User login
Cellulitis treatment recommendations
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
COVID-19 vaccines: New candidates & answers to commonly asked questions
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
Eruptive Annular Papules on the Trunk of an Organ Transplant Recipient
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.
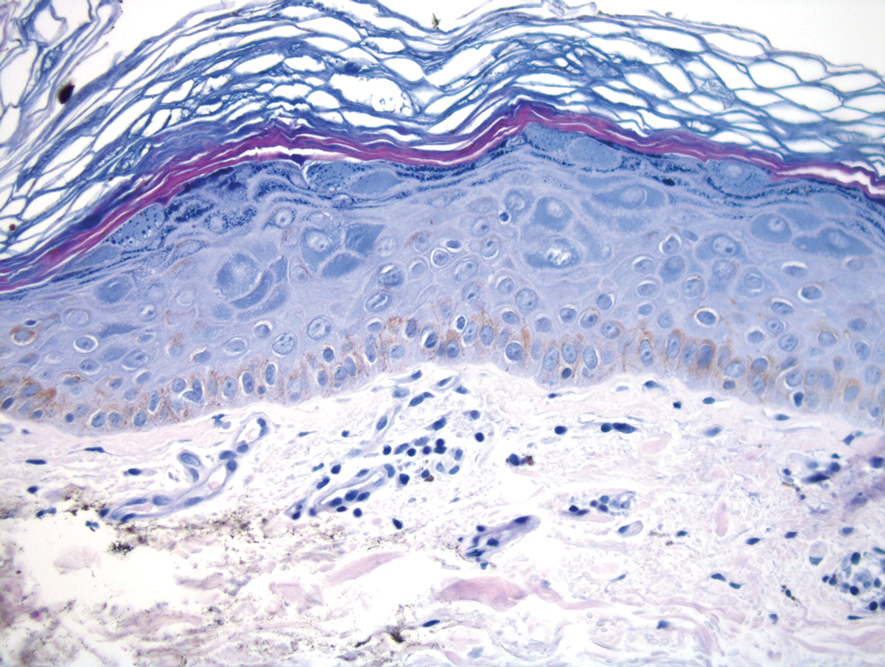
Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
A 50-year-old Black woman with systemic lupus erythematosus and a renal transplant 15 years prior due to lupus nephritis presented with a nonpruritic rash on the abdomen of 1 year’s duration. Her immunosuppressive regimen consisted of tacrolimus, azathioprine, and prednisone. Physical examination revealed numerous monomorphic, annular, hyperpigmented, and thin papules with central clearing present on the abdomen extending to the flanks and groin. The patient denied any family history of similar lesions. A 4-mm punch biopsy of an abdominal lesion was performed.
Influenza-related maternal morbidity has more than doubled over 15 years
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
FROM THE PREGNANCY MEETING
Vibrio vulnificus: Review of Mild to Life-threatening Skin Infections
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Practice Points
- Vibrio vulnificus infection should be high on the differential for patients who present with chronic liver disease and immunosuppression; a history of raw seafood consumption or exposure to brackish water; and bullae, cellulitis, necrotic lesions, or sepsis.
- Time to treatment is directly proportional to mortality rates in V vulnificus infections, and prompt treatment with antibiotics, wound care, debridement, and supportive measures is necessary to decrease mortality rates.
- The incidence of V vulnificus infection is rising in the United States, likely due to a combination of factors, including an aging population with multiple comorbidities, improvements in diagnosis, and climate change.
Dried blood spot tests show sensitivity as cCMV screen
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
FROM JAMA PEDIATRICS
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
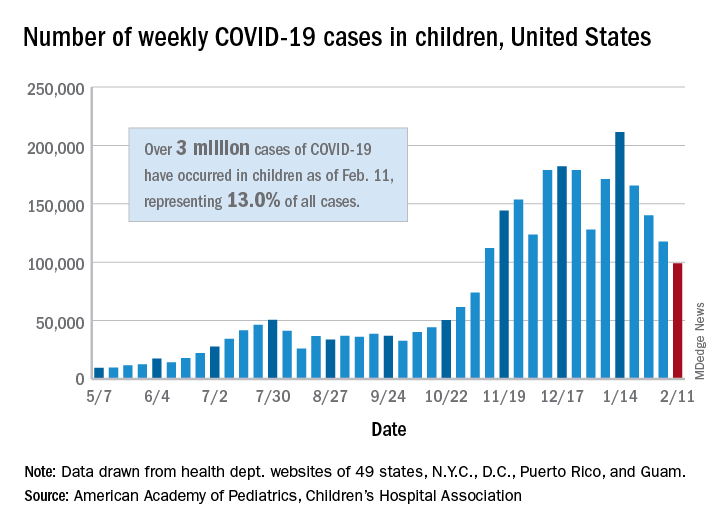
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
One-third of health care workers leery of getting COVID-19 vaccine, survey shows
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Prospective data support delaying antibiotics for pediatric respiratory infections
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
Zika vaccine candidate shows promise in phase 1 trial
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
FROM ANNALS OF INTERNAL MEDICINE