User login
Early PCSK9 inhibition in AMI yields plaque regression
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
FROM ACC 2022
Calcium scores predict sudden-death risk in preclinical CAD
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
Hybrid ACC 2022 resurrects the live scientific session
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Regardless of the pandemic’s sometimes mercurial behavior, the cardiology community appears set to reclaim valued traditions perhaps taken for granted in the pre-COVID era.
They include the bustling scientific congress and its myriad educational and networking prospects, along with pleiotropic effects like unplanned reunions with colleagues and catching up face-to-face with old friends.
That seems evident in the growing number of registrants for live attendance at at the annual scientific sessions of the American College of Cardiology, set for this Saturday through Monday in Washington as well as virtually, for a global reach that was unattainable in the pre-COVID era.
Registrations had hit the 11,000 mark and were picking up speed in recent weeks, ACC 2022 cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said at a mid-March presentation to the media.
They had reached about 12,880 and were still climbing a week before the conference, the ACC confirmed to this news organization. By then the professional registration had surpassed 9,900, of whom more than two-thirds reported plans to attend in person.
Dr. Morris said there had been 117 international submissions for what turned out to be 39 coveted spots on the meeting’s Late-Breaking Clinical Trial (LBCT) and Featured Clinical Research agenda spread across eight separate sessions.
On-site participants at the Walter E. Washington Convention Center should head for the Main Tent in Hall D for all LBCT presentations; venues for the Featured Clinical Research sessions are as noted below. Their real-time virtual equivalents will reside on the online platform’s Hot Topics channel. All noted session times are Eastern Daylight Time.
Saturday, April 2, 9:30 a.m.–10:30 a.m. Joint American College of Cardiology/Journal of the American College of Cardiology LBCT (I)
Leading off the conference’s first LBCT session, the randomized VALOR-HCM trial explored whether 16 weeks of mavacamten (MyoKardia) could help patients with severe obstructive hypertrophic cardiomyopathy (HCM) avoid septal reduction therapy, either surgical or by alcohol ablation.
The 22-center VALOR-HCM trial with an estimated enrollment of 100 follows EXPLORER-HCM, which in 2020 suggested the novel myosin-inhibiting agent could improve symptoms, exercise capacity, cardiac remodeling, and quality of life in such patients.
Simply advising people with heart failure (HF) to consume less salt is one thing, but it’s another to show them clinical trial evidence that it might help keep them out of the hospital. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure) study, conducted at 27 sites in six countries, sought to provide that evidence.
The trial randomly assigned 1,000 patients with NYHA class 2-3 HF to consume no more than 1,500 mg/day in sodium or to receive standard advice to limit sodium intake, and followed them for a year for the endpoint of death from any cause, cardiovascular (CV) hospitalization, or CV emergency department visit.
SODIUM-HF “may provide a rigorous evidence base for sodium restriction in patients with heart failure and may truly change our practice and how we recommend dietary modification,” ACC 2022 vice chair Douglas E. Drachman, MD, Massachusetts General Hospital, Boston, said at the media presentation.
In the same session, the CHAP (Chronic Hypertension and Pregnancy) study explored whether blood pressure (BP) control in pregnant women with new or untreated chronic hypertension could help avert preeclampsia, poor fetal outcomes, and other adverse events.
CHAP assigned about 2,400 women to receive either stepwise antihypertensive therapy to a BP goal of 140/90 mm Hg or lower or no such meds unless their BP reached or exceeded 160/105 mm Hg. Stepwise therapy featured either labetalol or extended-release nifedipine to start, the other agent added as necessary.
The LBCT block also includes the POISE-3 (Perioperative Ischemic Evaluation-3) comparison of the hemostatic agent tranexamic acid vs. placebo in nearly 10,000 patients undergoing noncardiac surgery. A separate randomization of the same cohort, to be reported at a Monday LBCT session, compared pre- and perioperative BP-control strategies.
Saturday, April 2, 12:00 p.m.–1:15 p.m. Featured Clinical Research I. Room 143A
This session features a subgroup analysis by age from the REVERSE-IT trial, which had previously showcased the monoclonal antibody bentracimab (PhaseBio Pharmaceuticals) for its ability to reverse the antiplatelet effects of ticagrelor.
REVERSE-IT is accompanied on the schedule by several secondary-endpoint presentations from trials whose primary outcomes have already been presented at meetings or in the journals.
They include the SCORED trial of sotagliflozin in patients with diabetes and chronic kidney disease (CKD); COMPLETE, which explored complete revascularization of multivessel coronary disease at primary stenting; and the FAME-3 comparison of coronary bypass surgery (CABG) vs. percutaneous coronary intervention (PCI) guided by fractional flow reserve (FFR) readings.
The session is to conclude with EDIT-CMD, which was a small, randomized assessment of diltiazem for improving microvascular dysfunction in patients with chronic angina despite nonobstructive coronary disease.
Sunday, April 3, 8:00 a.m.–9:15 a.m. Joint American College of Cardiology/Journal of the American Medical Association LBCT (II)
The SuperWIN (Supermarket Web Intervention) study tested an innovative strategy for community-based promotion of healthy lifestyle choices: point-of-purchase dietary education for grocery shoppers with an online instructional component, and follow-up to determine whether it influenced future food choices.
“Dietary interventions are notoriously difficult for us to implement, let alone to study scientifically,” Dr. Drachman observed. “So we think that there may be opportunity for dietary interventions to be best implemented at grocery stores where people are doing their shopping for food.”
SuperWIN compared supermarket shoppers with at least one CV risk factor who participated in the education intervention to a nonintervention control group for any changes in their DASH scores. The scores reflected consistency with the venerable DASH diet based on participants’ food purchases over 3 months.
In the same session, the MITIGATE trial explored whether daily administration of icosapent ethyl (Vascepa) might cut the risk of upper respiratory infection (especially from SARS-CoV-2 or seasonal influenza virus) in persons 50 or older with a history of clinical coronary, neurovascular, or peripheral vascular disease or revascularization. The trial has an estimated enrollment of 39,600.
Accompanying SuperWIN and MITIGATE are studies of several dyslipidemia drugs, including the discontinued antisense agent vupanorsen (Pfizer), as tested in TRANSLATE-TIMI 70; the PCSK9 inhibitor alirocumab (Praluent), explored for its effects on coronary plaque volume and composition in the PACMAN-AMI trial; and the APOLLO trial, a phase 1 evaluation of SLN360 (Silence Therapeutics), a short interfering ribonucleic acid (siRNA) that suppresses the molecular machinery in the liver that produces lipoprotein(a), or Lp(a).
The 32-patient APOLLO trial’s recently released top-line results suggested that SLN360 at varying dosages reduced Lp(a) levels by about one-half to more than 90%. Although elevated Lp(a) is known to track with CV risk, it remains to be shown whether dropping Lp(a) levels pharmacologically is protective.
Sunday, April 3, 9:45 a.m.–11:00 a.m. Joint American College of Cardiology/New England Journal of Medicine LBCT (III)
The meeting’s all-HF late-breaker session includes the METEORIC-HF trial, which compared the myotropic agent omecamtiv mecarbil (Cytokinetics) against placebo for effects on exercise performance over 20 weeks. The trial entered 276 patients with HF with reduced ejection fraction (HFrEF) and reduced peak VO2.
The GALACTIC-HF trial had previously suggested that the drug improved the risk of HF-related events or CV death in more than 8000 patients with HFrEF, those with the lowest ejection fractions benefiting the most.
This block of trials also features DIAMOND, the latest trial with a gemologic name to look at the potassium sequestrant patiromer (Veltassa) for any protection against hyperkalemia, a familiar side effect of renin-angiotensin-aldosterone inhibitors. DIAMOND tested patiromer in 878 patients with HFrEF who were on beta-blockers and other HF-appropriate medications and had a history of drug-associated hyperkalemia.
Previously, the AMBER trial of patients with CKD or refractory hypertension on spironolactone had suggested the drug might be protective enough against hyperkalemia to allow higher and more consistent dosing of BP-lowering agents.
Also in the session: the randomized IVVE (Influenza Vaccine to Prevent Adverse Vascular Events) trial, with an estimated 5,000 patients with HF in Africa, Asia, and the Middle East; PROMPT-HF, with a projected 1,310 HF patients and billed as a cluster-randomized pragmatic trial of a strategy for improving guideline-directed outpatient medical therapy; and MAVA-LTE, the long-term extension study of an estimated 310 patients who were in the MAVERICK-HCM and EXPLORER-HCM mavacamten trials.
Sunday, April 3, 12:15–1:30 p.m. Featured Clinical Research II. Main Tent, Hall D
The arrhythmia-centric session includes PARTITA, with its estimated 590 patients with primary- or secondary-prevention implantable cardioverter-defibrillators (ICDs). The trial followed them initially for burden of untreated nonsustained ventricular tachycardia (VT) or events treated with anti-tachycardia pacing. Then it randomly assigned those who experienced a first appropriate ICD shock to either immediate VT ablation or standard care. The latter included ablation on next occurrence of arrhythmic storm.
Investigational oral factor XIa inhibitors, viewed by many as potentially safer as anticoagulants than contemporary oral inhibitors of factor Xa, are now on the scene and include milvexian (Bristol-Myers Squibb/Janssen) and, lately, asundexian (BAY 2433334; Bayer). The latter agent was compared to the factor Xa inhibitor apixaban (Eliquis) in 753 patients with AF in the phase 2 PACIFIC-AF trial, which looked at the newer drug’s safety and optimal dosing.
Also on the bill: a long-term follow-up of the mAFA-2 (Mobile AF Application 2) extension study, which explored the value of a smartphone-based atrial fibrillation (AF) screening app for improving risk of AF-related events; a presentation billed as “Residual Leaks Post Left Atrial Appendage Occlusion”; and one that declares “low rates of guideline-directed care” to be “associated with higher mortality” in patients with pacemakers or ICDs.
Monday, April 4, 8:30 a.m.–9:45 a.m. LBCT IV
This session is to open with the PROTECT trial, which sought to determine whether perioperative “aggressive warming” may be cardioprotective in patients with CV risk factors undergoing noncardiac surgery. Its estimated 5,100 patients were randomly assigned to a procedure that achieves normothermia, that is 37° C (98.6° F), vs. standard care in which patients’ core temperature may decline to no further than 35.5° C (95.9° F).
Next on the list are a second POISE-3 comparison of BP-control strategies comparing hypotension avoidance vs. hypertension avoidance in patients undergoing noncardiac surgery; the pivotal CLASP 2 TR trial of patients with symptomatic tricuspid regurgitation on optimal medical therapy with vs. without treatment with the Edwards PASCAL Transcatheter Repair System; and one said to provide “insights from the Corevalve US Pivotal and SURTAVI trials” on 5-year incidence, timing, and predictors of hemodynamic valve deterioration transcatheter and surgical aortic bioprostheses.”
Rounding out the block of presentations: the ADAPT-TAVR comparison of the factor Xa inhibitor edoxaban (Lixiana) to dual-antiplatelet therapy for prevention of leaflet thrombosis after successful transcatheter aortic valve replacement (TAVR). The 235-patient trial was conducted at five centers in South Korea, Hong Kong, and Taiwan.
Monday, April 4, 11:00–12:15 p.m. LBCT V
This session includes the FLAVOUR randomized comparison of PCI guided by either FFR or intravascular ultrasound (IVUS) in 1,700 patients with 40%-70% stenoses. The patients from centers in China and South Korea were followed for death from any cause, MI, or any repeat revascularization at 24 months.
Also scheduled: the 2-year report on 4,000 patients with ST-segment elevation MI (STEMI) in the ACC-sponsored quality improvement program GHATI (Global Heart Attack Treatment Initiative); the GIPS-4 myocardial protection study of an estimated 380 patients with STEMI assigned to receive pre- and post-PCI infusions of sodium thiosulfate or placebo, with infarct size at 4 months as the primary endpoint; and a randomized test of an arrhythmia-monitoring implant for influence on clinical outcomes in 802 patients with a history of MI but no pacemaker or ICD indication, called BIO-GUARD-MI,
Last in the session: the Chocolate Touch Study of peripheral-artery angioplasty using a drug-coated balloon (DCB) with a confectionery name that treats lesions not with theobromine, but the antiproliferative mainstay paclitaxel.
The randomized comparison of the Chocolate Touch DCB (TriReme Medical) and the more established Lutonix DCB (Bard) assigned a projected 585 patients with symptomatic peripheral vascular disease to treatment of superficial femoral or popliteal artery lesions with one of the two paclitaxel-coated balloon catheters.
Monday, April 4, 12:45–2 p.m. Featured Clinical Research III. Room 143A
The final session features five subgroup analyses or other updates from trials that have already reported their primary outcomes. Among them is the SPYRAL HTN-ON MED trial, which helped to revitalize hopes for renal denervation therapy as a catheter-based treatment for drug-resistant hypertension by showing significant effects on both systolic and diastolic blood pressure. The new data follow the trial’s more than 400 patients out to 3 years.
There is also a symptom and quality-of-life analysis from the 530-patient EMPULSE trial of 530 patients with stabilized acute HF assigned in-hospital to start on empagliflozin (Jardiance) or placebo. The trial made a splash last year when it reported a significant improvement in risk for death or HF rehospitalization for its patients put on the SGLT2 inhibitor.
A secondary analysis from CANTOS is also featured; the trial had randomly assigned more than 10,000 patients with recent acute MI and elevated C-reactive protein (CRP) levels to receive or not receive the anti-inflammatory canakinumab (Ilaris). Those assigned to active therapy showed benefits for a range of outcomes, including CV mortality and stroke, but no decreases in cholesterol levels. Billing for the new CANTOS analysis promises insights on the “differential impact of residual inflammatory risk and residual cholesterol risk among atherosclerosis patients with and without chronic kidney disease.”
The session also features “trends and final results” from the NACMI (North American COVID-19 Myocardial Infarction) registry, which had shown excellent primary-PCI results without compromise of door-to-balloon times in patients with confirmed SARS-CoV-2 infection; and a FIDELITY analysis of cardiorenal endpoints by history of CV disease in the study’s more than 13,000 patients with diabetes and CKD assigned to placebo or finerenone (Kerendia), a mineralocorticoid receptor antagonist.
A version of this article first appeared on Medscape.com.
Handheld ECGs ease AFib screening in the very elderly
The use of handheld, single-lead electrocardiograms (ECGs) did not increase diagnoses of AFib overall in patients aged 65 and older, but it did in patients 85 and up, researchers reported in Circulation.
“Incorporating single-lead ECGs into routine medical assessments as a new vital sign was widely feasible. Over 90% of people who were offered screening agreed to it and underwent screening,” said Steven Lubitz, MD, of the Cardiac Arrhythmia Service and Cardiovascular Research Center at Massachusetts General Hospital, Boston, who led the study.
Because advanced age is associated with a substantially increased risk of both AFib and stroke, point-of-care screening might be an efficient use of handheld ECGs, Dr. Lubitz said.
“The technology simply requires patients to place their fingers on the device to record an electrocardiogram and can be easily embedded in the routine clinical practice of primary care physicians,” he said in an interview.
The typical person has a 30% lifetime risk of developing AFib, and the chances of experiencing a stroke associated with the arrhythmia can be reduced significantly with anticoagulants, Dr. Lubitz said.
Professional organizations are split about the utility of screening for AFib. The European Society of Cardiology recommends opportunistic screening with either pulse palpation or ECG rhythm strip at clinic visits for patients 65 and older. The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have issued similar guidelines.
However, screening for AFib is not considered standard of care in the United States – although Dr. Lubitz predicted that that would change.
“I think the guidelines in the United States will evolve in the next few years, because I think we’re getting closer to understanding who we should be screening for atrial fibrillation and how we should be screening,” Dr. Lubitz told this news organization.
‘Very reassuring’ results
The randomized controlled trial found that for patients 85 and older, use of handheld ECGs led to a nearly 2% increase in new diagnoses of AFib in the screening group compared to conventional care.
The researchers also demonstrated an increased likelihood of diagnosing AFib during the patient’s primary-care encounter than at other sites, such as the emergency department or inpatient settings that might be more costly and resource-intensive. Moreover, the study reported that point-of-care screening was associated with high rates of oral anticoagulation prescriptions written for patients with newly diagnosed AFib, a finding Dr. Lubitz called “very reassuring.”
The Mass General researchers used single-lead devices attached to a tablet computer to screen more than 35,000 men and women from 16 primary care sites affiliated with the hospital’s practice-based research network.
Half the sites were randomly selected to include the screening intervention, where medical assistants used handheld ECGs at the start of the visit while checking routine vital signs.
The 1-year study screened 91% of eligible patients, demonstrating that single-lead rhythm assessment is feasible as part of routine primary care practice, Dr. Lubitz said. This finding supports other studies suggesting that handheld devices can enable rapid and scalable mass screening.
“We demonstrated that integration into routine practice by clinical personnel – in this case, medical assistants – is feasible. No study has measured and demonstrated such a high integration with routine care, reflecting both patient interest in screening and feasibility of incorporating screening into busy clinical practices,” Dr. Lubitz said.
Mobile ECGs with the handheld device take about 30 seconds to perform. In contrast, standard ECGs used in outpatient practices are bulky, and recording the ECG can take roughly 10 minutes.
Anthony Leazzo, DO, chairman of family practice at Northwestern Medicine Delnor Hospital, in Geneva, Ill., noted that smartwatches provide an alternative technology for detecting AFib.
But “a handheld, one-lead device would be more beneficial and should be more sensitive by measuring electrical activity similar to a normal ECG,” he said.
However, Dr. Leazzo said using such technology would need to be cost-effective because the patients at highest risk for AFib usually are on fixed incomes. Consumer versions of the devices can cost under $100. Dr. Lubitz said the actual cost for devices and a software platform used for a medical enterprise may differ.
Handheld ECGs are gradually being integrated into clinical practices, a trend driven by the rapid growth of telemedicine to remotely assess patients, Dr. Lubitz said.
“Our work affirmed that single-lead devices generate information for the physician that is actionable, though the proportion of newly detected AFib cases using a point-of-care ECG screening approach is likely to be very small,” Dr. Lubitz said in an interview. “For that reason, we think handheld devices are best deployed for people at the highest risk of AFib and stroke, and age is an excellent surrogate for that determination.”
The study was funded by Bristol-Myers Squibb–Pfizer Alliance.
A version of this article first appeared on Medscape.com.
The use of handheld, single-lead electrocardiograms (ECGs) did not increase diagnoses of AFib overall in patients aged 65 and older, but it did in patients 85 and up, researchers reported in Circulation.
“Incorporating single-lead ECGs into routine medical assessments as a new vital sign was widely feasible. Over 90% of people who were offered screening agreed to it and underwent screening,” said Steven Lubitz, MD, of the Cardiac Arrhythmia Service and Cardiovascular Research Center at Massachusetts General Hospital, Boston, who led the study.
Because advanced age is associated with a substantially increased risk of both AFib and stroke, point-of-care screening might be an efficient use of handheld ECGs, Dr. Lubitz said.
“The technology simply requires patients to place their fingers on the device to record an electrocardiogram and can be easily embedded in the routine clinical practice of primary care physicians,” he said in an interview.
The typical person has a 30% lifetime risk of developing AFib, and the chances of experiencing a stroke associated with the arrhythmia can be reduced significantly with anticoagulants, Dr. Lubitz said.
Professional organizations are split about the utility of screening for AFib. The European Society of Cardiology recommends opportunistic screening with either pulse palpation or ECG rhythm strip at clinic visits for patients 65 and older. The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have issued similar guidelines.
However, screening for AFib is not considered standard of care in the United States – although Dr. Lubitz predicted that that would change.
“I think the guidelines in the United States will evolve in the next few years, because I think we’re getting closer to understanding who we should be screening for atrial fibrillation and how we should be screening,” Dr. Lubitz told this news organization.
‘Very reassuring’ results
The randomized controlled trial found that for patients 85 and older, use of handheld ECGs led to a nearly 2% increase in new diagnoses of AFib in the screening group compared to conventional care.
The researchers also demonstrated an increased likelihood of diagnosing AFib during the patient’s primary-care encounter than at other sites, such as the emergency department or inpatient settings that might be more costly and resource-intensive. Moreover, the study reported that point-of-care screening was associated with high rates of oral anticoagulation prescriptions written for patients with newly diagnosed AFib, a finding Dr. Lubitz called “very reassuring.”
The Mass General researchers used single-lead devices attached to a tablet computer to screen more than 35,000 men and women from 16 primary care sites affiliated with the hospital’s practice-based research network.
Half the sites were randomly selected to include the screening intervention, where medical assistants used handheld ECGs at the start of the visit while checking routine vital signs.
The 1-year study screened 91% of eligible patients, demonstrating that single-lead rhythm assessment is feasible as part of routine primary care practice, Dr. Lubitz said. This finding supports other studies suggesting that handheld devices can enable rapid and scalable mass screening.
“We demonstrated that integration into routine practice by clinical personnel – in this case, medical assistants – is feasible. No study has measured and demonstrated such a high integration with routine care, reflecting both patient interest in screening and feasibility of incorporating screening into busy clinical practices,” Dr. Lubitz said.
Mobile ECGs with the handheld device take about 30 seconds to perform. In contrast, standard ECGs used in outpatient practices are bulky, and recording the ECG can take roughly 10 minutes.
Anthony Leazzo, DO, chairman of family practice at Northwestern Medicine Delnor Hospital, in Geneva, Ill., noted that smartwatches provide an alternative technology for detecting AFib.
But “a handheld, one-lead device would be more beneficial and should be more sensitive by measuring electrical activity similar to a normal ECG,” he said.
However, Dr. Leazzo said using such technology would need to be cost-effective because the patients at highest risk for AFib usually are on fixed incomes. Consumer versions of the devices can cost under $100. Dr. Lubitz said the actual cost for devices and a software platform used for a medical enterprise may differ.
Handheld ECGs are gradually being integrated into clinical practices, a trend driven by the rapid growth of telemedicine to remotely assess patients, Dr. Lubitz said.
“Our work affirmed that single-lead devices generate information for the physician that is actionable, though the proportion of newly detected AFib cases using a point-of-care ECG screening approach is likely to be very small,” Dr. Lubitz said in an interview. “For that reason, we think handheld devices are best deployed for people at the highest risk of AFib and stroke, and age is an excellent surrogate for that determination.”
The study was funded by Bristol-Myers Squibb–Pfizer Alliance.
A version of this article first appeared on Medscape.com.
The use of handheld, single-lead electrocardiograms (ECGs) did not increase diagnoses of AFib overall in patients aged 65 and older, but it did in patients 85 and up, researchers reported in Circulation.
“Incorporating single-lead ECGs into routine medical assessments as a new vital sign was widely feasible. Over 90% of people who were offered screening agreed to it and underwent screening,” said Steven Lubitz, MD, of the Cardiac Arrhythmia Service and Cardiovascular Research Center at Massachusetts General Hospital, Boston, who led the study.
Because advanced age is associated with a substantially increased risk of both AFib and stroke, point-of-care screening might be an efficient use of handheld ECGs, Dr. Lubitz said.
“The technology simply requires patients to place their fingers on the device to record an electrocardiogram and can be easily embedded in the routine clinical practice of primary care physicians,” he said in an interview.
The typical person has a 30% lifetime risk of developing AFib, and the chances of experiencing a stroke associated with the arrhythmia can be reduced significantly with anticoagulants, Dr. Lubitz said.
Professional organizations are split about the utility of screening for AFib. The European Society of Cardiology recommends opportunistic screening with either pulse palpation or ECG rhythm strip at clinic visits for patients 65 and older. The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have issued similar guidelines.
However, screening for AFib is not considered standard of care in the United States – although Dr. Lubitz predicted that that would change.
“I think the guidelines in the United States will evolve in the next few years, because I think we’re getting closer to understanding who we should be screening for atrial fibrillation and how we should be screening,” Dr. Lubitz told this news organization.
‘Very reassuring’ results
The randomized controlled trial found that for patients 85 and older, use of handheld ECGs led to a nearly 2% increase in new diagnoses of AFib in the screening group compared to conventional care.
The researchers also demonstrated an increased likelihood of diagnosing AFib during the patient’s primary-care encounter than at other sites, such as the emergency department or inpatient settings that might be more costly and resource-intensive. Moreover, the study reported that point-of-care screening was associated with high rates of oral anticoagulation prescriptions written for patients with newly diagnosed AFib, a finding Dr. Lubitz called “very reassuring.”
The Mass General researchers used single-lead devices attached to a tablet computer to screen more than 35,000 men and women from 16 primary care sites affiliated with the hospital’s practice-based research network.
Half the sites were randomly selected to include the screening intervention, where medical assistants used handheld ECGs at the start of the visit while checking routine vital signs.
The 1-year study screened 91% of eligible patients, demonstrating that single-lead rhythm assessment is feasible as part of routine primary care practice, Dr. Lubitz said. This finding supports other studies suggesting that handheld devices can enable rapid and scalable mass screening.
“We demonstrated that integration into routine practice by clinical personnel – in this case, medical assistants – is feasible. No study has measured and demonstrated such a high integration with routine care, reflecting both patient interest in screening and feasibility of incorporating screening into busy clinical practices,” Dr. Lubitz said.
Mobile ECGs with the handheld device take about 30 seconds to perform. In contrast, standard ECGs used in outpatient practices are bulky, and recording the ECG can take roughly 10 minutes.
Anthony Leazzo, DO, chairman of family practice at Northwestern Medicine Delnor Hospital, in Geneva, Ill., noted that smartwatches provide an alternative technology for detecting AFib.
But “a handheld, one-lead device would be more beneficial and should be more sensitive by measuring electrical activity similar to a normal ECG,” he said.
However, Dr. Leazzo said using such technology would need to be cost-effective because the patients at highest risk for AFib usually are on fixed incomes. Consumer versions of the devices can cost under $100. Dr. Lubitz said the actual cost for devices and a software platform used for a medical enterprise may differ.
Handheld ECGs are gradually being integrated into clinical practices, a trend driven by the rapid growth of telemedicine to remotely assess patients, Dr. Lubitz said.
“Our work affirmed that single-lead devices generate information for the physician that is actionable, though the proportion of newly detected AFib cases using a point-of-care ECG screening approach is likely to be very small,” Dr. Lubitz said in an interview. “For that reason, we think handheld devices are best deployed for people at the highest risk of AFib and stroke, and age is an excellent surrogate for that determination.”
The study was funded by Bristol-Myers Squibb–Pfizer Alliance.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
How Lp(a) can help improve ASCVD risk assessment
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).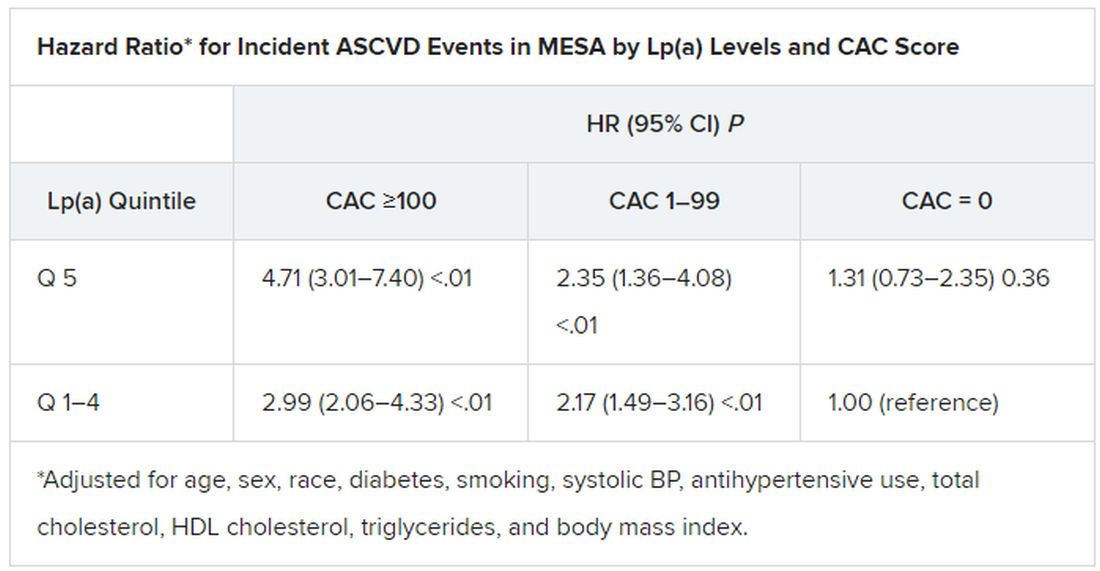
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).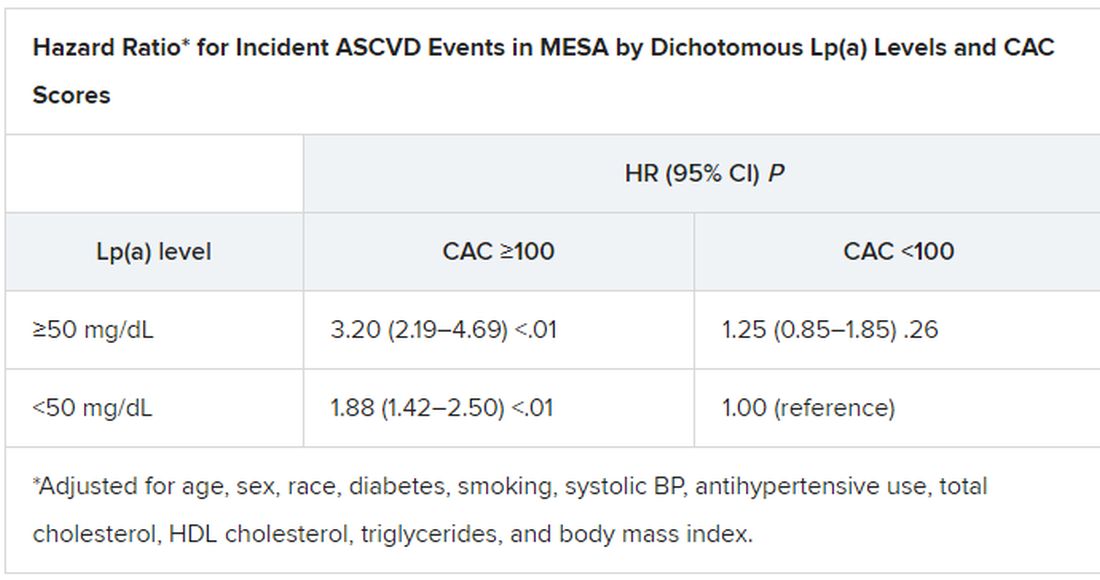
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Tiny hitchhikers like to ride in the trunk
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Junk (germs) in the trunk
It’s been a long drive, and you’ve got a long way to go. You pull into a rest stop to use the bathroom and get some food. Quick, which order do you do those things in?
If you’re not a crazy person, you’d use the bathroom and then get your food. Who would bring food into a dirty bathroom? That’s kind of gross. Most people would take care of business, grab food, then get back in the car, eating along the way. Unfortunately, if you’re searching for a sanitary eating environment, your car may not actually be much better than that bathroom, according to new research from Aston University in Birmingham, England.
Let’s start off with the good news. The steering wheels of the five used cars that were swabbed for bacteria were pretty clean. Definitely cleaner than either of the toilet seats analyzed, likely thanks to increased usage of sanitizer, courtesy of the current pandemic. It’s easy to wipe down the steering wheel. Things break down, though, once we look elsewhere. The interiors of the five cars all contained just as much, if not more, bacteria than the toilet seats, with fecal matter commonly appearing on the driver’s seat.
The car interiors were less than sanitary, but they paled in comparison with the real winner here: the trunk. In each of the five cars, bacteria levels there far exceeded those in the toilets, and included everyone’s favorites – Escherichia coli and Staphylococcus aureus.
So, snacking on a bag of chips as you drive along is probably okay, but the food that popped out of its bag and spent the last 5 minutes rolling around the back? Perhaps less okay. You may want to wash it. Or burn it. Or torch the entire car for good measure like we’re about to do. Next time we’ll buy a car without poop in it.
Shut the lid when you flush
Maybe you’ve never thought about this, but it’s actually extremely important to shut the toilet lid when you flush. Just think of all those germs flying around from the force of the flush. Is your toothbrush anywhere near the toilet? Ew. Those pesky little bacteria and viruses are everywhere, and we know we can’t really escape them, but we should really do our best once we’re made aware of where to find them.
It seems like a no-brainer these days since we’ve all been really focused on cleanliness during the pandemic, but according to a poll in the United Kingdom, 55% of the 2,000 participants said they don’t put the lid down while flushing.
The OnePoll survey commissioned by Harpic, a company that makes toilet-cleaning products, also advised that toilet water isn’t even completely clean after flushed several times and can still be contaminated with many germs. Company researchers took specialized pictures of flushing toilets and they looked like tiny little Fourth of July fireworks shows, minus the sparklers. The pictures proved that droplets can go all over the place, including on bathroom users.
“There has never been a more important time to take extra care around our homes, although the risks associated with germ spread in unhygienic bathrooms are high, the solution to keeping them clean is simple,” a Harpic researcher said. Since other studies have shown that coronavirus can be found in feces, it’s become increasingly important to keep ourselves and others safe. Fireworks are pretty, but not when they come out of your toilet.
The latest in MRI fashion
Do you see that photo just below? Looks like something you could buy at the Lego store, right? Well, it’s not. Nor is it the proverbial thinking cap come to life.
(Did someone just say “come to life”? That reminds us of our favorite scene from Frosty the Snowman.)
Anywaaay, about the photo. That funny-looking chapeau is what we in the science business call a metamaterial.
Nope, metamaterials have nothing to do with Facebook parent company Meta. We checked. According to a statement from Boston University, they are engineered structures “created from small unit cells that might be unspectacular alone, but when grouped together in a precise way, get new superpowers not found in nature.”
Superpowers, eh? Who doesn’t want superpowers? Even if they come with a funny hat.
The unit cells, known as resonators, are just plastic tubes wrapped in copper wiring, but when they are grouped in an array and precisely arranged into a helmet, they can channel the magnetic field of the MRI machine during a scan. In theory, that would create “crisper images that can be captured at twice the normal speed,” Xin Zhang, PhD, and her team at BU’s Photonics Center explained in the university statement.
In the future, the metamaterial device could “be used in conjunction with cheaper low-field MRI machines to make the technology more widely available, particularly in the developing world,” they suggested. Or, like so many other superpowers, it could fall into the wrong hands. Like those of Lex Luthor. Or Mark Zuckerberg. Or Frosty the Snowman.
The highway of the mind
How fast can you think on your feet? Well, according to a recently published study, it could be a legitimate measure of intelligence. Here’s the science.
Researchers from the University of Würzburg in Germany and Indiana University have suggested that a person’s intelligence score measures the ability, based on certain neuronal networks and their communication structures, to switch between resting state and different task states.
The investigators set up a study to observe almost 800 people while they completed seven tasks. By monitoring brain activity with functional magnetic resonance imaging, the teams found that subjects who had higher intelligence scores required “less adjustment when switching between different cognitive states,” they said in a separate statement.
It comes down to the network architecture of their brains.
Kirsten Hilger, PhD, head of the German group, described it in terms of highways. The resting state of the brain is normal traffic. It’s always moving. Holiday traffic is the task. The ability to handle the increased flow of commuters is a function of the highway infrastructure. The better the infrastructure, the higher the intelligence.
So the next time you’re stuck in traffic, think how efficient your brain would be with such a task. The quicker, the better.
Early, subtle, cardiac changes tied to midlife cognitive decline
new research suggests.
Cardiovascular disease risk factors such as high blood pressure, high cholesterol, and diabetes have been associated with an increased risk for cognitive impairment, but much less is known about heart structure and function and the risks for cognition.
“We showed for the first time that, even before the occurrence of cardiovascular disease, people with abnormalities in cardiac structure and function as early as in young adulthood have lower midlife cognition,” investigators Laure Rouch, PharmD, PhD, and Kristine Yaffe, MD, both with the department of psychiatry, University of California, San Francisco, said in a joint email.
“This study reminds us that heart health is key to brain health and that the overlap and interplay between the two is not limited to patients with end-stage heart disease,” Dr. Rouch and Dr. Yaffe said.
The findings were published online Jan. 26, 2022, in Neurology.
Heart/brain connection
The analysis included 2,653 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Echocardiograms were obtained at year 5, 25, and 30 study visits – at mean ages of 30, 50, and 55 years, respectively. At year 30, participants underwent a standard battery of tests measuring global cognition, processing speed, executive function, delayed verbal memory, and verbal fluency.
Over 25 years, there was an average increase in left ventricular mass of 0.27 g/m2 per year – from a mean of 80.5 g/m2 in year 5 to 86.0 g/m2 in year 30.
Left atrial volume increased by an average of 0.42 mL/m2 per year, from 16 mL/m2 in year 5 to 26 mL/m2 in year 30.
Left ventricular ejection fraction (LVEF) decreased by 0.11% per year, from 63.3% in year 5 to 59.7% in year 30.
After adjustment for demographics and education, an increase in left ventricular mass of at least 1 standard deviation over 25 years was associated with lower cognition on most tests (P ≤ .02).
An increase in left atrial volume over the study period was associated with lower global cognition (P = .04), whereas a decrease in LVEF was not associated with cognition. Further adjustment for cardiovascular risk factors yielded similar results.
“A more effective collaboration is needed between cardiologists and neurologists to promote healthy brain aging,” Dr. Rouch and Dr. Yaffe said.
“Echocardiography is a widely available, relatively inexpensive, and noninvasive imaging method that could be integrated into a risk assessment for cognitive impairment,” they added.
Looking ahead, the investigators noted there is a need for further research to determine whether interventions to improve cardiac structure and diastolic function could also benefit brain health.
They should also investigate the role of arterial stiffness and cerebral small vessel disease in the relationship between cardiac structure, function, and cognition, the researchers added.
First structural biomarker
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said the study is important because, “thus far, the connections have really been physiological parameters,” such as blood pressure and cognitive health.
“This is really strong evidence of a structural cardiac biomarker that can be measured before and independent of changes in physiology or diseased state,” said Dr. Lakhan, who was not involved with the research.
As more and more interventions are being introduced for addressing disorders of cognition, “this potential structural finding may serve as a solid biomarker to determine” what lifestyle or drug therapy should be taken, he added.
Also weighing in on the findings, Pierre Fayad, MD, professor in the department of neurological sciences and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said CARDIA is “an important study” providing “precious data.”
The reported changes in cardiac structure and function “precede the clinical symptomatology, as the follow-up stops before they enter into later adulthood, where the risk of clinical events dramatically rises. Meaning these patients still have not had stroke, congestive heart failure, heart attack or dementia, but some of them could be on that trajectory later in their life,” Dr. Fayad told this news organization.
Documenting such changes over time is “valuable to give an insight into what leads us to such progression,” he noted.
How reliably predictive the findings are for eventual clinical cognitive impairment “will need to be confirmed and verified” in future studies, he added.
“If verified, it could be helpful to provide interventions to those with the left atrial volume enlargement marker and see their effectiveness at preventing eventual clinical cognitive impairment,” said Dr. Fayad.
The CARDIA study is supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Rouch, Lakhan, and Dr. Fayad have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Cardiovascular disease risk factors such as high blood pressure, high cholesterol, and diabetes have been associated with an increased risk for cognitive impairment, but much less is known about heart structure and function and the risks for cognition.
“We showed for the first time that, even before the occurrence of cardiovascular disease, people with abnormalities in cardiac structure and function as early as in young adulthood have lower midlife cognition,” investigators Laure Rouch, PharmD, PhD, and Kristine Yaffe, MD, both with the department of psychiatry, University of California, San Francisco, said in a joint email.
“This study reminds us that heart health is key to brain health and that the overlap and interplay between the two is not limited to patients with end-stage heart disease,” Dr. Rouch and Dr. Yaffe said.
The findings were published online Jan. 26, 2022, in Neurology.
Heart/brain connection
The analysis included 2,653 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Echocardiograms were obtained at year 5, 25, and 30 study visits – at mean ages of 30, 50, and 55 years, respectively. At year 30, participants underwent a standard battery of tests measuring global cognition, processing speed, executive function, delayed verbal memory, and verbal fluency.
Over 25 years, there was an average increase in left ventricular mass of 0.27 g/m2 per year – from a mean of 80.5 g/m2 in year 5 to 86.0 g/m2 in year 30.
Left atrial volume increased by an average of 0.42 mL/m2 per year, from 16 mL/m2 in year 5 to 26 mL/m2 in year 30.
Left ventricular ejection fraction (LVEF) decreased by 0.11% per year, from 63.3% in year 5 to 59.7% in year 30.
After adjustment for demographics and education, an increase in left ventricular mass of at least 1 standard deviation over 25 years was associated with lower cognition on most tests (P ≤ .02).
An increase in left atrial volume over the study period was associated with lower global cognition (P = .04), whereas a decrease in LVEF was not associated with cognition. Further adjustment for cardiovascular risk factors yielded similar results.
“A more effective collaboration is needed between cardiologists and neurologists to promote healthy brain aging,” Dr. Rouch and Dr. Yaffe said.
“Echocardiography is a widely available, relatively inexpensive, and noninvasive imaging method that could be integrated into a risk assessment for cognitive impairment,” they added.
Looking ahead, the investigators noted there is a need for further research to determine whether interventions to improve cardiac structure and diastolic function could also benefit brain health.
They should also investigate the role of arterial stiffness and cerebral small vessel disease in the relationship between cardiac structure, function, and cognition, the researchers added.
First structural biomarker
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said the study is important because, “thus far, the connections have really been physiological parameters,” such as blood pressure and cognitive health.
“This is really strong evidence of a structural cardiac biomarker that can be measured before and independent of changes in physiology or diseased state,” said Dr. Lakhan, who was not involved with the research.
As more and more interventions are being introduced for addressing disorders of cognition, “this potential structural finding may serve as a solid biomarker to determine” what lifestyle or drug therapy should be taken, he added.
Also weighing in on the findings, Pierre Fayad, MD, professor in the department of neurological sciences and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said CARDIA is “an important study” providing “precious data.”
The reported changes in cardiac structure and function “precede the clinical symptomatology, as the follow-up stops before they enter into later adulthood, where the risk of clinical events dramatically rises. Meaning these patients still have not had stroke, congestive heart failure, heart attack or dementia, but some of them could be on that trajectory later in their life,” Dr. Fayad told this news organization.
Documenting such changes over time is “valuable to give an insight into what leads us to such progression,” he noted.
How reliably predictive the findings are for eventual clinical cognitive impairment “will need to be confirmed and verified” in future studies, he added.
“If verified, it could be helpful to provide interventions to those with the left atrial volume enlargement marker and see their effectiveness at preventing eventual clinical cognitive impairment,” said Dr. Fayad.
The CARDIA study is supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Rouch, Lakhan, and Dr. Fayad have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Cardiovascular disease risk factors such as high blood pressure, high cholesterol, and diabetes have been associated with an increased risk for cognitive impairment, but much less is known about heart structure and function and the risks for cognition.
“We showed for the first time that, even before the occurrence of cardiovascular disease, people with abnormalities in cardiac structure and function as early as in young adulthood have lower midlife cognition,” investigators Laure Rouch, PharmD, PhD, and Kristine Yaffe, MD, both with the department of psychiatry, University of California, San Francisco, said in a joint email.
“This study reminds us that heart health is key to brain health and that the overlap and interplay between the two is not limited to patients with end-stage heart disease,” Dr. Rouch and Dr. Yaffe said.
The findings were published online Jan. 26, 2022, in Neurology.
Heart/brain connection
The analysis included 2,653 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Echocardiograms were obtained at year 5, 25, and 30 study visits – at mean ages of 30, 50, and 55 years, respectively. At year 30, participants underwent a standard battery of tests measuring global cognition, processing speed, executive function, delayed verbal memory, and verbal fluency.
Over 25 years, there was an average increase in left ventricular mass of 0.27 g/m2 per year – from a mean of 80.5 g/m2 in year 5 to 86.0 g/m2 in year 30.
Left atrial volume increased by an average of 0.42 mL/m2 per year, from 16 mL/m2 in year 5 to 26 mL/m2 in year 30.
Left ventricular ejection fraction (LVEF) decreased by 0.11% per year, from 63.3% in year 5 to 59.7% in year 30.
After adjustment for demographics and education, an increase in left ventricular mass of at least 1 standard deviation over 25 years was associated with lower cognition on most tests (P ≤ .02).
An increase in left atrial volume over the study period was associated with lower global cognition (P = .04), whereas a decrease in LVEF was not associated with cognition. Further adjustment for cardiovascular risk factors yielded similar results.
“A more effective collaboration is needed between cardiologists and neurologists to promote healthy brain aging,” Dr. Rouch and Dr. Yaffe said.
“Echocardiography is a widely available, relatively inexpensive, and noninvasive imaging method that could be integrated into a risk assessment for cognitive impairment,” they added.
Looking ahead, the investigators noted there is a need for further research to determine whether interventions to improve cardiac structure and diastolic function could also benefit brain health.
They should also investigate the role of arterial stiffness and cerebral small vessel disease in the relationship between cardiac structure, function, and cognition, the researchers added.
First structural biomarker
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said the study is important because, “thus far, the connections have really been physiological parameters,” such as blood pressure and cognitive health.
“This is really strong evidence of a structural cardiac biomarker that can be measured before and independent of changes in physiology or diseased state,” said Dr. Lakhan, who was not involved with the research.
As more and more interventions are being introduced for addressing disorders of cognition, “this potential structural finding may serve as a solid biomarker to determine” what lifestyle or drug therapy should be taken, he added.
Also weighing in on the findings, Pierre Fayad, MD, professor in the department of neurological sciences and director of the Nebraska Stroke Center, University of Nebraska Medical Center, Omaha, said CARDIA is “an important study” providing “precious data.”
The reported changes in cardiac structure and function “precede the clinical symptomatology, as the follow-up stops before they enter into later adulthood, where the risk of clinical events dramatically rises. Meaning these patients still have not had stroke, congestive heart failure, heart attack or dementia, but some of them could be on that trajectory later in their life,” Dr. Fayad told this news organization.
Documenting such changes over time is “valuable to give an insight into what leads us to such progression,” he noted.
How reliably predictive the findings are for eventual clinical cognitive impairment “will need to be confirmed and verified” in future studies, he added.
“If verified, it could be helpful to provide interventions to those with the left atrial volume enlargement marker and see their effectiveness at preventing eventual clinical cognitive impairment,” said Dr. Fayad.
The CARDIA study is supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Rouch, Lakhan, and Dr. Fayad have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Unraveling plaque changes in CAD With elevated Lp(a)
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AHA advice for diabetes patients to stay heart healthy
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Valentin Fuster: ‘Atherosclerosis starts in the femoral artery’
Advances in technology and genomics have given rise to many issues, such as the extent to which genetic and lifestyle factors contribute to the individual-level risk for coronary artery disease, and the extent one’s genetic risk can be offset by a healthy lifestyle.
Over the years, Valentin Fuster, MD, PhD, director of Mount Sinai Heart and physician-in-chief at the Mount Sinai Hospital, both in New York, has focused much of his research on this topic. At the virtual ACC Latin America 2021 conference, the cardiologist spoke about his hypotheses and findings during his opening plenary on imaging genomics, an emerging field that is rapidly identifying genes that influence the brain, cognition, and risk for disease.
Dr. Fuster discussed his research (J Am Coll Cardiol. 2021;77:2777-91; J Am Coll Cardiol. 2020;75:1617-27; J Am Coll Cardiol. 2019;73:1371-82; J Am Coll Cardiol. 2017;70:2979-91; Circulation. 2015;131:2104-13) and spoke about his innovative program that looks at cardiovascular health in people from young children to senior citizens. The work has been a process of learning and discovery. “We’re beginning to understand how the disease can develop earlier and how we can prevent it from getting worse. There’s nothing more beneficial than beginning to see how the disease starts in the arteries – something that we’re able to do with imaging technologies that, in the next 2 years, will be available worldwide.” And “by using imaging biomarkers in conjunction with genomic biomarkers, we’re beginning to get an idea earlier on as to whether the person is at risk.”
We need to be talking more about health and healthy arteries and trying to come up with epistemologies that are more modern, Dr. Fuster said. “To be able to see who we actually are is fascinating, and all of this is completely new” with imaging genomics.
Developing cardiovascular disease can be identified in people aged 40-60 years when seven risk factors – obesity, metabolic syndrome, blood pressure, diabetes, smoking, sedentary lifestyle, and poor nutrition” – are grouped together, he explained. In their 2015 study, Dr. Fuster and colleagues explored, using high-quality three-dimensional ultrasonography, five areas of the body – right and left carotids, aorta, and right and left iliofemorals – in more than 4,000 people with no history of cardiovascular disease.
“The first thing I want to point out is that the disease originates in a territory that is not commonly evaluated. And we had no idea. We only learned about this development through imaging tests, assessing plaques. The disease starts in the femoral artery and, in fact, it starts with an inflammatory process – seen at autopsy – that can lead to fibrosis and, in later years, can form lipid-rich vulnerable plaque,” he said.
His work has shown an increase in disease progression in groups of people who have been monitored for 20 years. What is most interesting is the way lesions are silent and evolve as the years go by.
“Atherosclerosis appears as a silent phenomenon initially and worsens in the presence of risk factors that trigger its progression,” he said.
But can subclinical disease be identified in people who have few or no risk factors? “What we call normal is not, in fact, normal,” said Dr. Fuster. To not have subclinical disease, LDL cholesterol needs to be 70 mg/dL and hemoglobin A1c needs to be 5%-6%, according to a 2020 study by Dr. Fuster and colleagues.
“The fact that we’re seeing people with no apparent risk factors develop atherosclerosis is the reason what we consider normal is not,” he said. It is necessary to take into account what happened in the first 40 years of these individuals’ lives, he added.
Dr. Fuster presented findings on 6,000 people aged 60-100 years underwent three-dimensional ultrasonography and were monitored for 12 years. The data have yet to be published, but they indicate that, with this disease, more than just risk factors are at play; atherosclerosis is related to what happens early on in one’s life.
In their 2016 study of more than 55,000 participants, Dr. Fuster and associates quantified the genetic risk for coronary artery disease with a polygenic risk score derived from an analysis of up to 50 genetic polymorphisms that had been associated with coronary artery disease in previous studies. On the basis of this score, the participants were divided into subgroups by genetic risk: low, intermediate, and high. Genetic and lifestyle factors were independently associated with susceptibility to coronary artery disease. For participants at high genetic risk, a favorable lifestyle was associated with a relative risk for coronary artery disease nearly 50% lower than an unfavorable lifestyle.
The risk factors cause the bone marrow to be activated and, when this happens, an inflammatory process occurs in the arteries. This activation is a defense mechanism designed to help monocytes heal the arteries. “When we’re dealing with a disease in the arteries, inflammation starts in the bone marrow, where cholesterol is deposited, and there are macrophages that, because there’s too much to clean up and they can’t keep up, will actually kill themselves. When that happens, they will release substances that will damage the arteries,” Dr. Fuster reported.
In elderly people, risk factors have an impact not only on the great vessels, they can also lead to cerebral small vessel disease.
“The problem is that, before, we didn’t have the technology to make this observation. And this is something critical with respect to late-onset dementia,” he said, citing a 2016 study on Alzheimer’s disease. Even if risk factors are increasing, the person will not necessarily develop the disease, but there is a greater chance that they will.
Education
Playful activities have a major impact in childhood. With this in mind, Dr. Fuster instituted a 6-month, 60-hour educational program for children aged 3-6 years. The approach was aimed at teaching children about healthy eating habits and how the human body works. “Children are able to absorb everything we say, but then at age 10, it all goes away,” he said. With another intervention that involved the same children, he showed that the benefits were greater than those seen in the first intervention.
“Our hypothesis is that, regardless of age, any program that has to do with prevention needs to be repeated,” Dr. Fuster said. “Repetition will bring more benefits every x years. That’s what we’re learning.
“We learned that when these children go home, they tell their parents what to do. The program had a greater impact on the children than their parents. So we need to use repetition in prevention efforts directed at young children. And we need to remember that the later we start this kind of work, the less impact it will have. The sooner things start, the greater the benefit and the lower the cost,” he concluded.
A version of this article first appeared on Medscape.com.
Advances in technology and genomics have given rise to many issues, such as the extent to which genetic and lifestyle factors contribute to the individual-level risk for coronary artery disease, and the extent one’s genetic risk can be offset by a healthy lifestyle.
Over the years, Valentin Fuster, MD, PhD, director of Mount Sinai Heart and physician-in-chief at the Mount Sinai Hospital, both in New York, has focused much of his research on this topic. At the virtual ACC Latin America 2021 conference, the cardiologist spoke about his hypotheses and findings during his opening plenary on imaging genomics, an emerging field that is rapidly identifying genes that influence the brain, cognition, and risk for disease.
Dr. Fuster discussed his research (J Am Coll Cardiol. 2021;77:2777-91; J Am Coll Cardiol. 2020;75:1617-27; J Am Coll Cardiol. 2019;73:1371-82; J Am Coll Cardiol. 2017;70:2979-91; Circulation. 2015;131:2104-13) and spoke about his innovative program that looks at cardiovascular health in people from young children to senior citizens. The work has been a process of learning and discovery. “We’re beginning to understand how the disease can develop earlier and how we can prevent it from getting worse. There’s nothing more beneficial than beginning to see how the disease starts in the arteries – something that we’re able to do with imaging technologies that, in the next 2 years, will be available worldwide.” And “by using imaging biomarkers in conjunction with genomic biomarkers, we’re beginning to get an idea earlier on as to whether the person is at risk.”
We need to be talking more about health and healthy arteries and trying to come up with epistemologies that are more modern, Dr. Fuster said. “To be able to see who we actually are is fascinating, and all of this is completely new” with imaging genomics.
Developing cardiovascular disease can be identified in people aged 40-60 years when seven risk factors – obesity, metabolic syndrome, blood pressure, diabetes, smoking, sedentary lifestyle, and poor nutrition” – are grouped together, he explained. In their 2015 study, Dr. Fuster and colleagues explored, using high-quality three-dimensional ultrasonography, five areas of the body – right and left carotids, aorta, and right and left iliofemorals – in more than 4,000 people with no history of cardiovascular disease.
“The first thing I want to point out is that the disease originates in a territory that is not commonly evaluated. And we had no idea. We only learned about this development through imaging tests, assessing plaques. The disease starts in the femoral artery and, in fact, it starts with an inflammatory process – seen at autopsy – that can lead to fibrosis and, in later years, can form lipid-rich vulnerable plaque,” he said.
His work has shown an increase in disease progression in groups of people who have been monitored for 20 years. What is most interesting is the way lesions are silent and evolve as the years go by.
“Atherosclerosis appears as a silent phenomenon initially and worsens in the presence of risk factors that trigger its progression,” he said.
But can subclinical disease be identified in people who have few or no risk factors? “What we call normal is not, in fact, normal,” said Dr. Fuster. To not have subclinical disease, LDL cholesterol needs to be 70 mg/dL and hemoglobin A1c needs to be 5%-6%, according to a 2020 study by Dr. Fuster and colleagues.
“The fact that we’re seeing people with no apparent risk factors develop atherosclerosis is the reason what we consider normal is not,” he said. It is necessary to take into account what happened in the first 40 years of these individuals’ lives, he added.
Dr. Fuster presented findings on 6,000 people aged 60-100 years underwent three-dimensional ultrasonography and were monitored for 12 years. The data have yet to be published, but they indicate that, with this disease, more than just risk factors are at play; atherosclerosis is related to what happens early on in one’s life.
In their 2016 study of more than 55,000 participants, Dr. Fuster and associates quantified the genetic risk for coronary artery disease with a polygenic risk score derived from an analysis of up to 50 genetic polymorphisms that had been associated with coronary artery disease in previous studies. On the basis of this score, the participants were divided into subgroups by genetic risk: low, intermediate, and high. Genetic and lifestyle factors were independently associated with susceptibility to coronary artery disease. For participants at high genetic risk, a favorable lifestyle was associated with a relative risk for coronary artery disease nearly 50% lower than an unfavorable lifestyle.
The risk factors cause the bone marrow to be activated and, when this happens, an inflammatory process occurs in the arteries. This activation is a defense mechanism designed to help monocytes heal the arteries. “When we’re dealing with a disease in the arteries, inflammation starts in the bone marrow, where cholesterol is deposited, and there are macrophages that, because there’s too much to clean up and they can’t keep up, will actually kill themselves. When that happens, they will release substances that will damage the arteries,” Dr. Fuster reported.
In elderly people, risk factors have an impact not only on the great vessels, they can also lead to cerebral small vessel disease.
“The problem is that, before, we didn’t have the technology to make this observation. And this is something critical with respect to late-onset dementia,” he said, citing a 2016 study on Alzheimer’s disease. Even if risk factors are increasing, the person will not necessarily develop the disease, but there is a greater chance that they will.
Education
Playful activities have a major impact in childhood. With this in mind, Dr. Fuster instituted a 6-month, 60-hour educational program for children aged 3-6 years. The approach was aimed at teaching children about healthy eating habits and how the human body works. “Children are able to absorb everything we say, but then at age 10, it all goes away,” he said. With another intervention that involved the same children, he showed that the benefits were greater than those seen in the first intervention.
“Our hypothesis is that, regardless of age, any program that has to do with prevention needs to be repeated,” Dr. Fuster said. “Repetition will bring more benefits every x years. That’s what we’re learning.
“We learned that when these children go home, they tell their parents what to do. The program had a greater impact on the children than their parents. So we need to use repetition in prevention efforts directed at young children. And we need to remember that the later we start this kind of work, the less impact it will have. The sooner things start, the greater the benefit and the lower the cost,” he concluded.
A version of this article first appeared on Medscape.com.
Advances in technology and genomics have given rise to many issues, such as the extent to which genetic and lifestyle factors contribute to the individual-level risk for coronary artery disease, and the extent one’s genetic risk can be offset by a healthy lifestyle.
Over the years, Valentin Fuster, MD, PhD, director of Mount Sinai Heart and physician-in-chief at the Mount Sinai Hospital, both in New York, has focused much of his research on this topic. At the virtual ACC Latin America 2021 conference, the cardiologist spoke about his hypotheses and findings during his opening plenary on imaging genomics, an emerging field that is rapidly identifying genes that influence the brain, cognition, and risk for disease.
Dr. Fuster discussed his research (J Am Coll Cardiol. 2021;77:2777-91; J Am Coll Cardiol. 2020;75:1617-27; J Am Coll Cardiol. 2019;73:1371-82; J Am Coll Cardiol. 2017;70:2979-91; Circulation. 2015;131:2104-13) and spoke about his innovative program that looks at cardiovascular health in people from young children to senior citizens. The work has been a process of learning and discovery. “We’re beginning to understand how the disease can develop earlier and how we can prevent it from getting worse. There’s nothing more beneficial than beginning to see how the disease starts in the arteries – something that we’re able to do with imaging technologies that, in the next 2 years, will be available worldwide.” And “by using imaging biomarkers in conjunction with genomic biomarkers, we’re beginning to get an idea earlier on as to whether the person is at risk.”
We need to be talking more about health and healthy arteries and trying to come up with epistemologies that are more modern, Dr. Fuster said. “To be able to see who we actually are is fascinating, and all of this is completely new” with imaging genomics.
Developing cardiovascular disease can be identified in people aged 40-60 years when seven risk factors – obesity, metabolic syndrome, blood pressure, diabetes, smoking, sedentary lifestyle, and poor nutrition” – are grouped together, he explained. In their 2015 study, Dr. Fuster and colleagues explored, using high-quality three-dimensional ultrasonography, five areas of the body – right and left carotids, aorta, and right and left iliofemorals – in more than 4,000 people with no history of cardiovascular disease.
“The first thing I want to point out is that the disease originates in a territory that is not commonly evaluated. And we had no idea. We only learned about this development through imaging tests, assessing plaques. The disease starts in the femoral artery and, in fact, it starts with an inflammatory process – seen at autopsy – that can lead to fibrosis and, in later years, can form lipid-rich vulnerable plaque,” he said.
His work has shown an increase in disease progression in groups of people who have been monitored for 20 years. What is most interesting is the way lesions are silent and evolve as the years go by.
“Atherosclerosis appears as a silent phenomenon initially and worsens in the presence of risk factors that trigger its progression,” he said.
But can subclinical disease be identified in people who have few or no risk factors? “What we call normal is not, in fact, normal,” said Dr. Fuster. To not have subclinical disease, LDL cholesterol needs to be 70 mg/dL and hemoglobin A1c needs to be 5%-6%, according to a 2020 study by Dr. Fuster and colleagues.
“The fact that we’re seeing people with no apparent risk factors develop atherosclerosis is the reason what we consider normal is not,” he said. It is necessary to take into account what happened in the first 40 years of these individuals’ lives, he added.
Dr. Fuster presented findings on 6,000 people aged 60-100 years underwent three-dimensional ultrasonography and were monitored for 12 years. The data have yet to be published, but they indicate that, with this disease, more than just risk factors are at play; atherosclerosis is related to what happens early on in one’s life.
In their 2016 study of more than 55,000 participants, Dr. Fuster and associates quantified the genetic risk for coronary artery disease with a polygenic risk score derived from an analysis of up to 50 genetic polymorphisms that had been associated with coronary artery disease in previous studies. On the basis of this score, the participants were divided into subgroups by genetic risk: low, intermediate, and high. Genetic and lifestyle factors were independently associated with susceptibility to coronary artery disease. For participants at high genetic risk, a favorable lifestyle was associated with a relative risk for coronary artery disease nearly 50% lower than an unfavorable lifestyle.
The risk factors cause the bone marrow to be activated and, when this happens, an inflammatory process occurs in the arteries. This activation is a defense mechanism designed to help monocytes heal the arteries. “When we’re dealing with a disease in the arteries, inflammation starts in the bone marrow, where cholesterol is deposited, and there are macrophages that, because there’s too much to clean up and they can’t keep up, will actually kill themselves. When that happens, they will release substances that will damage the arteries,” Dr. Fuster reported.
In elderly people, risk factors have an impact not only on the great vessels, they can also lead to cerebral small vessel disease.
“The problem is that, before, we didn’t have the technology to make this observation. And this is something critical with respect to late-onset dementia,” he said, citing a 2016 study on Alzheimer’s disease. Even if risk factors are increasing, the person will not necessarily develop the disease, but there is a greater chance that they will.
Education
Playful activities have a major impact in childhood. With this in mind, Dr. Fuster instituted a 6-month, 60-hour educational program for children aged 3-6 years. The approach was aimed at teaching children about healthy eating habits and how the human body works. “Children are able to absorb everything we say, but then at age 10, it all goes away,” he said. With another intervention that involved the same children, he showed that the benefits were greater than those seen in the first intervention.
“Our hypothesis is that, regardless of age, any program that has to do with prevention needs to be repeated,” Dr. Fuster said. “Repetition will bring more benefits every x years. That’s what we’re learning.
“We learned that when these children go home, they tell their parents what to do. The program had a greater impact on the children than their parents. So we need to use repetition in prevention efforts directed at young children. And we need to remember that the later we start this kind of work, the less impact it will have. The sooner things start, the greater the benefit and the lower the cost,” he concluded.
A version of this article first appeared on Medscape.com.