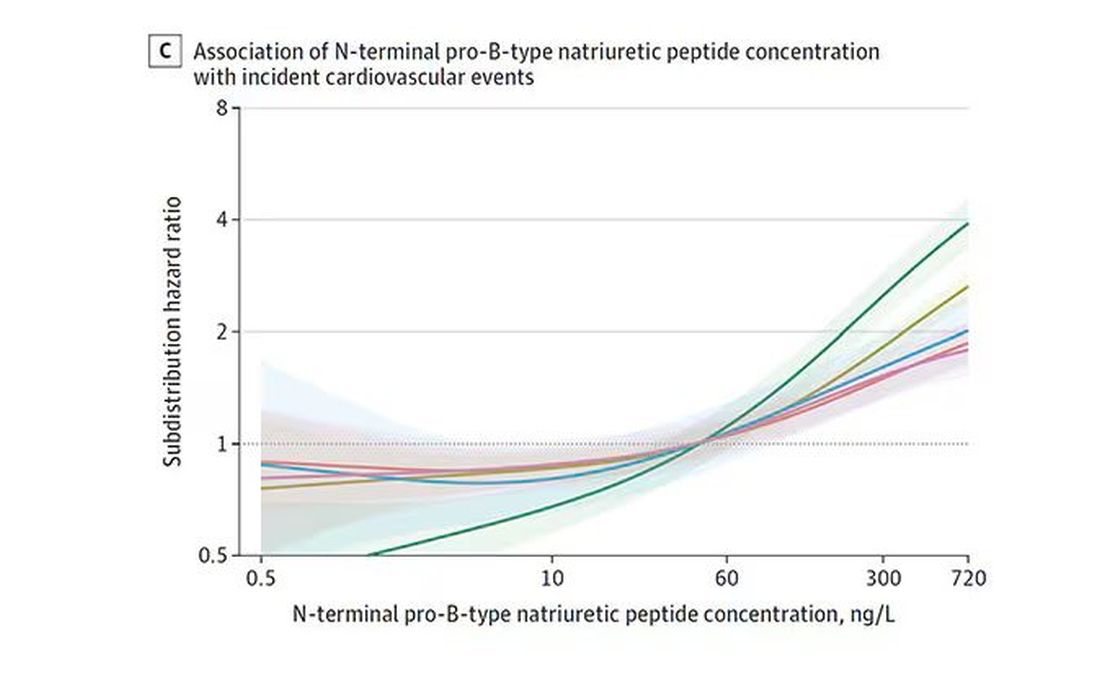
And CRP does a similar thing, with levels above 1.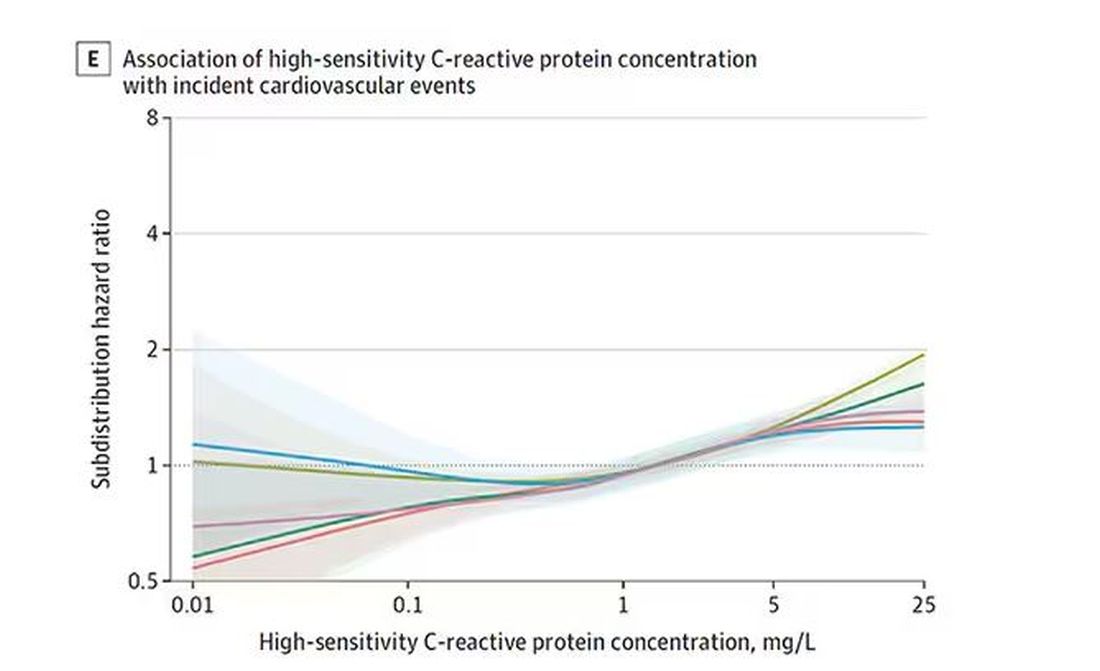
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.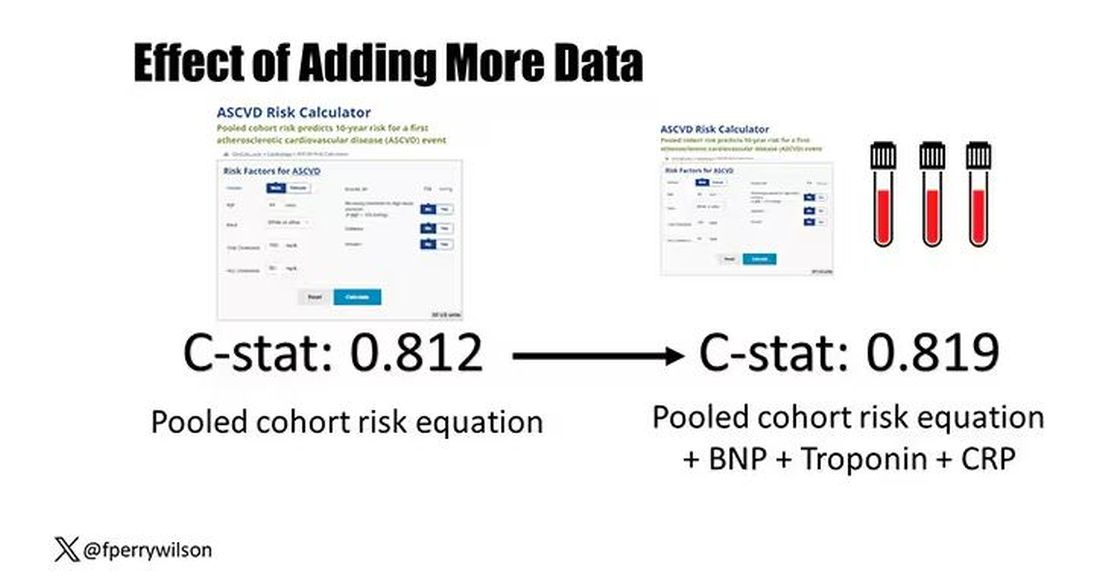
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Neutrophils Take Center Stage in Growing Understanding of Colchicine’s Role in Treating Atherosclerotic Cardiovascular Disease
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
Which Emergencies Are Genuine Emergencies?
WIESBADEN, GERMANY — Crowded waiting rooms, long wait times, irritable patients, and aggression toward nursing staff and doctors are increasingly the reality in German emergency rooms. Clearly, emergencies belong in the emergency room. However, “In about half of all patients in the emergency room, there is no urgent medical emergency,” Norbert Schütz, MD, director of geriatrics and rheumatology at Helios Dr. Horst Schmidt Hospital in Wiesbaden, Germany, said at a press conference for the 130th Annual Meeting of the German Society of Internal Medicine (DGIM).
“In our daily medical practice, we repeatedly experience people either accessing our emergency departments and ambulances too quickly or lingering at home for too long when they have severe symptoms,” said Dr. Schütz, who organized the Patient Day during the Internist Congress.
DGIM Educates Patients
What is an emergency? “I think the public is quite well informed about conditions associated with loss of consciousness, severe pain, chest pain, or paralysis: Think stroke or heart attack. This is undoubtedly a success of recent years. The difficulty arises with everything in between. For instance, should I go to the hospital with severe headaches?” asked Dr. Schütz.
When is a patient a case for the emergency room, the physician on-call service, or the general practitioner? At the Patient Day in Wiesbaden, DGIM aims to educate and train interested parties with a dedicated lecture. The focus is on recognizing an emergency, specifically emergencies in children and mental illnesses.
“Our Patient Day aims to contribute to making the right decisions. We want to inform, answer questions, and alleviate fears,” said Dr. Schütz. Interested parties can refresh their emergency knowledge, tour ambulances, and have the equipment explained. The public also has the opportunity to learn about resuscitation techniques theoretically and practically.
“Should, for whatever reason, the general practitioner not be reachable, the physician on-call service can be reached,” said Dr. Schütz. It may happen, however, that neither the general practitioner nor the on-call physician is immediately available.
What Are Emergencies?
In cases of severe health impairment, urgency is required, and a severe emergency should be assumed in the following cases:
- Chest pain
- Circulatory disorder
- Disorders of consciousness
- Breathing difficulties
- Sudden weakness or numbness/paralysis
- Severe bleeding
- Allergic shock
“In such cases, the emergency departments of the hospitals are available around the clock, and if necessary, an emergency doctor should be present during transportation to the hospital,” said Dr. Schütz.
Classifying emergencies is challenging, especially with children. “Children often find it difficult to clearly categorize or describe symptoms,” said Dr. Schütz. A situation is critical if, for example, the child’s breathing or consciousness is impaired.
Mental emergencies pose a particular challenge for patients and relatives because the patient and relatives are often overwhelmed by the situation. If there are suicidal thoughts, the patient should present him- or herself immediately to an emergency room.
“Patients who come to the emergency room because they cannot get appointments with their general practitioner or specialist, for whatever reason, are no emergency. We also see this in the emergency room from time to time,” said Dr. Schütz. Emergency rooms are not intended for this purpose. “And generally, these are not emergencies.”
Four of 10 Cases
The number of patients in emergency rooms has steadily increased in recent years. Statistically, only 4 out of 10 cases are genuine emergencies, as detailed surveys of patients in the emergency rooms of northern German hospitals have shown.
In the PiNo Nord cross-sectional study, Martin Scherer, MD, of University Hospital Hamburg-Eppendorf in Hamburg, Germany, and his team examined the reasons why patients visit the emergency room. They interviewed 1175 patients in five hospitals and documented the medical diagnoses. Patients classified as “immediately” or “very urgently” in need of treatment were excluded.
The surveyed patients were on average 41.8 years old, 52.9% were men, and 54.7% of the patients indicated a low urgency of treatment. About 41% of the patients visited the emergency room on their own initiative, 17% stated they were referred or entrusted by their general practitioner, and 8% were referred by a specialist in the emergency room.
The strongest predictors for low subjective treatment urgency were musculoskeletal trauma (odds ratio [OR], 2.18), skin afflictions (OR, 2.15), and the unavailability of an open general practitioner’s office (OR, 1.70).
According to Dr. Scherer and his colleagues, the reasons for visiting an emergency room are diverse and can be based on the perceived structural conditions and individual patient preferences in addition to the urgency of the health problem.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
WIESBADEN, GERMANY — Crowded waiting rooms, long wait times, irritable patients, and aggression toward nursing staff and doctors are increasingly the reality in German emergency rooms. Clearly, emergencies belong in the emergency room. However, “In about half of all patients in the emergency room, there is no urgent medical emergency,” Norbert Schütz, MD, director of geriatrics and rheumatology at Helios Dr. Horst Schmidt Hospital in Wiesbaden, Germany, said at a press conference for the 130th Annual Meeting of the German Society of Internal Medicine (DGIM).
“In our daily medical practice, we repeatedly experience people either accessing our emergency departments and ambulances too quickly or lingering at home for too long when they have severe symptoms,” said Dr. Schütz, who organized the Patient Day during the Internist Congress.
DGIM Educates Patients
What is an emergency? “I think the public is quite well informed about conditions associated with loss of consciousness, severe pain, chest pain, or paralysis: Think stroke or heart attack. This is undoubtedly a success of recent years. The difficulty arises with everything in between. For instance, should I go to the hospital with severe headaches?” asked Dr. Schütz.
When is a patient a case for the emergency room, the physician on-call service, or the general practitioner? At the Patient Day in Wiesbaden, DGIM aims to educate and train interested parties with a dedicated lecture. The focus is on recognizing an emergency, specifically emergencies in children and mental illnesses.
“Our Patient Day aims to contribute to making the right decisions. We want to inform, answer questions, and alleviate fears,” said Dr. Schütz. Interested parties can refresh their emergency knowledge, tour ambulances, and have the equipment explained. The public also has the opportunity to learn about resuscitation techniques theoretically and practically.
“Should, for whatever reason, the general practitioner not be reachable, the physician on-call service can be reached,” said Dr. Schütz. It may happen, however, that neither the general practitioner nor the on-call physician is immediately available.
What Are Emergencies?
In cases of severe health impairment, urgency is required, and a severe emergency should be assumed in the following cases:
- Chest pain
- Circulatory disorder
- Disorders of consciousness
- Breathing difficulties
- Sudden weakness or numbness/paralysis
- Severe bleeding
- Allergic shock
“In such cases, the emergency departments of the hospitals are available around the clock, and if necessary, an emergency doctor should be present during transportation to the hospital,” said Dr. Schütz.
Classifying emergencies is challenging, especially with children. “Children often find it difficult to clearly categorize or describe symptoms,” said Dr. Schütz. A situation is critical if, for example, the child’s breathing or consciousness is impaired.
Mental emergencies pose a particular challenge for patients and relatives because the patient and relatives are often overwhelmed by the situation. If there are suicidal thoughts, the patient should present him- or herself immediately to an emergency room.
“Patients who come to the emergency room because they cannot get appointments with their general practitioner or specialist, for whatever reason, are no emergency. We also see this in the emergency room from time to time,” said Dr. Schütz. Emergency rooms are not intended for this purpose. “And generally, these are not emergencies.”
Four of 10 Cases
The number of patients in emergency rooms has steadily increased in recent years. Statistically, only 4 out of 10 cases are genuine emergencies, as detailed surveys of patients in the emergency rooms of northern German hospitals have shown.
In the PiNo Nord cross-sectional study, Martin Scherer, MD, of University Hospital Hamburg-Eppendorf in Hamburg, Germany, and his team examined the reasons why patients visit the emergency room. They interviewed 1175 patients in five hospitals and documented the medical diagnoses. Patients classified as “immediately” or “very urgently” in need of treatment were excluded.
The surveyed patients were on average 41.8 years old, 52.9% were men, and 54.7% of the patients indicated a low urgency of treatment. About 41% of the patients visited the emergency room on their own initiative, 17% stated they were referred or entrusted by their general practitioner, and 8% were referred by a specialist in the emergency room.
The strongest predictors for low subjective treatment urgency were musculoskeletal trauma (odds ratio [OR], 2.18), skin afflictions (OR, 2.15), and the unavailability of an open general practitioner’s office (OR, 1.70).
According to Dr. Scherer and his colleagues, the reasons for visiting an emergency room are diverse and can be based on the perceived structural conditions and individual patient preferences in addition to the urgency of the health problem.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
WIESBADEN, GERMANY — Crowded waiting rooms, long wait times, irritable patients, and aggression toward nursing staff and doctors are increasingly the reality in German emergency rooms. Clearly, emergencies belong in the emergency room. However, “In about half of all patients in the emergency room, there is no urgent medical emergency,” Norbert Schütz, MD, director of geriatrics and rheumatology at Helios Dr. Horst Schmidt Hospital in Wiesbaden, Germany, said at a press conference for the 130th Annual Meeting of the German Society of Internal Medicine (DGIM).
“In our daily medical practice, we repeatedly experience people either accessing our emergency departments and ambulances too quickly or lingering at home for too long when they have severe symptoms,” said Dr. Schütz, who organized the Patient Day during the Internist Congress.
DGIM Educates Patients
What is an emergency? “I think the public is quite well informed about conditions associated with loss of consciousness, severe pain, chest pain, or paralysis: Think stroke or heart attack. This is undoubtedly a success of recent years. The difficulty arises with everything in between. For instance, should I go to the hospital with severe headaches?” asked Dr. Schütz.
When is a patient a case for the emergency room, the physician on-call service, or the general practitioner? At the Patient Day in Wiesbaden, DGIM aims to educate and train interested parties with a dedicated lecture. The focus is on recognizing an emergency, specifically emergencies in children and mental illnesses.
“Our Patient Day aims to contribute to making the right decisions. We want to inform, answer questions, and alleviate fears,” said Dr. Schütz. Interested parties can refresh their emergency knowledge, tour ambulances, and have the equipment explained. The public also has the opportunity to learn about resuscitation techniques theoretically and practically.
“Should, for whatever reason, the general practitioner not be reachable, the physician on-call service can be reached,” said Dr. Schütz. It may happen, however, that neither the general practitioner nor the on-call physician is immediately available.
What Are Emergencies?
In cases of severe health impairment, urgency is required, and a severe emergency should be assumed in the following cases:
- Chest pain
- Circulatory disorder
- Disorders of consciousness
- Breathing difficulties
- Sudden weakness or numbness/paralysis
- Severe bleeding
- Allergic shock
“In such cases, the emergency departments of the hospitals are available around the clock, and if necessary, an emergency doctor should be present during transportation to the hospital,” said Dr. Schütz.
Classifying emergencies is challenging, especially with children. “Children often find it difficult to clearly categorize or describe symptoms,” said Dr. Schütz. A situation is critical if, for example, the child’s breathing or consciousness is impaired.
Mental emergencies pose a particular challenge for patients and relatives because the patient and relatives are often overwhelmed by the situation. If there are suicidal thoughts, the patient should present him- or herself immediately to an emergency room.
“Patients who come to the emergency room because they cannot get appointments with their general practitioner or specialist, for whatever reason, are no emergency. We also see this in the emergency room from time to time,” said Dr. Schütz. Emergency rooms are not intended for this purpose. “And generally, these are not emergencies.”
Four of 10 Cases
The number of patients in emergency rooms has steadily increased in recent years. Statistically, only 4 out of 10 cases are genuine emergencies, as detailed surveys of patients in the emergency rooms of northern German hospitals have shown.
In the PiNo Nord cross-sectional study, Martin Scherer, MD, of University Hospital Hamburg-Eppendorf in Hamburg, Germany, and his team examined the reasons why patients visit the emergency room. They interviewed 1175 patients in five hospitals and documented the medical diagnoses. Patients classified as “immediately” or “very urgently” in need of treatment were excluded.
The surveyed patients were on average 41.8 years old, 52.9% were men, and 54.7% of the patients indicated a low urgency of treatment. About 41% of the patients visited the emergency room on their own initiative, 17% stated they were referred or entrusted by their general practitioner, and 8% were referred by a specialist in the emergency room.
The strongest predictors for low subjective treatment urgency were musculoskeletal trauma (odds ratio [OR], 2.18), skin afflictions (OR, 2.15), and the unavailability of an open general practitioner’s office (OR, 1.70).
According to Dr. Scherer and his colleagues, the reasons for visiting an emergency room are diverse and can be based on the perceived structural conditions and individual patient preferences in addition to the urgency of the health problem.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Telemedicine Reduces Rehospitalization, Revascularization in Post-PCI ACS Patients
ATLANTA — Patients with acute coronary syndrome (ACS) who had a myocardial infarction or unstable angina and underwent percutaneous coronary intervention (PCI) had a 76% lower rate of hospital readmission after 6 months if they participated in a remote monitoring protocol compared with similar patients who had standard post-discharge care, results of a new trial suggest.
The TELE-ACS trial showed that at 6 months, telemedicine patients also had statistically significantly lower rates of post-discharge emergency department visits, unplanned coronary revascularizations, and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness. However, the rates of major adverse cardiovascular events (MACE) were similar between the two groups. The protocol included consultation with a cardiologist who reviewed home-monitoring data.
“The team was able to aid in preventing unnecessary presentations and advised the patients to seek emergency care whenever was necessary,” Nasser Alshahrani, MSc, a clinical research fellow at Imperial College London, said while presenting the results at the American College of Cardiology meeting. “The TELE-ACS protocol provided a significant reduction in readmission rates post-ACS and other adverse events.”
The study findings were published online simultaneously in the Journal of the American College of Cardiology.
Telemedicine Protocol
The trial, conducted from January 2022 to April 2023, randomly assigned 337 patients to telemedicine or standard care when they were discharged after PCI and had at least one cardiovascular risk factor. The telemedicine protocol consisted of 12-lead electrocardiogram belt, an automated blood-pressure monitor, and a pulse oximeter.
Patients in the telemedicine arm initiated the remote monitoring protocol if they thought they had cardiac symptoms. The majority (86%) were men with what the study described as “a high preponderance of cardiovascular risk factors.” Average age was 58.1 years.
If a telemedicine patient initiated the protocol, a cardiologist remotely assessed the patient’s symptoms and channeled the patient to the appropriate care pathway, whether reassuring the patient or sending them to a primary care physician or emergency department, or to call emergency services. Patients who didn’t get a call back from the cardiologist within 15 minutes were told to seek care in the standard clinical pathway.
Telemedicine patients were given the telemonitoring package and training in how to use the devices before they were discharged. They also received three follow-up quality control calls in the first two months to ensure they were using the equipment correctly. They kept the telemonitoring equipment for 8 months, but were followed out to 9 months. Six telemedicine patients dropped out while one standard care patient withdrew from the study.
Results showed that at 6 months, telemedicine patients had statistically significantly lower rates of post-discharge emergency department visits (25% vs 37%, P < .001), unplanned coronary revascularizations (3% vs 9%, P < .01) and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness (a 13% to 18% difference for each symptom, P < .01).
MACE rates were similar between the two groups.
At 9 months, 3 months after the protocol ended, 20 telemedicine patients and 50 standard-care patients were readmitted to the hospital, while 52 and 73, respectively, went to the emergency department.
The telemedicine patients also had shorter hospital stays: an average of 0.5 and 1.2 days at 6 and 9 months, respectively, vs 1.5 and 1.8 days in the standard treatment arm (P < .001 for both).
Mr. Alshahrani noted several limitations with the study, namely that 86% of participants were men, and that the intervention was only offered to people who had smartphones. “The high level of support for the telemedicine group, with prompt cardiology responses, may be challenging to replicate outside a trial setting, requiring significant investment and training,” he added.
Human Element Key
In an interview from London after the presentation, lead author Ramzi Khamis, MB ChB, PhD, said, “This was quite a basic study. Really what we did was we integrated a clinical decision-making algorithm that we perfected with some quite novel but basic technology.” Future research should strive to add a home troponin test to the protocol and an artificial intelligence component, he said.
However, Dr. Khamis noted that human interaction was key to the success of the TELE-ACS trial. “The human factor is very important here and I think it would be really interesting to have a head-to-head comparison of human interaction with remote monitoring vs an AI-driven interaction,” he said. “I have my doubts that AI would be able to beat the human factor here.”
Lawrence Phillips, MD, medical director of outpatient cardiology at NYU Langone Heart, told this news organization that the study was appropriately powered to evaluate the telemedicine protocol, and that it could serve as a template for other studies of remote monitoring in cardiology.
“I think that this study is forming the foundation of evolving telemedicine data,” he said. “It shows really interesting results, and I’m sure it’s going to be reproduced in different ways going forward.”
While other studies have shown the utility of telemedicine to decrease unnecessary hospitalizations, this study went one step further, Dr. Phillips said. “What was unique about this study was the package that they put together,” he added. “It was a combination of telehealth and being able to speak with someone when you have concerns with objective data of an electrocardiogram, blood-pressure cuff, and oxygen level assessment, which is an interesting approach having that ejective data with [a] subjective element.”
The trial received funding from the British Heart Foundation; King Khalid University, Abha, Saudi Arabia via The Saudi Arabian Cultural Bureau; Sansour Fund, Imperial Healthcare Charity; and Safwan Sobhan Fund at Imperial College London. Mr. Alshahrani and Dr. Khamis have no relevant relationships to disclose. Dr. Phillips has no relevant disclosures.
A version of this article first appeared on Medscape.com.
ATLANTA — Patients with acute coronary syndrome (ACS) who had a myocardial infarction or unstable angina and underwent percutaneous coronary intervention (PCI) had a 76% lower rate of hospital readmission after 6 months if they participated in a remote monitoring protocol compared with similar patients who had standard post-discharge care, results of a new trial suggest.
The TELE-ACS trial showed that at 6 months, telemedicine patients also had statistically significantly lower rates of post-discharge emergency department visits, unplanned coronary revascularizations, and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness. However, the rates of major adverse cardiovascular events (MACE) were similar between the two groups. The protocol included consultation with a cardiologist who reviewed home-monitoring data.
“The team was able to aid in preventing unnecessary presentations and advised the patients to seek emergency care whenever was necessary,” Nasser Alshahrani, MSc, a clinical research fellow at Imperial College London, said while presenting the results at the American College of Cardiology meeting. “The TELE-ACS protocol provided a significant reduction in readmission rates post-ACS and other adverse events.”
The study findings were published online simultaneously in the Journal of the American College of Cardiology.
Telemedicine Protocol
The trial, conducted from January 2022 to April 2023, randomly assigned 337 patients to telemedicine or standard care when they were discharged after PCI and had at least one cardiovascular risk factor. The telemedicine protocol consisted of 12-lead electrocardiogram belt, an automated blood-pressure monitor, and a pulse oximeter.
Patients in the telemedicine arm initiated the remote monitoring protocol if they thought they had cardiac symptoms. The majority (86%) were men with what the study described as “a high preponderance of cardiovascular risk factors.” Average age was 58.1 years.
If a telemedicine patient initiated the protocol, a cardiologist remotely assessed the patient’s symptoms and channeled the patient to the appropriate care pathway, whether reassuring the patient or sending them to a primary care physician or emergency department, or to call emergency services. Patients who didn’t get a call back from the cardiologist within 15 minutes were told to seek care in the standard clinical pathway.
Telemedicine patients were given the telemonitoring package and training in how to use the devices before they were discharged. They also received three follow-up quality control calls in the first two months to ensure they were using the equipment correctly. They kept the telemonitoring equipment for 8 months, but were followed out to 9 months. Six telemedicine patients dropped out while one standard care patient withdrew from the study.
Results showed that at 6 months, telemedicine patients had statistically significantly lower rates of post-discharge emergency department visits (25% vs 37%, P < .001), unplanned coronary revascularizations (3% vs 9%, P < .01) and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness (a 13% to 18% difference for each symptom, P < .01).
MACE rates were similar between the two groups.
At 9 months, 3 months after the protocol ended, 20 telemedicine patients and 50 standard-care patients were readmitted to the hospital, while 52 and 73, respectively, went to the emergency department.
The telemedicine patients also had shorter hospital stays: an average of 0.5 and 1.2 days at 6 and 9 months, respectively, vs 1.5 and 1.8 days in the standard treatment arm (P < .001 for both).
Mr. Alshahrani noted several limitations with the study, namely that 86% of participants were men, and that the intervention was only offered to people who had smartphones. “The high level of support for the telemedicine group, with prompt cardiology responses, may be challenging to replicate outside a trial setting, requiring significant investment and training,” he added.
Human Element Key
In an interview from London after the presentation, lead author Ramzi Khamis, MB ChB, PhD, said, “This was quite a basic study. Really what we did was we integrated a clinical decision-making algorithm that we perfected with some quite novel but basic technology.” Future research should strive to add a home troponin test to the protocol and an artificial intelligence component, he said.
However, Dr. Khamis noted that human interaction was key to the success of the TELE-ACS trial. “The human factor is very important here and I think it would be really interesting to have a head-to-head comparison of human interaction with remote monitoring vs an AI-driven interaction,” he said. “I have my doubts that AI would be able to beat the human factor here.”
Lawrence Phillips, MD, medical director of outpatient cardiology at NYU Langone Heart, told this news organization that the study was appropriately powered to evaluate the telemedicine protocol, and that it could serve as a template for other studies of remote monitoring in cardiology.
“I think that this study is forming the foundation of evolving telemedicine data,” he said. “It shows really interesting results, and I’m sure it’s going to be reproduced in different ways going forward.”
While other studies have shown the utility of telemedicine to decrease unnecessary hospitalizations, this study went one step further, Dr. Phillips said. “What was unique about this study was the package that they put together,” he added. “It was a combination of telehealth and being able to speak with someone when you have concerns with objective data of an electrocardiogram, blood-pressure cuff, and oxygen level assessment, which is an interesting approach having that ejective data with [a] subjective element.”
The trial received funding from the British Heart Foundation; King Khalid University, Abha, Saudi Arabia via The Saudi Arabian Cultural Bureau; Sansour Fund, Imperial Healthcare Charity; and Safwan Sobhan Fund at Imperial College London. Mr. Alshahrani and Dr. Khamis have no relevant relationships to disclose. Dr. Phillips has no relevant disclosures.
A version of this article first appeared on Medscape.com.
ATLANTA — Patients with acute coronary syndrome (ACS) who had a myocardial infarction or unstable angina and underwent percutaneous coronary intervention (PCI) had a 76% lower rate of hospital readmission after 6 months if they participated in a remote monitoring protocol compared with similar patients who had standard post-discharge care, results of a new trial suggest.
The TELE-ACS trial showed that at 6 months, telemedicine patients also had statistically significantly lower rates of post-discharge emergency department visits, unplanned coronary revascularizations, and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness. However, the rates of major adverse cardiovascular events (MACE) were similar between the two groups. The protocol included consultation with a cardiologist who reviewed home-monitoring data.
“The team was able to aid in preventing unnecessary presentations and advised the patients to seek emergency care whenever was necessary,” Nasser Alshahrani, MSc, a clinical research fellow at Imperial College London, said while presenting the results at the American College of Cardiology meeting. “The TELE-ACS protocol provided a significant reduction in readmission rates post-ACS and other adverse events.”
The study findings were published online simultaneously in the Journal of the American College of Cardiology.
Telemedicine Protocol
The trial, conducted from January 2022 to April 2023, randomly assigned 337 patients to telemedicine or standard care when they were discharged after PCI and had at least one cardiovascular risk factor. The telemedicine protocol consisted of 12-lead electrocardiogram belt, an automated blood-pressure monitor, and a pulse oximeter.
Patients in the telemedicine arm initiated the remote monitoring protocol if they thought they had cardiac symptoms. The majority (86%) were men with what the study described as “a high preponderance of cardiovascular risk factors.” Average age was 58.1 years.
If a telemedicine patient initiated the protocol, a cardiologist remotely assessed the patient’s symptoms and channeled the patient to the appropriate care pathway, whether reassuring the patient or sending them to a primary care physician or emergency department, or to call emergency services. Patients who didn’t get a call back from the cardiologist within 15 minutes were told to seek care in the standard clinical pathway.
Telemedicine patients were given the telemonitoring package and training in how to use the devices before they were discharged. They also received three follow-up quality control calls in the first two months to ensure they were using the equipment correctly. They kept the telemonitoring equipment for 8 months, but were followed out to 9 months. Six telemedicine patients dropped out while one standard care patient withdrew from the study.
Results showed that at 6 months, telemedicine patients had statistically significantly lower rates of post-discharge emergency department visits (25% vs 37%, P < .001), unplanned coronary revascularizations (3% vs 9%, P < .01) and cardiovascular symptoms, such as chest pain, shortness of breath and dizziness (a 13% to 18% difference for each symptom, P < .01).
MACE rates were similar between the two groups.
At 9 months, 3 months after the protocol ended, 20 telemedicine patients and 50 standard-care patients were readmitted to the hospital, while 52 and 73, respectively, went to the emergency department.
The telemedicine patients also had shorter hospital stays: an average of 0.5 and 1.2 days at 6 and 9 months, respectively, vs 1.5 and 1.8 days in the standard treatment arm (P < .001 for both).
Mr. Alshahrani noted several limitations with the study, namely that 86% of participants were men, and that the intervention was only offered to people who had smartphones. “The high level of support for the telemedicine group, with prompt cardiology responses, may be challenging to replicate outside a trial setting, requiring significant investment and training,” he added.
Human Element Key
In an interview from London after the presentation, lead author Ramzi Khamis, MB ChB, PhD, said, “This was quite a basic study. Really what we did was we integrated a clinical decision-making algorithm that we perfected with some quite novel but basic technology.” Future research should strive to add a home troponin test to the protocol and an artificial intelligence component, he said.
However, Dr. Khamis noted that human interaction was key to the success of the TELE-ACS trial. “The human factor is very important here and I think it would be really interesting to have a head-to-head comparison of human interaction with remote monitoring vs an AI-driven interaction,” he said. “I have my doubts that AI would be able to beat the human factor here.”
Lawrence Phillips, MD, medical director of outpatient cardiology at NYU Langone Heart, told this news organization that the study was appropriately powered to evaluate the telemedicine protocol, and that it could serve as a template for other studies of remote monitoring in cardiology.
“I think that this study is forming the foundation of evolving telemedicine data,” he said. “It shows really interesting results, and I’m sure it’s going to be reproduced in different ways going forward.”
While other studies have shown the utility of telemedicine to decrease unnecessary hospitalizations, this study went one step further, Dr. Phillips said. “What was unique about this study was the package that they put together,” he added. “It was a combination of telehealth and being able to speak with someone when you have concerns with objective data of an electrocardiogram, blood-pressure cuff, and oxygen level assessment, which is an interesting approach having that ejective data with [a] subjective element.”
The trial received funding from the British Heart Foundation; King Khalid University, Abha, Saudi Arabia via The Saudi Arabian Cultural Bureau; Sansour Fund, Imperial Healthcare Charity; and Safwan Sobhan Fund at Imperial College London. Mr. Alshahrani and Dr. Khamis have no relevant relationships to disclose. Dr. Phillips has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Prolonged Sitting at Work Ups CVD and All-Cause Mortality, Daily Breaks May Help
However, daily breaks from sitting and leisure-time activity can help mitigate the “serious” risks associated with prolonged occupational sitting, the researchers say.
“As part of modern lifestyles, prolonged occupational sitting is considered normal and has not received due attention, even though its deleterious effect on health outcomes has been demonstrated,” wrote the authors, led by Wayne Gao, PhD, with Taipei Medical University College of Public Health, Taipei City, Taiwan.
“The importance of physical activity and moving around can never be overstated,” Michelle Bloom, MD, director of the cardio-oncology program at NYU Langone Health in New York, who wasn’t involved in the study, told this news organization.
“As a cardiologist, I bring this up at almost every visit with every patient regardless of why they’re seeing me, because I think that patients respond better when their doctor says it than when they just kind of know it in the back of their mind,” said Dr. Bloom, who is also a professor in the Division of Cardiology, NYU Grossman Long Island School of Medicine, New York.
The study was published online in JAMA Network Open.
Prolonged Sitting Hard on the Heart
2020 marked the first time that guidelines on physical activity from the World Health Organization recommended reducing sedentary behaviors owing to their health consequences. Less is known on the specific association of prolonged occupational sitting with health outcomes, especially in the context of low physical activity.
For their study, Dr. Gao and colleagues quantified health risks associated with prolonged sitting on the job and determined whether a certain threshold of physical activity may attenuate this risk.
Participants included 481,688 adults (mean age, 39 years; 53% women) in a health surveillance program in Taiwan. Data on occupational sitting, leisure-time physical activity, lifestyle, and metabolic parameters were collected.
During an average follow up of nearly 13 years, 26,257 participants died; more than half (57%) of the deaths occurred in individuals who mostly sat at work. There were 5371 CVD-related deaths, with 60% occurring in the mostly sitting group.
In multivariate analysis that adjusted for sex, age, education, smoking, drinking, and body mass index, adults who mostly sat at work had a 16% higher risk of dying of any cause (hazard ratio [HR], 1.16; 95% CI, 1.11-1.20) and a 34% increased risk of dying of CVD (HR, 1.34; 95% CI, 1.22-1.46) compared with those who mostly did not sit at work.
Adults who mostly alternated between sitting and not sitting at work were not at increased risk of all-cause mortality compared with individuals who mostly did not at work (HR, 1.01; 95% CI, 0.97-1.05).
Among adults who mostly sat at work and engaged in low (15-29 minutes) or no (< 15 minutes) daily leisure-time activity, increasing activity by 15 and 30 minutes per day, respectively, lowered the risk for mortality to a level similar to that of inactive individuals who mostly do not sit at work.
“Overall, our findings from a large prospective cohort help to strengthen the increasingly accumulating evidence linking a sedentary lifestyle and health risks,” the authors wrote.
“Systemic changes, such as more frequent breaks, standing desks, designated workplace areas for physical activity, and gym membership benefits, can help reduce risk,” they added.
Simple Yet Profound Message
Reached for comment, Anu Lala, MD, with Icahn School of Medicine at Mount Sinai and Mount Sinai Fuster Heart Hospital in New York, said this study provides a “simple yet profound message” about the dangers of prolonged sitting.
The finding of a 16% higher all-cause mortality in those who mostly sat at work after adjustment for major risk factors is “pretty remarkable. And for CVD mortality, it’s double that,” Dr. Lala told this news organization.
“I think we undervalue the importance of movement, however simple it is. Even simple actions, like squatting and standing up have benefits for the heart,” Dr. Lala added.
Dr. Bloom said she tells her patients, “You don’t have to go out tomorrow and run a marathon. Just get up a few times a day, walk a few laps in your office, walk back and forth from the mailbox, walk up and down your steps a couple of times — just do something more than you’re doing already.”
The study had no commercial funding. Dr. Gao and Dr. Bloom have no relevant disclosures. Dr. Lala has serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Novartis, AstraZeneca, Merck, Bayer, Novo Nordisk, Cordio, Zoll, and Sequana Medical.
A version of this article appeared on Medscape.com.
However, daily breaks from sitting and leisure-time activity can help mitigate the “serious” risks associated with prolonged occupational sitting, the researchers say.
“As part of modern lifestyles, prolonged occupational sitting is considered normal and has not received due attention, even though its deleterious effect on health outcomes has been demonstrated,” wrote the authors, led by Wayne Gao, PhD, with Taipei Medical University College of Public Health, Taipei City, Taiwan.
“The importance of physical activity and moving around can never be overstated,” Michelle Bloom, MD, director of the cardio-oncology program at NYU Langone Health in New York, who wasn’t involved in the study, told this news organization.
“As a cardiologist, I bring this up at almost every visit with every patient regardless of why they’re seeing me, because I think that patients respond better when their doctor says it than when they just kind of know it in the back of their mind,” said Dr. Bloom, who is also a professor in the Division of Cardiology, NYU Grossman Long Island School of Medicine, New York.
The study was published online in JAMA Network Open.
Prolonged Sitting Hard on the Heart
2020 marked the first time that guidelines on physical activity from the World Health Organization recommended reducing sedentary behaviors owing to their health consequences. Less is known on the specific association of prolonged occupational sitting with health outcomes, especially in the context of low physical activity.
For their study, Dr. Gao and colleagues quantified health risks associated with prolonged sitting on the job and determined whether a certain threshold of physical activity may attenuate this risk.
Participants included 481,688 adults (mean age, 39 years; 53% women) in a health surveillance program in Taiwan. Data on occupational sitting, leisure-time physical activity, lifestyle, and metabolic parameters were collected.
During an average follow up of nearly 13 years, 26,257 participants died; more than half (57%) of the deaths occurred in individuals who mostly sat at work. There were 5371 CVD-related deaths, with 60% occurring in the mostly sitting group.
In multivariate analysis that adjusted for sex, age, education, smoking, drinking, and body mass index, adults who mostly sat at work had a 16% higher risk of dying of any cause (hazard ratio [HR], 1.16; 95% CI, 1.11-1.20) and a 34% increased risk of dying of CVD (HR, 1.34; 95% CI, 1.22-1.46) compared with those who mostly did not sit at work.
Adults who mostly alternated between sitting and not sitting at work were not at increased risk of all-cause mortality compared with individuals who mostly did not at work (HR, 1.01; 95% CI, 0.97-1.05).
Among adults who mostly sat at work and engaged in low (15-29 minutes) or no (< 15 minutes) daily leisure-time activity, increasing activity by 15 and 30 minutes per day, respectively, lowered the risk for mortality to a level similar to that of inactive individuals who mostly do not sit at work.
“Overall, our findings from a large prospective cohort help to strengthen the increasingly accumulating evidence linking a sedentary lifestyle and health risks,” the authors wrote.
“Systemic changes, such as more frequent breaks, standing desks, designated workplace areas for physical activity, and gym membership benefits, can help reduce risk,” they added.
Simple Yet Profound Message
Reached for comment, Anu Lala, MD, with Icahn School of Medicine at Mount Sinai and Mount Sinai Fuster Heart Hospital in New York, said this study provides a “simple yet profound message” about the dangers of prolonged sitting.
The finding of a 16% higher all-cause mortality in those who mostly sat at work after adjustment for major risk factors is “pretty remarkable. And for CVD mortality, it’s double that,” Dr. Lala told this news organization.
“I think we undervalue the importance of movement, however simple it is. Even simple actions, like squatting and standing up have benefits for the heart,” Dr. Lala added.
Dr. Bloom said she tells her patients, “You don’t have to go out tomorrow and run a marathon. Just get up a few times a day, walk a few laps in your office, walk back and forth from the mailbox, walk up and down your steps a couple of times — just do something more than you’re doing already.”
The study had no commercial funding. Dr. Gao and Dr. Bloom have no relevant disclosures. Dr. Lala has serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Novartis, AstraZeneca, Merck, Bayer, Novo Nordisk, Cordio, Zoll, and Sequana Medical.
A version of this article appeared on Medscape.com.
However, daily breaks from sitting and leisure-time activity can help mitigate the “serious” risks associated with prolonged occupational sitting, the researchers say.
“As part of modern lifestyles, prolonged occupational sitting is considered normal and has not received due attention, even though its deleterious effect on health outcomes has been demonstrated,” wrote the authors, led by Wayne Gao, PhD, with Taipei Medical University College of Public Health, Taipei City, Taiwan.
“The importance of physical activity and moving around can never be overstated,” Michelle Bloom, MD, director of the cardio-oncology program at NYU Langone Health in New York, who wasn’t involved in the study, told this news organization.
“As a cardiologist, I bring this up at almost every visit with every patient regardless of why they’re seeing me, because I think that patients respond better when their doctor says it than when they just kind of know it in the back of their mind,” said Dr. Bloom, who is also a professor in the Division of Cardiology, NYU Grossman Long Island School of Medicine, New York.
The study was published online in JAMA Network Open.
Prolonged Sitting Hard on the Heart
2020 marked the first time that guidelines on physical activity from the World Health Organization recommended reducing sedentary behaviors owing to their health consequences. Less is known on the specific association of prolonged occupational sitting with health outcomes, especially in the context of low physical activity.
For their study, Dr. Gao and colleagues quantified health risks associated with prolonged sitting on the job and determined whether a certain threshold of physical activity may attenuate this risk.
Participants included 481,688 adults (mean age, 39 years; 53% women) in a health surveillance program in Taiwan. Data on occupational sitting, leisure-time physical activity, lifestyle, and metabolic parameters were collected.
During an average follow up of nearly 13 years, 26,257 participants died; more than half (57%) of the deaths occurred in individuals who mostly sat at work. There were 5371 CVD-related deaths, with 60% occurring in the mostly sitting group.
In multivariate analysis that adjusted for sex, age, education, smoking, drinking, and body mass index, adults who mostly sat at work had a 16% higher risk of dying of any cause (hazard ratio [HR], 1.16; 95% CI, 1.11-1.20) and a 34% increased risk of dying of CVD (HR, 1.34; 95% CI, 1.22-1.46) compared with those who mostly did not sit at work.
Adults who mostly alternated between sitting and not sitting at work were not at increased risk of all-cause mortality compared with individuals who mostly did not at work (HR, 1.01; 95% CI, 0.97-1.05).
Among adults who mostly sat at work and engaged in low (15-29 minutes) or no (< 15 minutes) daily leisure-time activity, increasing activity by 15 and 30 minutes per day, respectively, lowered the risk for mortality to a level similar to that of inactive individuals who mostly do not sit at work.
“Overall, our findings from a large prospective cohort help to strengthen the increasingly accumulating evidence linking a sedentary lifestyle and health risks,” the authors wrote.
“Systemic changes, such as more frequent breaks, standing desks, designated workplace areas for physical activity, and gym membership benefits, can help reduce risk,” they added.
Simple Yet Profound Message
Reached for comment, Anu Lala, MD, with Icahn School of Medicine at Mount Sinai and Mount Sinai Fuster Heart Hospital in New York, said this study provides a “simple yet profound message” about the dangers of prolonged sitting.
The finding of a 16% higher all-cause mortality in those who mostly sat at work after adjustment for major risk factors is “pretty remarkable. And for CVD mortality, it’s double that,” Dr. Lala told this news organization.
“I think we undervalue the importance of movement, however simple it is. Even simple actions, like squatting and standing up have benefits for the heart,” Dr. Lala added.
Dr. Bloom said she tells her patients, “You don’t have to go out tomorrow and run a marathon. Just get up a few times a day, walk a few laps in your office, walk back and forth from the mailbox, walk up and down your steps a couple of times — just do something more than you’re doing already.”
The study had no commercial funding. Dr. Gao and Dr. Bloom have no relevant disclosures. Dr. Lala has serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Novartis, AstraZeneca, Merck, Bayer, Novo Nordisk, Cordio, Zoll, and Sequana Medical.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Traumatic Brain Injury and CVD: What’s the Link?
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.