User login
Multifocal Annular Pink Plaques With a Central Violaceous Hue
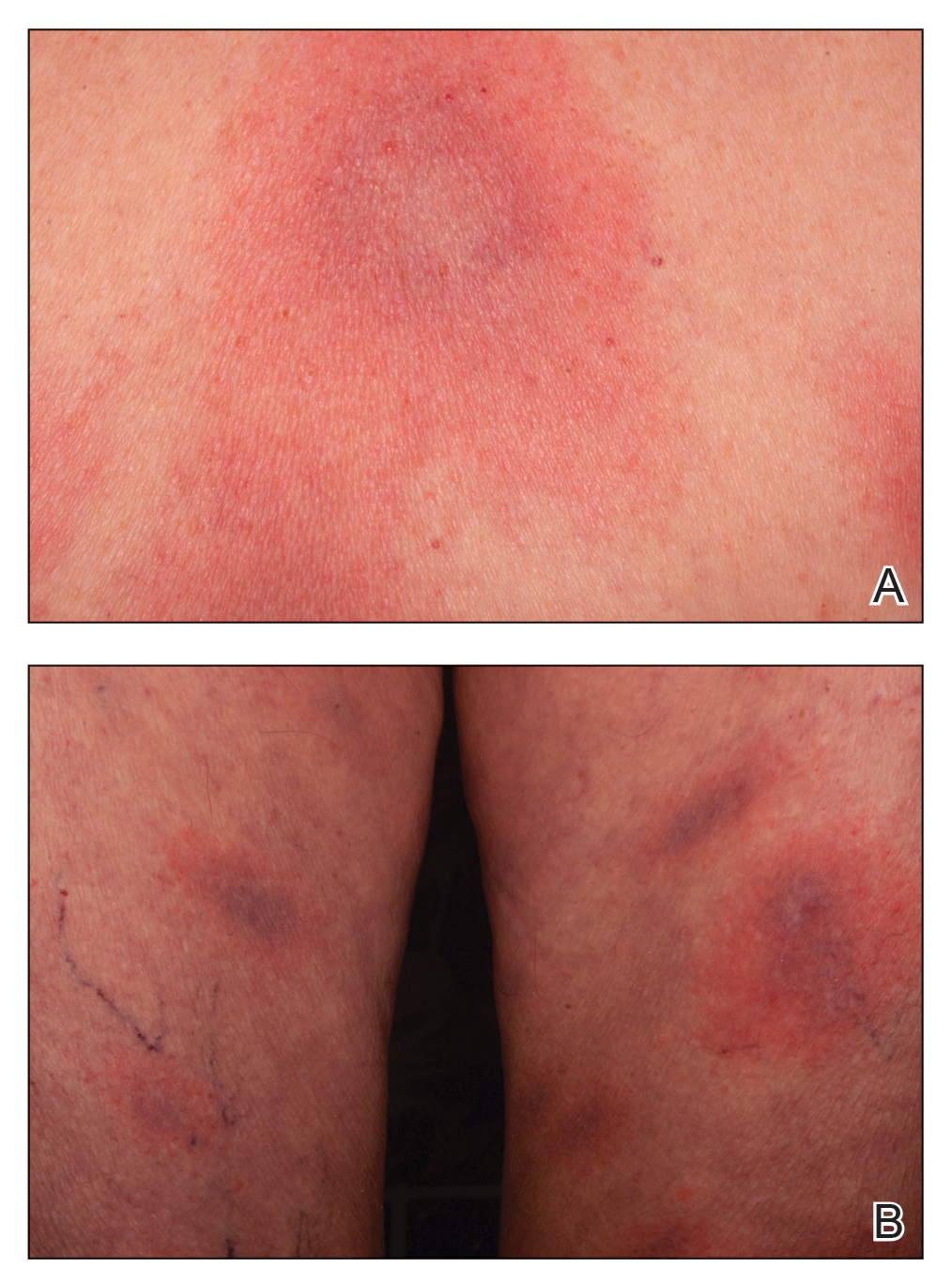
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.
The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.
The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.
The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
An otherwise healthy 78-year-old woman presented with a diffuse, mildly itchy rash of 5 days’ duration with associated fatigue, chills, decreased appetite, and nausea. She reported waking up with her arms “feeling like they weigh a ton.” She denied any pain, bleeding, or oozing and was unsure if new spots were continuing to develop. The patient reported having allergies to numerous medications but denied any new medications or recent illnesses. She had recently spent time on a farm in Minnesota, and upon further questioning she recalled a tick bite 2 months prior to presentation. She stated that she removed the nonengorged tick and that it could not have been attached for more than 24 hours. Her medical and family history were unremarkable. Physical examination showed multiple annular pink plaques with a central violaceous hue in a generalized distribution involving the face, trunk, arms, and legs with mild erythema of the palms. The plantar surfaces were clear, and there was no evidence of lymphadenopathy. The remainder of the physical examination and review of systems was negative. Laboratory screening was notable for an elevated erythrocyte sedimentation rate and C-reactive protein level with negative antinuclear antibodies.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
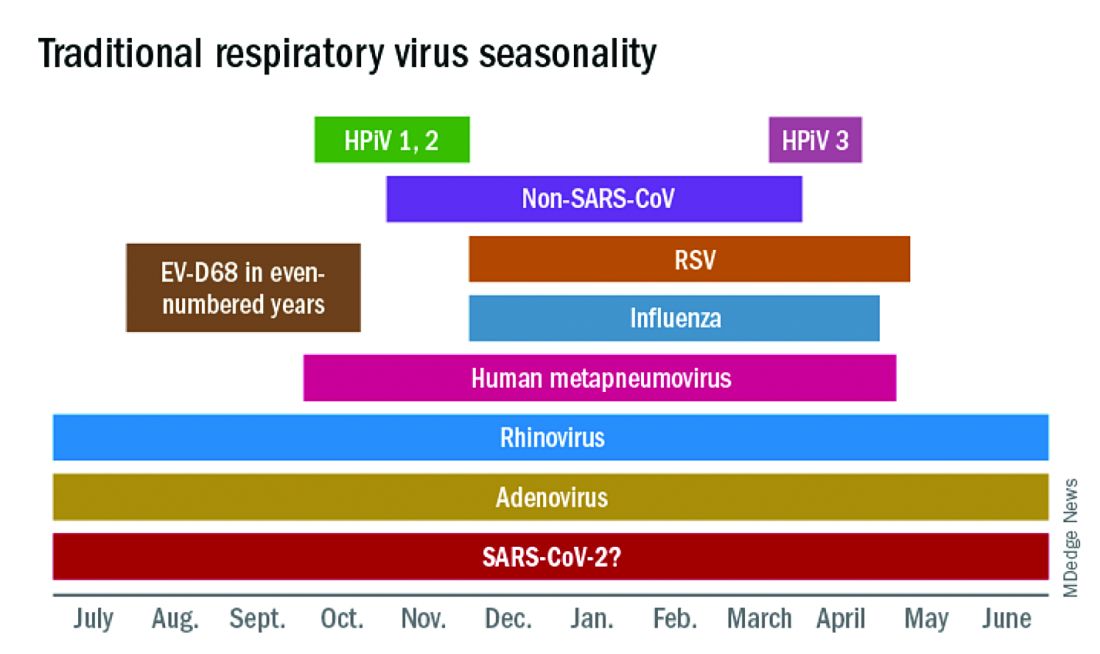
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).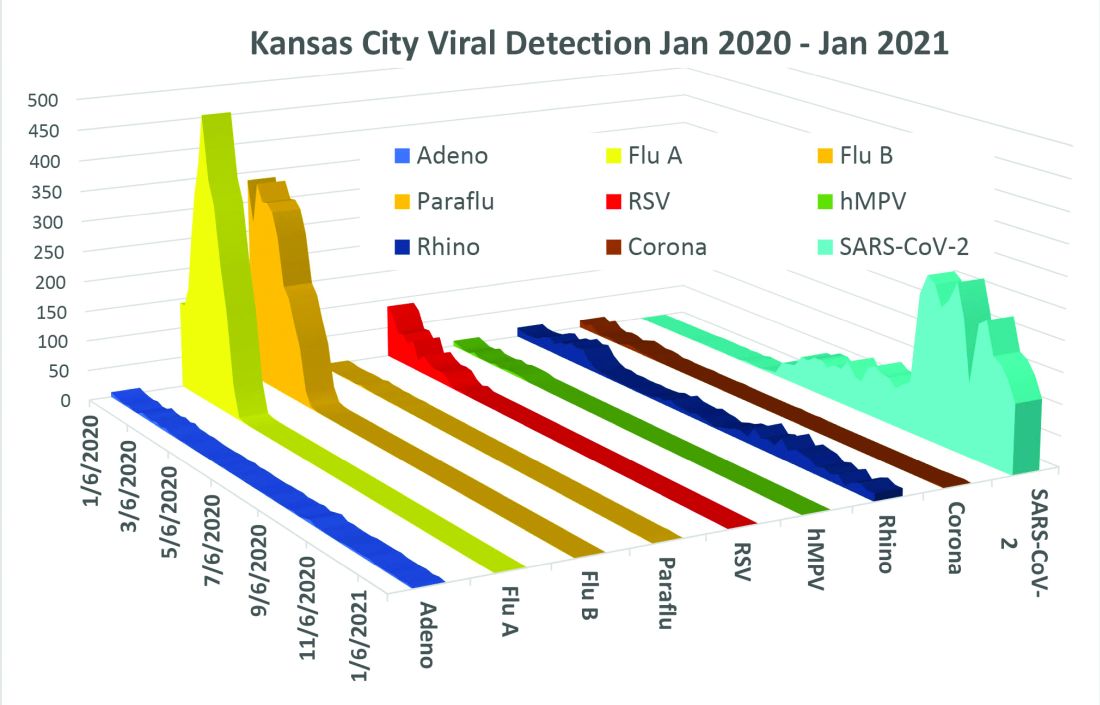
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Infectious diseases ‘giant’ John Bartlett: His ‘impact will endure’
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The changing brain signature of HIV
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
Scrub Typhus in Chile
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
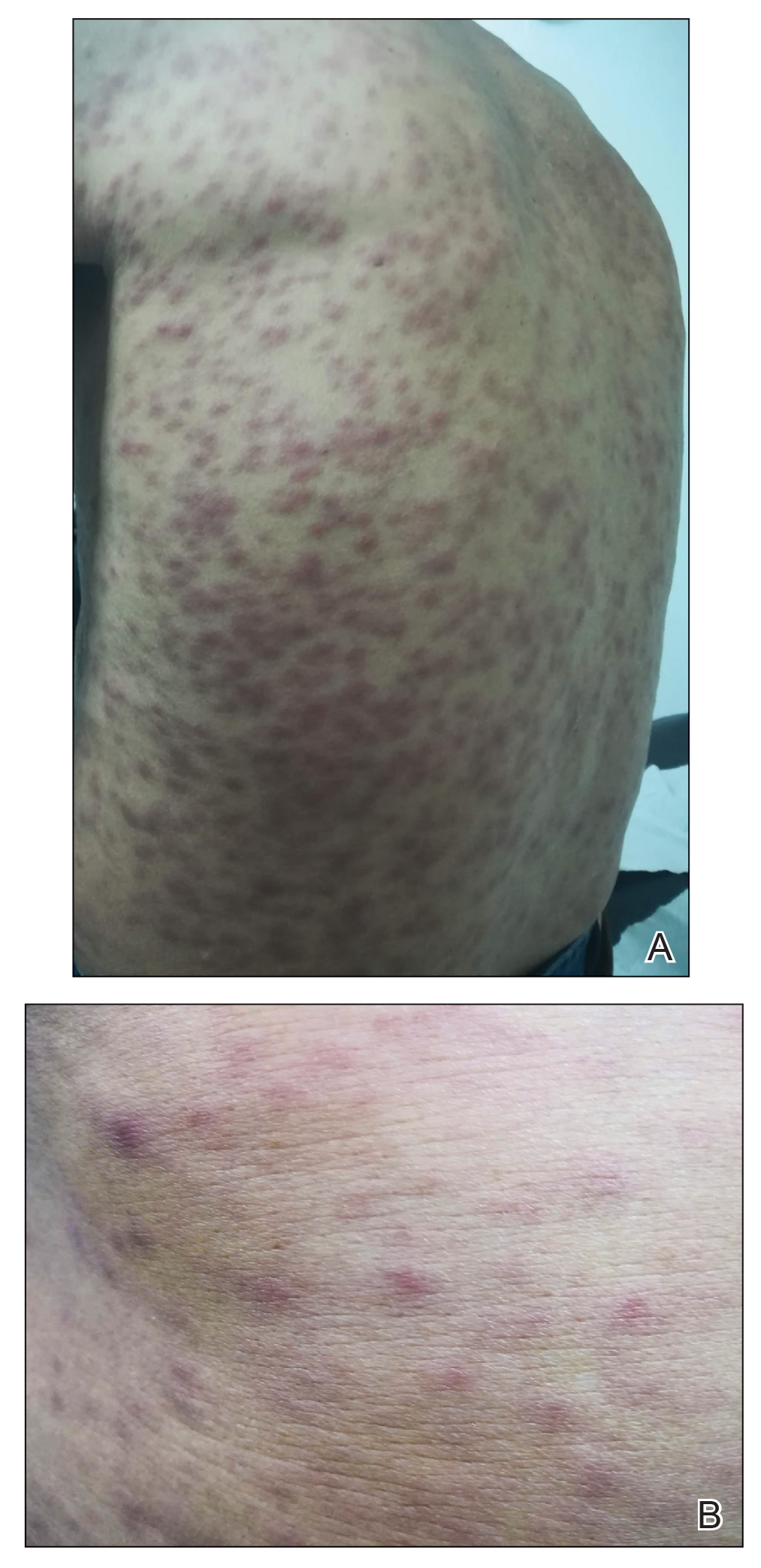
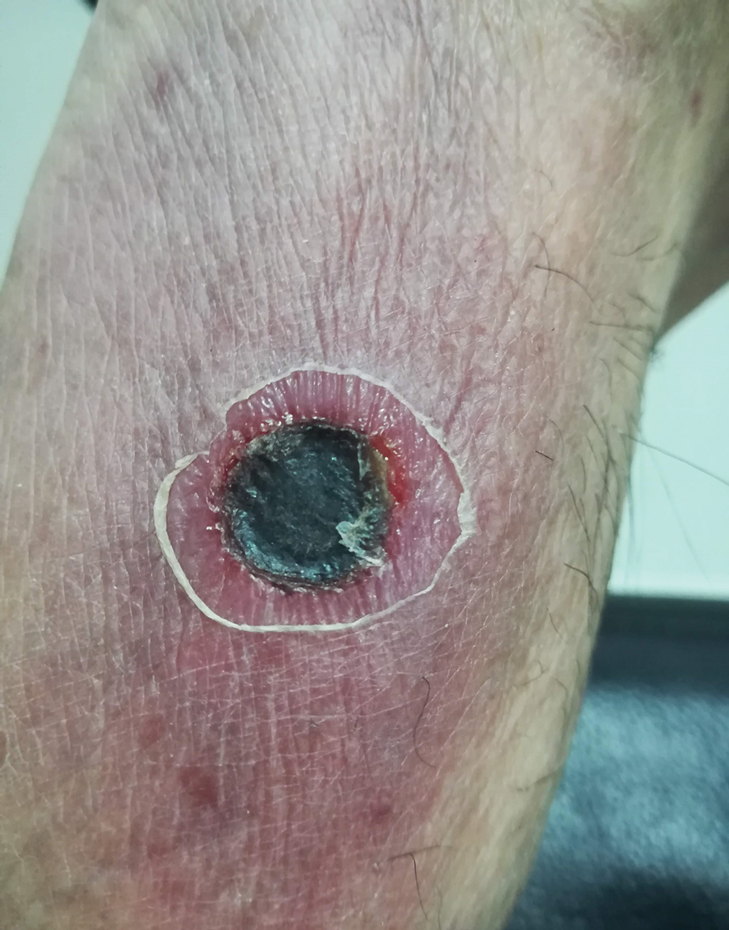
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).
A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
Practice Points
- Scrub typhus is clinically suspected in patients who present with a febrile macular or papular rash and a characteristic necrotic eschar known as tache noire while residing in or traveling to rural areas.
- Scrub typhus can lead to serious complications. Due to its changing epidemiology, dermatologists outside the usual area of distribution should be aware in the event that new cases emerge.
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
Expert calls for paradigm shift in lab monitoring of some dermatology drugs
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
FROM ODAC 2021
Is COVID-19 accelerating progress toward high-value care?
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
COVID-19 in children: New cases down for third straight week
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
U.K. COVID-19 variant doubling every 10 days in the U.S.: Study
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.