User login
Recurrent Bleeding in Small-Intestinal Angiodysplasia Reduced by Thalidomide
, according to results of a new placebo-controlled trial.
At 1 year follow-up, thalidomide doses of 100 mg/day and 50 mg/day outperformed placebo in reducing by at least 50% the number of bleeding episodes, compared with the year prior to treatment, according to the study published online in the New England Journal of Medicine.
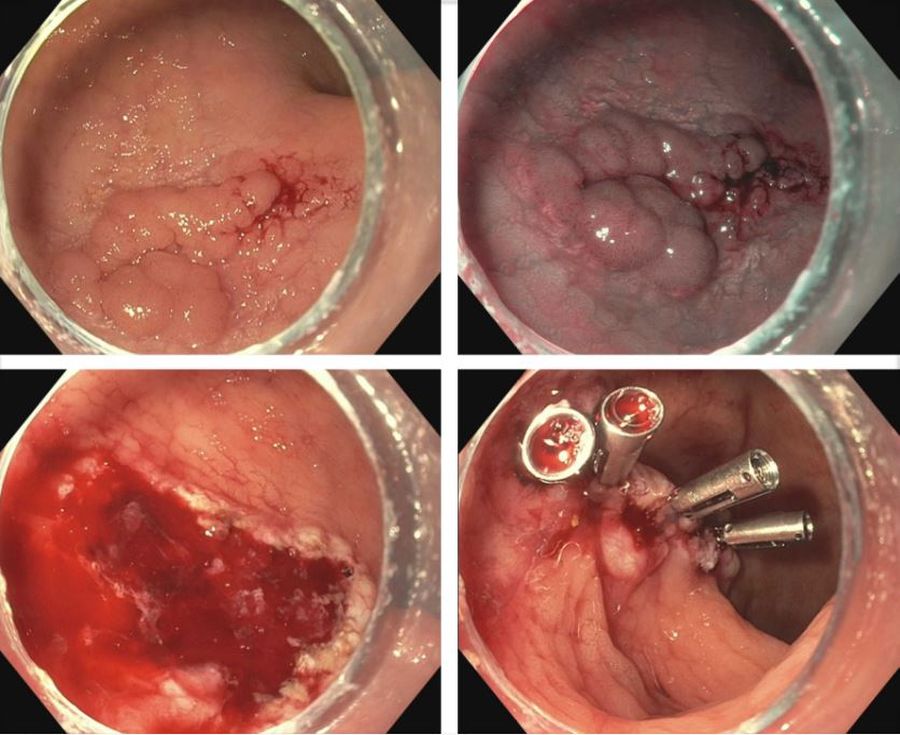
SIA, an increasingly recognized cause of repeat obscure gastrointestinal bleeding and iron-deficiency anemia, is a distinct vascular abnormality in the mucosa and submucosa characterized by focal accumulation of ectatic vessels. It is the most common cause of small intestine bleeding, especially among patients older than 50.
There is a high unmet need among patients with SIA for an effective and relatively safe oral medication, given substantial recurrent bleeding risks following endoscopic or surgical procedures, and only observational studies suggest treatment with somatostatin and octreotide, noted senior author Zhizheng Ge, MD, Shanghai Jiao Tong University, Shanghai, China.
SIA is characterized by dilated and tortuous arterial or venous capillaries between thin-walled and immature veins and capillaries without a smooth-muscle layer. Its pathologic process involves chronic hypoxia and vessel sprouting.
Dr. Ge and colleagues postulated that thalidomide’s ability to decrease the expression of proangiogenic factors and angiogenesis would have a long-lasting ameliorating effect on bleeding episodes of angiodysplasia, and thus a continued benefit with respect to bleeding cessation. Their previous small, single-center, open-label, randomized controlled trial of thalidomide for SIA showed a benefit, but it required larger confirmatory trials.
For their current trial, the researchers explored whether a short treatment period, selected to avoid treatment nonadherence, could have a long-term effect. They randomly assigned on a 1:1:1 basis 150 patients with recurrent SIA-related bleeding, defined as at least four episodes during the previous year, to an oral daily dose of 100 mg of thalidomide, 50 mg of thalidomide, or placebo for 4 months.
The patients (median age, 62.2 years; 88% aged 50 years or older) were followed for at least 1 year after treatment. The trial was conducted at 10 sites in China.
The primary endpoint was effective response, defined as a reduction of at least 50% in the number of bleeding episodes in the year following thalidomide treatment, compared with the number in the year before treatment. Bleeding was defined as the presence of overt bleeding or a positive fecal occult blood test.
The percentages of patients with effective response at 1-year follow-up were 68.6% in the 100-mg thalidomide group, 51% in the 50-mg thalidomide group, and 16% in the placebo group.
Among secondary endpoints, the incidence of rebleeding during the 4-month treatment period was 27.5% (14 of 51 patients) in the 100-mg thalidomide group, 42.9% (21 of 49 patients) in the 50-mg thalidomide group, and 90% (45 of 50 patients) in the placebo group. The percentage of patients who received a blood transfusion during the 1-year follow-up period were 17.6% in the 100-mg thalidomide group, 24.5% in the 50-mg thalidomide group, and 62% in the placebo group.
Cessation of bleeding, defined by two consecutive negative fecal occult blood tests on different days, during 1 year of follow-up was observed in 44 patients: 26 (51%) of patients in the 100-mg thalidomide group, 16 (32.7%) in the 50-mg thalidomide group, and 2 (4%) in the placebo group. The authors urge further exploration of the duration of benefit and the efficacy of longer courses of treatment.
Adverse events, all grade 1 or 2, resolved after treatment of symptoms, completion of treatment, or discontinuation of thalidomide or placebo.
Retreatment May Be Necessary
In an accompanying editorial, Loren Laine, MD, chief of the section of digestive diseases, internal medicine, and medical chief, digestive health, Yale School of Medicine, New Haven, Connecticut, affirmed the authors’ conclusions and commended the quality of evidence they provided.
“Their results suggest that thalidomide may be disease-modifying, with efficacy persisting after discontinuation,” wrote Dr. Laine, also a Yale professor of medicine and digestive diseases.
While thalidomide effectively prevented rebleeding for 42 patients during the year after therapy was stopped, suggesting an alteration of angiodysplasias, rebleeding during the subsequent 3-27 months occurred among 20 of those patients, Dr. Laine noted. That finding, “suggests that retreatment will be needed,” although the appropriate duration of treatment before retreatment and the duration of retreatment remain unclear, he added.
The study’s reliance on bleeding episodes that were defined by positive fecal occult blood tests, which may be clinically unimportant, is a weakness in the trial, Dr. Laine wrote.
Despite the study’s positive findings, clinicians may still prefer somatostatin analogues because of their potential for better safety and, with once-monthly injections versus daily thalidomide pills, their likelihood for better adherence, Dr. Laine wrote. “[They] will reserve thalidomide for use in patients who have continued bleeding or side effects with somatostatin analogues,” he added.
Somatostatin is rarely used in the treatment of SIA bleeding in China, where thalidomide is relatively easy to obtain and is being used clinically, Dr. Ge told this news organization in response to Dr. Laine’s editorial. “The clinical application of thalidomide has been taken up in other [Chinese] hospitals that have seen our research,” he added.
Future research may include randomized controlled trials of somatostatin, since Chinese experience with it is so limited, Dr. Ge said. “We would want to compare efficacy, safety, feasibility and cost-effectiveness between somatostatin and thalidomide,” he added.
The study was supported by grants from the National Natural Science Foundation of China and a grant from the Shanghai Municipal Education Commission, Gaofeng Clinical Medicine. The author disclosures can be found with the original article.
, according to results of a new placebo-controlled trial.
At 1 year follow-up, thalidomide doses of 100 mg/day and 50 mg/day outperformed placebo in reducing by at least 50% the number of bleeding episodes, compared with the year prior to treatment, according to the study published online in the New England Journal of Medicine.
SIA, an increasingly recognized cause of repeat obscure gastrointestinal bleeding and iron-deficiency anemia, is a distinct vascular abnormality in the mucosa and submucosa characterized by focal accumulation of ectatic vessels. It is the most common cause of small intestine bleeding, especially among patients older than 50.
There is a high unmet need among patients with SIA for an effective and relatively safe oral medication, given substantial recurrent bleeding risks following endoscopic or surgical procedures, and only observational studies suggest treatment with somatostatin and octreotide, noted senior author Zhizheng Ge, MD, Shanghai Jiao Tong University, Shanghai, China.
SIA is characterized by dilated and tortuous arterial or venous capillaries between thin-walled and immature veins and capillaries without a smooth-muscle layer. Its pathologic process involves chronic hypoxia and vessel sprouting.
Dr. Ge and colleagues postulated that thalidomide’s ability to decrease the expression of proangiogenic factors and angiogenesis would have a long-lasting ameliorating effect on bleeding episodes of angiodysplasia, and thus a continued benefit with respect to bleeding cessation. Their previous small, single-center, open-label, randomized controlled trial of thalidomide for SIA showed a benefit, but it required larger confirmatory trials.
For their current trial, the researchers explored whether a short treatment period, selected to avoid treatment nonadherence, could have a long-term effect. They randomly assigned on a 1:1:1 basis 150 patients with recurrent SIA-related bleeding, defined as at least four episodes during the previous year, to an oral daily dose of 100 mg of thalidomide, 50 mg of thalidomide, or placebo for 4 months.
The patients (median age, 62.2 years; 88% aged 50 years or older) were followed for at least 1 year after treatment. The trial was conducted at 10 sites in China.
The primary endpoint was effective response, defined as a reduction of at least 50% in the number of bleeding episodes in the year following thalidomide treatment, compared with the number in the year before treatment. Bleeding was defined as the presence of overt bleeding or a positive fecal occult blood test.
The percentages of patients with effective response at 1-year follow-up were 68.6% in the 100-mg thalidomide group, 51% in the 50-mg thalidomide group, and 16% in the placebo group.
Among secondary endpoints, the incidence of rebleeding during the 4-month treatment period was 27.5% (14 of 51 patients) in the 100-mg thalidomide group, 42.9% (21 of 49 patients) in the 50-mg thalidomide group, and 90% (45 of 50 patients) in the placebo group. The percentage of patients who received a blood transfusion during the 1-year follow-up period were 17.6% in the 100-mg thalidomide group, 24.5% in the 50-mg thalidomide group, and 62% in the placebo group.
Cessation of bleeding, defined by two consecutive negative fecal occult blood tests on different days, during 1 year of follow-up was observed in 44 patients: 26 (51%) of patients in the 100-mg thalidomide group, 16 (32.7%) in the 50-mg thalidomide group, and 2 (4%) in the placebo group. The authors urge further exploration of the duration of benefit and the efficacy of longer courses of treatment.
Adverse events, all grade 1 or 2, resolved after treatment of symptoms, completion of treatment, or discontinuation of thalidomide or placebo.
Retreatment May Be Necessary
In an accompanying editorial, Loren Laine, MD, chief of the section of digestive diseases, internal medicine, and medical chief, digestive health, Yale School of Medicine, New Haven, Connecticut, affirmed the authors’ conclusions and commended the quality of evidence they provided.
“Their results suggest that thalidomide may be disease-modifying, with efficacy persisting after discontinuation,” wrote Dr. Laine, also a Yale professor of medicine and digestive diseases.
While thalidomide effectively prevented rebleeding for 42 patients during the year after therapy was stopped, suggesting an alteration of angiodysplasias, rebleeding during the subsequent 3-27 months occurred among 20 of those patients, Dr. Laine noted. That finding, “suggests that retreatment will be needed,” although the appropriate duration of treatment before retreatment and the duration of retreatment remain unclear, he added.
The study’s reliance on bleeding episodes that were defined by positive fecal occult blood tests, which may be clinically unimportant, is a weakness in the trial, Dr. Laine wrote.
Despite the study’s positive findings, clinicians may still prefer somatostatin analogues because of their potential for better safety and, with once-monthly injections versus daily thalidomide pills, their likelihood for better adherence, Dr. Laine wrote. “[They] will reserve thalidomide for use in patients who have continued bleeding or side effects with somatostatin analogues,” he added.
Somatostatin is rarely used in the treatment of SIA bleeding in China, where thalidomide is relatively easy to obtain and is being used clinically, Dr. Ge told this news organization in response to Dr. Laine’s editorial. “The clinical application of thalidomide has been taken up in other [Chinese] hospitals that have seen our research,” he added.
Future research may include randomized controlled trials of somatostatin, since Chinese experience with it is so limited, Dr. Ge said. “We would want to compare efficacy, safety, feasibility and cost-effectiveness between somatostatin and thalidomide,” he added.
The study was supported by grants from the National Natural Science Foundation of China and a grant from the Shanghai Municipal Education Commission, Gaofeng Clinical Medicine. The author disclosures can be found with the original article.
, according to results of a new placebo-controlled trial.
At 1 year follow-up, thalidomide doses of 100 mg/day and 50 mg/day outperformed placebo in reducing by at least 50% the number of bleeding episodes, compared with the year prior to treatment, according to the study published online in the New England Journal of Medicine.
SIA, an increasingly recognized cause of repeat obscure gastrointestinal bleeding and iron-deficiency anemia, is a distinct vascular abnormality in the mucosa and submucosa characterized by focal accumulation of ectatic vessels. It is the most common cause of small intestine bleeding, especially among patients older than 50.
There is a high unmet need among patients with SIA for an effective and relatively safe oral medication, given substantial recurrent bleeding risks following endoscopic or surgical procedures, and only observational studies suggest treatment with somatostatin and octreotide, noted senior author Zhizheng Ge, MD, Shanghai Jiao Tong University, Shanghai, China.
SIA is characterized by dilated and tortuous arterial or venous capillaries between thin-walled and immature veins and capillaries without a smooth-muscle layer. Its pathologic process involves chronic hypoxia and vessel sprouting.
Dr. Ge and colleagues postulated that thalidomide’s ability to decrease the expression of proangiogenic factors and angiogenesis would have a long-lasting ameliorating effect on bleeding episodes of angiodysplasia, and thus a continued benefit with respect to bleeding cessation. Their previous small, single-center, open-label, randomized controlled trial of thalidomide for SIA showed a benefit, but it required larger confirmatory trials.
For their current trial, the researchers explored whether a short treatment period, selected to avoid treatment nonadherence, could have a long-term effect. They randomly assigned on a 1:1:1 basis 150 patients with recurrent SIA-related bleeding, defined as at least four episodes during the previous year, to an oral daily dose of 100 mg of thalidomide, 50 mg of thalidomide, or placebo for 4 months.
The patients (median age, 62.2 years; 88% aged 50 years or older) were followed for at least 1 year after treatment. The trial was conducted at 10 sites in China.
The primary endpoint was effective response, defined as a reduction of at least 50% in the number of bleeding episodes in the year following thalidomide treatment, compared with the number in the year before treatment. Bleeding was defined as the presence of overt bleeding or a positive fecal occult blood test.
The percentages of patients with effective response at 1-year follow-up were 68.6% in the 100-mg thalidomide group, 51% in the 50-mg thalidomide group, and 16% in the placebo group.
Among secondary endpoints, the incidence of rebleeding during the 4-month treatment period was 27.5% (14 of 51 patients) in the 100-mg thalidomide group, 42.9% (21 of 49 patients) in the 50-mg thalidomide group, and 90% (45 of 50 patients) in the placebo group. The percentage of patients who received a blood transfusion during the 1-year follow-up period were 17.6% in the 100-mg thalidomide group, 24.5% in the 50-mg thalidomide group, and 62% in the placebo group.
Cessation of bleeding, defined by two consecutive negative fecal occult blood tests on different days, during 1 year of follow-up was observed in 44 patients: 26 (51%) of patients in the 100-mg thalidomide group, 16 (32.7%) in the 50-mg thalidomide group, and 2 (4%) in the placebo group. The authors urge further exploration of the duration of benefit and the efficacy of longer courses of treatment.
Adverse events, all grade 1 or 2, resolved after treatment of symptoms, completion of treatment, or discontinuation of thalidomide or placebo.
Retreatment May Be Necessary
In an accompanying editorial, Loren Laine, MD, chief of the section of digestive diseases, internal medicine, and medical chief, digestive health, Yale School of Medicine, New Haven, Connecticut, affirmed the authors’ conclusions and commended the quality of evidence they provided.
“Their results suggest that thalidomide may be disease-modifying, with efficacy persisting after discontinuation,” wrote Dr. Laine, also a Yale professor of medicine and digestive diseases.
While thalidomide effectively prevented rebleeding for 42 patients during the year after therapy was stopped, suggesting an alteration of angiodysplasias, rebleeding during the subsequent 3-27 months occurred among 20 of those patients, Dr. Laine noted. That finding, “suggests that retreatment will be needed,” although the appropriate duration of treatment before retreatment and the duration of retreatment remain unclear, he added.
The study’s reliance on bleeding episodes that were defined by positive fecal occult blood tests, which may be clinically unimportant, is a weakness in the trial, Dr. Laine wrote.
Despite the study’s positive findings, clinicians may still prefer somatostatin analogues because of their potential for better safety and, with once-monthly injections versus daily thalidomide pills, their likelihood for better adherence, Dr. Laine wrote. “[They] will reserve thalidomide for use in patients who have continued bleeding or side effects with somatostatin analogues,” he added.
Somatostatin is rarely used in the treatment of SIA bleeding in China, where thalidomide is relatively easy to obtain and is being used clinically, Dr. Ge told this news organization in response to Dr. Laine’s editorial. “The clinical application of thalidomide has been taken up in other [Chinese] hospitals that have seen our research,” he added.
Future research may include randomized controlled trials of somatostatin, since Chinese experience with it is so limited, Dr. Ge said. “We would want to compare efficacy, safety, feasibility and cost-effectiveness between somatostatin and thalidomide,” he added.
The study was supported by grants from the National Natural Science Foundation of China and a grant from the Shanghai Municipal Education Commission, Gaofeng Clinical Medicine. The author disclosures can be found with the original article.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Caring for LGBTQ+ Patients with IBD
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
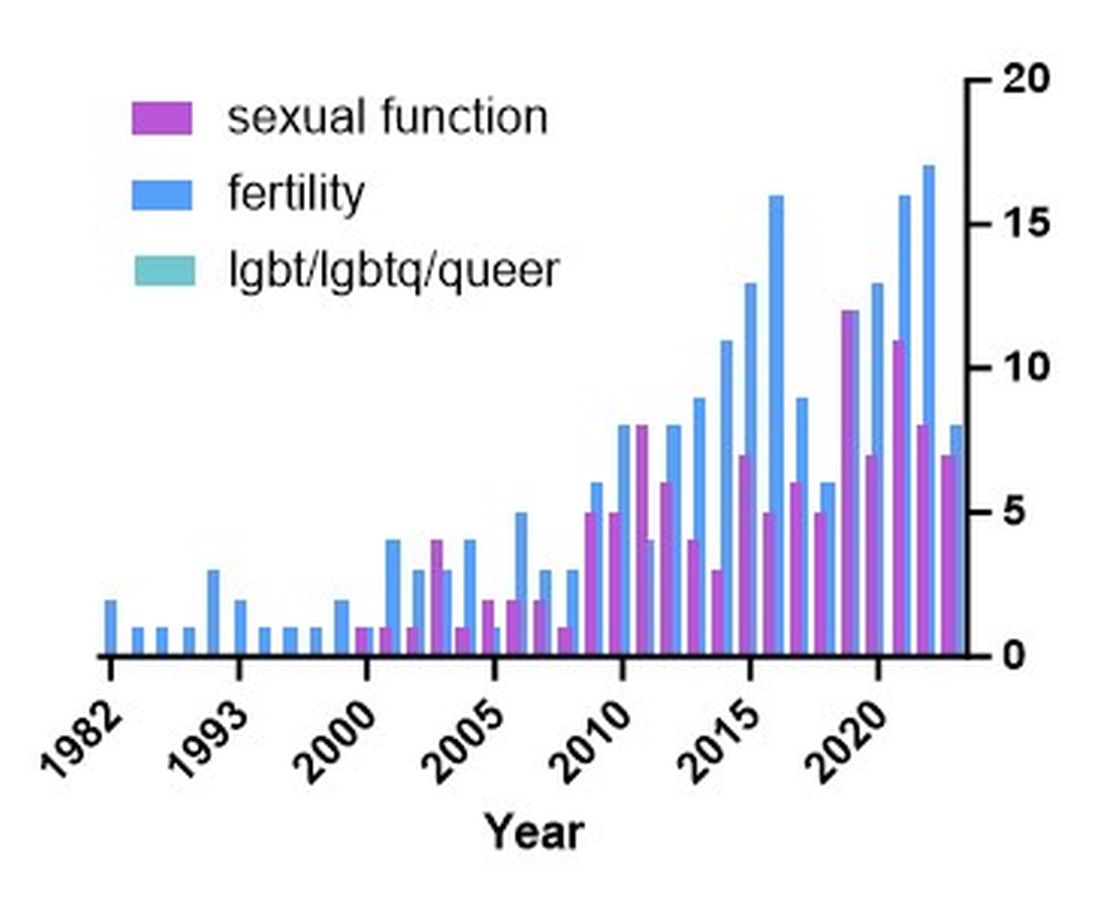
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.
Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.
Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.
Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Cases
Patient 1: 55-year-old cis-male, who identifies as gay, has ulcerative colitis that has been refractory to multiple biologic therapies. His provider recommends a total proctocolectomy with ileal pouch anal anastomosis (TPC with IPAA), but the patient has questions regarding sexual function following surgery. Specifically, he is wondering when, or if, he can resume receptive anal intercourse. How would you counsel him?
Patient 2: 25-year-old, trans-female, status-post vaginoplasty with use of sigmoid colon and with well-controlled ulcerative colitis, presents with vaginal discharge, weight loss, and rectal bleeding. How do you explain what has happened to her? During your discussion, she also asks you why her chart continues to use her “dead name.” How do you respond?
Patient 3: 32-year-old, cis-female, G2P2, who identifies as a lesbian, has active ulcerative colitis. She wants to discuss medical or surgical therapy and future pregnancies. How would you counsel her?
Many gastroenterologists would likely know how to address patient 3’s concerns, but the concerns of patients 1 and 2 often go unaddressed or dismissed. Numerous studies and surveys have been conducted on patients with inflammatory bowel disease (IBD), but the focus of these studies has always been through a heteronormative cisgender lens. The focus of many studies is on fertility or sexual health and function in cisgender, heteronormative individuals.1-3 In the last few years, however, there has been increasing awareness of the health disparities, stigma, and discrimination that sexual and gender minorities (SGM) experience.4-6 For the purposes of this discussion, individuals within the lesbian, gay, bisexual, transgender, queer/questioning, intersex, and asexual (LGBTQIA+) community will be referred to as SGM. We recognize that even this exhaustive listing above does not acknowledge the full spectrum of diversity within the SGM community.
Clinical Care/Competency for SGM with IBD is Lacking
Almost 10% of the US population identifies as some form of SGM, and that number can be higher within the younger generations.4 SGM patients tend to delay or avoid seeking health care due to concern for provider mistreatment or lack of regard for their individual concerns. Additionally, there are several gaps in clinical knowledge about caring for SGM individuals. Little is known regarding the incidence or prevalence of IBD in SGM populations, but it is perceived to be similar to cisgender heterosexual individuals. Furthermore, as Newman et al. highlighted in their systematic review published in May 2023, there is a lack of guidance regarding sexual activity in the setting of IBD in SGM individuals.5 There is also a significant lack of knowledge on the impact of gender-affirming care on the natural history and treatments of IBD in transgender and gender non-conforming (TGNC) individuals. This can impact providers’ comfort and competence in caring for TGNC individuals.
Another important point to make is that the SGM community still faces discrimination due to sexual orientation or gender identity to this day, which impacts the quality and delivery of their care.7 Culturally-competent care should include care that is free from stigma, implicit and explicit biases, and discrimination. In 2011, an Institute of Medicine report documented, among other issues, provider discomfort in delivering care to SGM patients.8 While SGM individuals prefer a provider who acknowledges their sexual orientation and gender identity and treats them with the dignity and respect they deserve, many SGM individuals share valid concerns regarding their safety, which impact their desire to disclose their identity to health care providers.9 This certainly can have an impact on the quality of care they receive, including important health maintenance milestones and cancer screenings.10
An internal survey at our institution of providers (nurses, physician assistants, surgeons, and physicians) found that among 85 responders, 70% have cared for SGM who have undergone TPC with ileal pouch anal anastomosis (IPAA). Of these, 75% did not ask about sexual orientation or practices before pouch formation (though almost all of them agreed it would be important to ask). A total of 55% were comfortable in discussing SGM-related concerns; 53% did not feel comfortable discussing sexual orientation or practices; and in particular when it came to anoreceptive intercourse (ARI), 73% did not feel confident discussing recommendations.11
All of these issues highlight the importance of developing curricula that focus on reducing implicit and explicit biases towards SGM individuals and increasing the competence of providers to take care of SGM individuals in a safe space.
Additionally, it further justifies the need for ethical research that focuses on the needs of SGM individuals to guide evidence-based approaches to care. Given the implicit and explicit heterosexism and transphobia in society and many health care systems, Rainbows in Gastro was formed as an advocacy group for SGM patients, trainees, and staff in gastroenterology and hepatology.4
Research in SGM and IBD is lacking
There are additional needs for research in IBD and how it pertains to the needs of SGM individuals. Figure 1 highlights the lack of PubMed results for the search terms “IBD + LGBT,” “IBD + LGBTQ,” or “IBD + queer.” In contrast, the search terms “IBD + fertility” and “IBD + sexual dysfunction” generate many results. Even a systemic review conducted by Newman et al. of multiple databases in 2022 found only seven articles that demonstrated appropriately performed studies on SGM patients with IBD.5 This highlights the significant dearth of research in the realm of SGM health in IBD.

Newman and colleagues have recently published research considerations for SGM individuals. They highlighted the need to include understanding the “unique combination of psychosocial, biomedical, and legal experiences” that results in different needs and outcomes. There were several areas identified, including minority stress, which comes from existence of being SGM, especially as transgender individuals face increasing legal challenges in a variety of settings, not just healthcare.6 In a retrospective chart review investigating social determinants of health in SGM-IBD populations,12 36% of patients reported some level of social isolation, and almost 50% reported some level of stress. A total of 40% of them self-reported some perceived level of risk with respect to employment, and 17% reported depression. Given that this was a chart review and not a strict questionnaire, this study was certainly limited, and we would hypothesize that these numbers are therefore underestimating the true proportion of SGM-IBD patients who deal with employment concerns, social isolation, or psychological distress.
What Next? Back to the Patients
Circling back to our patients from the introduction, how would you counsel each of them? In patient 1’s case, we would inform him that pelvic surgery can increase the risk for sexual dysfunction, such as erectile dysfunction. He additionally would be advised during a staged TPC with IPAA, he may experience issues with body image. However, should he desire to participate in receptive anal intercourse after completion of his surgeries, the general recommendation would be to wait at least 6 months and with proven remission. It should further be noted that these are not formalized recommendations, only highlighting the need for more research and consensus on standards of care for SGM patients. He should finally be told that because he has ulcerative colitis, removal of the colon does not remove the risk for future intestinal involvement such as possible pouchitis.
In patient 2’s case, she is likely experiencing diversion vaginitis related to use of her colon for her neo-vagina. She should undergo colonoscopy and vaginoscopy in addition to standard work-up for her known ulcerative colitis.13 Management should be done in a multidisciplinary approach between the IBD provider, gynecologist, and gender-affirming provider. The electronic medical record should be updated to reflect the patient’s preferred name, pronouns, and gender identity, and her medical records, including automated clinical reports, should be updated accordingly.
As for patient 3, she would be counseled according to well-documented guidelines on pregnancy and IBD, including risks of medications (such as Jak inhibitors or methotrexate) versus the risk of uncontrolled IBD during pregnancy.1
Regardless of a patient’s gender identity or sexual orientation, patient-centered, culturally competent, and sensitive care should be provided. At Mayo Clinic in Rochester, we started one of the first Pride in IBD Clinics, which focuses on the care of SGM individuals with IBD. Our focus is to address the needs of patients who belong to the SGM community in a wholistic approach within a safe space (https://www.youtube.com/watch?v=pYa_zYaCA6M; https://www.mayoclinic.org/departments-centers/inflammatory-bowel-disease-clinic/overview/ovc-20357763). Our process of developing the clinic included training all staff on proper communication and cultural sensitivity for the SGM community.
Furthermore, providing welcoming and affirming signs of inclusivity for SGM individuals at the provider’s office — including but not limited to rainbow progressive flags, gender-neutral bathroom signs, or pronoun pins on provider identification badges (see Figure 2) — are usually appreciated by patients. Ensuring that patient education materials do not assume gender (for example, using the term “parents” rather than “mother and father”) and using gender neutral terms on intake forms is very important. Inclusive communication includes providers introducing themselves by preferred name and pronouns, asking the patients to introduce themselves, and welcoming them to share their pronouns. These simple actions can provide an atmosphere of safety for SGM patients, which would serve to enhance the quality of care we can provide for them.
For Resources and Further Reading: CDC,14 the Fenway Institute’s National LGBTQIA+ Health Education Center,15 and US Department of Health and Human Services.16
Dr. Chiang and Dr. Chedid are both in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Chedid is also with the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic. Neither of the authors have any relevant conflicts of interest. They are on X, formerly Twitter: @dr_davidchiang , @VictorChedidMD .
CITATIONS
1. Mahadevan U et al. Inflammatory bowel disease in pregnancy clinical care pathway: A report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-24.
2. Pires F et al. A survey on the impact of IBD in sexual health: Into intimacy. Medicine (Baltimore). 2022;101:e32279.
3. Mules TC et al. The impact of disease activity on sexual and erectile dysfunction in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1244-54.
4. Duong N et al. Overcoming disparities for sexual and gender minority patients and providers in gastroenterology and hepatology: Introduction to Rainbows in Gastro. Lancet Gastroenterol Hepatol. 2023;8:299-301.
5. Newman KL et al. A systematic review of inflammatory bowel disease epidemiology and health outcomes in sexual and gender minority individuals. Gastroenterology. 2023;164:866-71.
6. Newman KL et al. Research considerations in Digestive and liver disease in transgender and gender-diverse populations. Gastroenterology. 2023;165:523-28 e1.
7. Velez C et al. Digestive health in sexual and gender minority populations. Am J Gastroenterol. 2022;117:865-75.
8. Medicine Io. Washington (DC): The National Academies Press, 2011.
9. Austin EL. Sexual orientation disclosure to health care providers among urban and non-urban southern lesbians. Women Health. 2013;53:41-55.
10. Oladeru OT et al. Breast and cervical cancer screening disparities in transgender people. Am J Clin Oncol. 2022;45:116-21.
11. Vinsard DG et al. Healthcare providers’ perspectives on anoreceptive intercourse in sexual and gender minorities with ileal pouch anal anastomosis. Digestive Disease Week (DDW). Chicago, IL, 2023.
12. Ghusn W et al. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. American College of Gastroenterology (ACG). Charlotte, NC, 2022.
13. Grasman ME et al. Neovaginal sparing in a transgender woman with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:e73-4.
14. Prevention CfDCa. Lesbian, Gay, Bisexual, and Transgender Health — https://www.cdc.gov/lgbthealth/index.htm.
15. Institute TF. National LGBTQIA+ Health Education Center — https://www.lgbtqiahealtheducation.org/.
16. Services UDoHaH. LGBTQI+ Resources — https://www.hhs.gov/programs/topic-sites/lgbtqi/resources/index.html.
Real-world evidence: Early ileocecal resection outperforms anti-TNF therapy for Crohn’s disease
These findings add weight to previously reported data from the LIR!C trial, suggesting that ileocecal resection should be considered a first-line treatment option for CD, reported principal investigator Kristine H. Allin, MD, PhD, of Aalborg University, Copenhagen.
“The LIR!C randomized clinical trial has demonstrated comparable quality of life with ileocecal resection and infliximab as a first-line treatment for limited, nonstricturing ileocecal CD at 1 year of follow-up, and improved outcomes with ileocecal resection on retrospective analysis of long-term follow-up data,” the investigators wrote in Gastroenterology. “However, in the real world, the long-term impact of early ileocecal resection for CD, compared with medical therapy, remains largely unexplored.”
To gather these real-world data, the investigators turned to the Danish National Patient Registry and the Danish National Prescription Registry, which included 1,279 individuals diagnosed with CD between 2003 and 2018 who received anti-TNF therapy or underwent ileocecal resection within 1 year of diagnosis. Within this group, slightly less than half underwent ileocecal resection (45.4%) while the remainder (54.6%) received anti-TNF therapy.
The primary outcome was a composite of one or more events: perianal CD, CD-related surgery, systemic corticosteroid exposure, and CD-related hospitalization. Secondary analyses evaluated the relative risks of these same four events as independent entities.
Multifactor-adjusted Cox proportional hazards regression analysis revealed that patients who underwent ileocecal resection had a 33% lower risk of the composite outcome compared with those who received anti-TNF therapy (adjusted hazard ratio [aHR], 0.67; 95% CI, 0.54-0.83).
In the secondary analyses, which examined risks for each component of the composite outcome, the surgery group had a significantly lower risk of CD-related surgery (aHR, 0.56; 95% CI, 0.39-0.80) and corticosteroid exposure (aHR, 0.71; 95% CI, 0.54-0.92), but not perianal CD or CD-related hospitalization.
After 5 years, half of the patients (49.7%) who underwent ileocecal resection were not receiving any treatment for CD. At the same timepoint, a slightly lower percentage of this group (46.3%) had started immunomodulator therapy, while 16.8% started anti-TNF therapy. Just 1.8% of these patients required a second intestinal resection.
“To our knowledge, these are the first real-world data in a population-based cohort with long-term follow-up of early ileocecal resection compared with anti-TNF therapy for newly diagnosed ileal and ileocecal CD,” the investigators wrote. “These data suggest that ileocecal resection may have a role as first-line therapy in Crohn’s disease management and challenge the current paradigm of reserving surgery for complicated Crohn’s disease refractory or intolerant to medications.”
Corresponding author Manasi Agrawal, MD, of Icahn School of Medicine at Mount Sinai, New York, suggested that “validation of our findings in external cohorts [is needed], and understanding of factors associated with improved outcomes following ileocecal resection.”
For clinicians and patients choosing between first-line anti-TNF therapy versus ileocecal resection using currently available evidence, Dr. Agrawal suggested that a variety of factors need to be considered, including disease location, extent of terminal ileum involved, presence of complications such as stricture, fistula, comorbid conditions, access to biologics, financial considerations, and patient preferences.
Benjamin Cohen, MD, staff physician and co-section head and clinical director for inflammatory bowel diseases in the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic, called this “an important study” because it offers the first real-world evidence to support the findings from the LIR!C trial.
Dr. Cohen agreed with Dr. Agrawal that more work is needed to determine which patients benefit most from early ileocecal resection, although he suggested that known risk factors for worse outcomes — such as early age at diagnosis, penetrating features of disease, or perianal disease — may increase strength of surgical candidacy.
Still, based on the “fairly strong” body of data now available, he suggested that all patients should be educated about first-line ileocecal resection, as it is “reasonable” approach.
“It’s always important to present surgery as a treatment option,” Dr. Cohen said in an interview. “We don’t want to think of surgery as a last resort, or a failure, because that really colors it in a negative light, and then that ultimately impacts patients’ quality of life, and their perception of outcomes.”
The study was supported by the Danish National Research Foundation. The investigators disclosed no conflicts of interest. Dr. Cohen disclosed consulting and speaking honoraria from AbbVie.
These findings add weight to previously reported data from the LIR!C trial, suggesting that ileocecal resection should be considered a first-line treatment option for CD, reported principal investigator Kristine H. Allin, MD, PhD, of Aalborg University, Copenhagen.
“The LIR!C randomized clinical trial has demonstrated comparable quality of life with ileocecal resection and infliximab as a first-line treatment for limited, nonstricturing ileocecal CD at 1 year of follow-up, and improved outcomes with ileocecal resection on retrospective analysis of long-term follow-up data,” the investigators wrote in Gastroenterology. “However, in the real world, the long-term impact of early ileocecal resection for CD, compared with medical therapy, remains largely unexplored.”
To gather these real-world data, the investigators turned to the Danish National Patient Registry and the Danish National Prescription Registry, which included 1,279 individuals diagnosed with CD between 2003 and 2018 who received anti-TNF therapy or underwent ileocecal resection within 1 year of diagnosis. Within this group, slightly less than half underwent ileocecal resection (45.4%) while the remainder (54.6%) received anti-TNF therapy.
The primary outcome was a composite of one or more events: perianal CD, CD-related surgery, systemic corticosteroid exposure, and CD-related hospitalization. Secondary analyses evaluated the relative risks of these same four events as independent entities.
Multifactor-adjusted Cox proportional hazards regression analysis revealed that patients who underwent ileocecal resection had a 33% lower risk of the composite outcome compared with those who received anti-TNF therapy (adjusted hazard ratio [aHR], 0.67; 95% CI, 0.54-0.83).
In the secondary analyses, which examined risks for each component of the composite outcome, the surgery group had a significantly lower risk of CD-related surgery (aHR, 0.56; 95% CI, 0.39-0.80) and corticosteroid exposure (aHR, 0.71; 95% CI, 0.54-0.92), but not perianal CD or CD-related hospitalization.
After 5 years, half of the patients (49.7%) who underwent ileocecal resection were not receiving any treatment for CD. At the same timepoint, a slightly lower percentage of this group (46.3%) had started immunomodulator therapy, while 16.8% started anti-TNF therapy. Just 1.8% of these patients required a second intestinal resection.
“To our knowledge, these are the first real-world data in a population-based cohort with long-term follow-up of early ileocecal resection compared with anti-TNF therapy for newly diagnosed ileal and ileocecal CD,” the investigators wrote. “These data suggest that ileocecal resection may have a role as first-line therapy in Crohn’s disease management and challenge the current paradigm of reserving surgery for complicated Crohn’s disease refractory or intolerant to medications.”
Corresponding author Manasi Agrawal, MD, of Icahn School of Medicine at Mount Sinai, New York, suggested that “validation of our findings in external cohorts [is needed], and understanding of factors associated with improved outcomes following ileocecal resection.”
For clinicians and patients choosing between first-line anti-TNF therapy versus ileocecal resection using currently available evidence, Dr. Agrawal suggested that a variety of factors need to be considered, including disease location, extent of terminal ileum involved, presence of complications such as stricture, fistula, comorbid conditions, access to biologics, financial considerations, and patient preferences.
Benjamin Cohen, MD, staff physician and co-section head and clinical director for inflammatory bowel diseases in the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic, called this “an important study” because it offers the first real-world evidence to support the findings from the LIR!C trial.
Dr. Cohen agreed with Dr. Agrawal that more work is needed to determine which patients benefit most from early ileocecal resection, although he suggested that known risk factors for worse outcomes — such as early age at diagnosis, penetrating features of disease, or perianal disease — may increase strength of surgical candidacy.
Still, based on the “fairly strong” body of data now available, he suggested that all patients should be educated about first-line ileocecal resection, as it is “reasonable” approach.
“It’s always important to present surgery as a treatment option,” Dr. Cohen said in an interview. “We don’t want to think of surgery as a last resort, or a failure, because that really colors it in a negative light, and then that ultimately impacts patients’ quality of life, and their perception of outcomes.”
The study was supported by the Danish National Research Foundation. The investigators disclosed no conflicts of interest. Dr. Cohen disclosed consulting and speaking honoraria from AbbVie.
These findings add weight to previously reported data from the LIR!C trial, suggesting that ileocecal resection should be considered a first-line treatment option for CD, reported principal investigator Kristine H. Allin, MD, PhD, of Aalborg University, Copenhagen.
“The LIR!C randomized clinical trial has demonstrated comparable quality of life with ileocecal resection and infliximab as a first-line treatment for limited, nonstricturing ileocecal CD at 1 year of follow-up, and improved outcomes with ileocecal resection on retrospective analysis of long-term follow-up data,” the investigators wrote in Gastroenterology. “However, in the real world, the long-term impact of early ileocecal resection for CD, compared with medical therapy, remains largely unexplored.”
To gather these real-world data, the investigators turned to the Danish National Patient Registry and the Danish National Prescription Registry, which included 1,279 individuals diagnosed with CD between 2003 and 2018 who received anti-TNF therapy or underwent ileocecal resection within 1 year of diagnosis. Within this group, slightly less than half underwent ileocecal resection (45.4%) while the remainder (54.6%) received anti-TNF therapy.
The primary outcome was a composite of one or more events: perianal CD, CD-related surgery, systemic corticosteroid exposure, and CD-related hospitalization. Secondary analyses evaluated the relative risks of these same four events as independent entities.
Multifactor-adjusted Cox proportional hazards regression analysis revealed that patients who underwent ileocecal resection had a 33% lower risk of the composite outcome compared with those who received anti-TNF therapy (adjusted hazard ratio [aHR], 0.67; 95% CI, 0.54-0.83).
In the secondary analyses, which examined risks for each component of the composite outcome, the surgery group had a significantly lower risk of CD-related surgery (aHR, 0.56; 95% CI, 0.39-0.80) and corticosteroid exposure (aHR, 0.71; 95% CI, 0.54-0.92), but not perianal CD or CD-related hospitalization.
After 5 years, half of the patients (49.7%) who underwent ileocecal resection were not receiving any treatment for CD. At the same timepoint, a slightly lower percentage of this group (46.3%) had started immunomodulator therapy, while 16.8% started anti-TNF therapy. Just 1.8% of these patients required a second intestinal resection.
“To our knowledge, these are the first real-world data in a population-based cohort with long-term follow-up of early ileocecal resection compared with anti-TNF therapy for newly diagnosed ileal and ileocecal CD,” the investigators wrote. “These data suggest that ileocecal resection may have a role as first-line therapy in Crohn’s disease management and challenge the current paradigm of reserving surgery for complicated Crohn’s disease refractory or intolerant to medications.”
Corresponding author Manasi Agrawal, MD, of Icahn School of Medicine at Mount Sinai, New York, suggested that “validation of our findings in external cohorts [is needed], and understanding of factors associated with improved outcomes following ileocecal resection.”
For clinicians and patients choosing between first-line anti-TNF therapy versus ileocecal resection using currently available evidence, Dr. Agrawal suggested that a variety of factors need to be considered, including disease location, extent of terminal ileum involved, presence of complications such as stricture, fistula, comorbid conditions, access to biologics, financial considerations, and patient preferences.
Benjamin Cohen, MD, staff physician and co-section head and clinical director for inflammatory bowel diseases in the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic, called this “an important study” because it offers the first real-world evidence to support the findings from the LIR!C trial.
Dr. Cohen agreed with Dr. Agrawal that more work is needed to determine which patients benefit most from early ileocecal resection, although he suggested that known risk factors for worse outcomes — such as early age at diagnosis, penetrating features of disease, or perianal disease — may increase strength of surgical candidacy.
Still, based on the “fairly strong” body of data now available, he suggested that all patients should be educated about first-line ileocecal resection, as it is “reasonable” approach.
“It’s always important to present surgery as a treatment option,” Dr. Cohen said in an interview. “We don’t want to think of surgery as a last resort, or a failure, because that really colors it in a negative light, and then that ultimately impacts patients’ quality of life, and their perception of outcomes.”
The study was supported by the Danish National Research Foundation. The investigators disclosed no conflicts of interest. Dr. Cohen disclosed consulting and speaking honoraria from AbbVie.
FROM GASTROENTEROLOGY
AGA clinical practice guideline affirms role of biomarkers in Crohn’s disease management
, offering the most specific evidence-based recommendations yet for the use of fecal calprotectin (FCP) and serum C-reactive protein (CRP) in assessing disease activity.
Repeated monitoring with endoscopy allows for an objective assessment of inflammation and mucosal healing compared with symptoms alone. However, relying solely on endoscopy to guide management is an approach “limited by cost and resource utilization, invasiveness, and reduced patient acceptability,” wrote guideline authors on behalf of the AGA Clinical Guidelines Committee. The guideline was published online Nov. 17 in Gastroenterology.
“Use of biomarkers is no longer considered experimental and should be an integral part of IBD care and monitoring,” said Ashwin Ananthakrishnan, MBBS, MPH, a gastroenterologist with Massachusetts General Hospital in Boston and first author of the guideline. “We need further studies to define their optimal longitudinal use, but at a given time point, there is now abundant evidence that biomarkers provide significant incremental benefit over symptoms alone in assessing a patient’s status.”
Using evidence from randomized controlled trials and observational studies, and applying it to common clinical scenarios, there are conditional recommendations on the use of biomarkers in patients with established, diagnosed disease who were asymptomatic, symptomatic, or in surgically induced remission. Those recommendations, laid out in a detailed Clinical Decision Support Tool, include the following:
For asymptomatic patients: Check CRP and FCP every 6-12 months. Patients with normal levels, and who have endoscopically confirmed remission within the last 3 years without any subsequent change in symptoms or treatment, need not undergo endoscopy and can be followed with biomarker and clinical checks alone. If CRP or FCP are elevated (defined as CRP ≥ 5 mg/L, FCP ≥ 150 mcg/g), consider repeating biomarkers and/or performing endoscopic assessment of disease activity before adjusting treatment.
For mildly symptomatic patients: Role of biomarker testing may be limited and endoscopic or radiologic assessment may be required to assess active inflammation given the higher rate of false positive and false negative results with biomarkers in this population.
For patients with more severe symptoms: Elevated CRP or FCP can be used to guide treatment adjustment without endoscopic confirmation in certain situations. Normal levels may be false negative and should be confirmed by endoscopic assessment of disease activity.
For patients in surgically induced remission with a low likelihood of recurrence: FCP levels below 50 mcg/g can be used in lieu of routine endoscopic assessment within the first year after surgery. Higher FCP levels should prompt endoscopic assessment.
For patients in surgically induced remission with a high risk of recurrence: Do not rely on biomarkers. Perform endoscopic assessment.
All recommendations were deemed of low to moderate certainty based on results from randomized clinical trials and observational studies that utilized these biomarkers in patients with Crohn’s disease. Citing a dearth of quality evidence, the guideline authors determined they could not make recommendations on the use of a third proprietary biomarker — the endoscopic healing index (EHI).
Recent AGA Clinical Practice Guidelines on the role of biomarkers in ulcerative colitis, published in March, also support a strong role for fecal and blood biomarkers, determining when these can be used to avoid unneeded endoscopic assessments. However, in patients with Crohn’s disease, symptoms correlate less well with endoscopic activity.
As a result, “biomarker performance was acceptable only in asymptomatic individuals who had recently confirmed endoscopic remission; in those without recent endoscopic assessment, test performance was suboptimal.” In addition, the weaker correlation between symptoms and endoscopic activity in Crohn’s “reduced the utility of biomarker measurement to infer disease activity in those with mild symptoms.”
The guidelines were fully funded by the AGA Institute. The authors disclosed a number of potential conflicts of interest, including receiving research grants, as well as consulting and speaking fees, from pharmaceutical companies.
, offering the most specific evidence-based recommendations yet for the use of fecal calprotectin (FCP) and serum C-reactive protein (CRP) in assessing disease activity.
Repeated monitoring with endoscopy allows for an objective assessment of inflammation and mucosal healing compared with symptoms alone. However, relying solely on endoscopy to guide management is an approach “limited by cost and resource utilization, invasiveness, and reduced patient acceptability,” wrote guideline authors on behalf of the AGA Clinical Guidelines Committee. The guideline was published online Nov. 17 in Gastroenterology.
“Use of biomarkers is no longer considered experimental and should be an integral part of IBD care and monitoring,” said Ashwin Ananthakrishnan, MBBS, MPH, a gastroenterologist with Massachusetts General Hospital in Boston and first author of the guideline. “We need further studies to define their optimal longitudinal use, but at a given time point, there is now abundant evidence that biomarkers provide significant incremental benefit over symptoms alone in assessing a patient’s status.”
Using evidence from randomized controlled trials and observational studies, and applying it to common clinical scenarios, there are conditional recommendations on the use of biomarkers in patients with established, diagnosed disease who were asymptomatic, symptomatic, or in surgically induced remission. Those recommendations, laid out in a detailed Clinical Decision Support Tool, include the following:
For asymptomatic patients: Check CRP and FCP every 6-12 months. Patients with normal levels, and who have endoscopically confirmed remission within the last 3 years without any subsequent change in symptoms or treatment, need not undergo endoscopy and can be followed with biomarker and clinical checks alone. If CRP or FCP are elevated (defined as CRP ≥ 5 mg/L, FCP ≥ 150 mcg/g), consider repeating biomarkers and/or performing endoscopic assessment of disease activity before adjusting treatment.
For mildly symptomatic patients: Role of biomarker testing may be limited and endoscopic or radiologic assessment may be required to assess active inflammation given the higher rate of false positive and false negative results with biomarkers in this population.
For patients with more severe symptoms: Elevated CRP or FCP can be used to guide treatment adjustment without endoscopic confirmation in certain situations. Normal levels may be false negative and should be confirmed by endoscopic assessment of disease activity.
For patients in surgically induced remission with a low likelihood of recurrence: FCP levels below 50 mcg/g can be used in lieu of routine endoscopic assessment within the first year after surgery. Higher FCP levels should prompt endoscopic assessment.
For patients in surgically induced remission with a high risk of recurrence: Do not rely on biomarkers. Perform endoscopic assessment.
All recommendations were deemed of low to moderate certainty based on results from randomized clinical trials and observational studies that utilized these biomarkers in patients with Crohn’s disease. Citing a dearth of quality evidence, the guideline authors determined they could not make recommendations on the use of a third proprietary biomarker — the endoscopic healing index (EHI).
Recent AGA Clinical Practice Guidelines on the role of biomarkers in ulcerative colitis, published in March, also support a strong role for fecal and blood biomarkers, determining when these can be used to avoid unneeded endoscopic assessments. However, in patients with Crohn’s disease, symptoms correlate less well with endoscopic activity.
As a result, “biomarker performance was acceptable only in asymptomatic individuals who had recently confirmed endoscopic remission; in those without recent endoscopic assessment, test performance was suboptimal.” In addition, the weaker correlation between symptoms and endoscopic activity in Crohn’s “reduced the utility of biomarker measurement to infer disease activity in those with mild symptoms.”
The guidelines were fully funded by the AGA Institute. The authors disclosed a number of potential conflicts of interest, including receiving research grants, as well as consulting and speaking fees, from pharmaceutical companies.
, offering the most specific evidence-based recommendations yet for the use of fecal calprotectin (FCP) and serum C-reactive protein (CRP) in assessing disease activity.
Repeated monitoring with endoscopy allows for an objective assessment of inflammation and mucosal healing compared with symptoms alone. However, relying solely on endoscopy to guide management is an approach “limited by cost and resource utilization, invasiveness, and reduced patient acceptability,” wrote guideline authors on behalf of the AGA Clinical Guidelines Committee. The guideline was published online Nov. 17 in Gastroenterology.
“Use of biomarkers is no longer considered experimental and should be an integral part of IBD care and monitoring,” said Ashwin Ananthakrishnan, MBBS, MPH, a gastroenterologist with Massachusetts General Hospital in Boston and first author of the guideline. “We need further studies to define their optimal longitudinal use, but at a given time point, there is now abundant evidence that biomarkers provide significant incremental benefit over symptoms alone in assessing a patient’s status.”
Using evidence from randomized controlled trials and observational studies, and applying it to common clinical scenarios, there are conditional recommendations on the use of biomarkers in patients with established, diagnosed disease who were asymptomatic, symptomatic, or in surgically induced remission. Those recommendations, laid out in a detailed Clinical Decision Support Tool, include the following:
For asymptomatic patients: Check CRP and FCP every 6-12 months. Patients with normal levels, and who have endoscopically confirmed remission within the last 3 years without any subsequent change in symptoms or treatment, need not undergo endoscopy and can be followed with biomarker and clinical checks alone. If CRP or FCP are elevated (defined as CRP ≥ 5 mg/L, FCP ≥ 150 mcg/g), consider repeating biomarkers and/or performing endoscopic assessment of disease activity before adjusting treatment.
For mildly symptomatic patients: Role of biomarker testing may be limited and endoscopic or radiologic assessment may be required to assess active inflammation given the higher rate of false positive and false negative results with biomarkers in this population.
For patients with more severe symptoms: Elevated CRP or FCP can be used to guide treatment adjustment without endoscopic confirmation in certain situations. Normal levels may be false negative and should be confirmed by endoscopic assessment of disease activity.
For patients in surgically induced remission with a low likelihood of recurrence: FCP levels below 50 mcg/g can be used in lieu of routine endoscopic assessment within the first year after surgery. Higher FCP levels should prompt endoscopic assessment.
For patients in surgically induced remission with a high risk of recurrence: Do not rely on biomarkers. Perform endoscopic assessment.
All recommendations were deemed of low to moderate certainty based on results from randomized clinical trials and observational studies that utilized these biomarkers in patients with Crohn’s disease. Citing a dearth of quality evidence, the guideline authors determined they could not make recommendations on the use of a third proprietary biomarker — the endoscopic healing index (EHI).
Recent AGA Clinical Practice Guidelines on the role of biomarkers in ulcerative colitis, published in March, also support a strong role for fecal and blood biomarkers, determining when these can be used to avoid unneeded endoscopic assessments. However, in patients with Crohn’s disease, symptoms correlate less well with endoscopic activity.
As a result, “biomarker performance was acceptable only in asymptomatic individuals who had recently confirmed endoscopic remission; in those without recent endoscopic assessment, test performance was suboptimal.” In addition, the weaker correlation between symptoms and endoscopic activity in Crohn’s “reduced the utility of biomarker measurement to infer disease activity in those with mild symptoms.”
The guidelines were fully funded by the AGA Institute. The authors disclosed a number of potential conflicts of interest, including receiving research grants, as well as consulting and speaking fees, from pharmaceutical companies.
FROM GASTROENTEROLOGY
Acyclcarnitines could drive IBD via dysbiosis
These findings improve our understanding of IBD pathogenesis and disease course, and could prove valuable in biomarker research, reported lead author Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
In health, carnitine and acylcarnitines aid in fatty acid transport, the investigators wrote in September in Cellular and Molecular Gastroenterology and Hepatology. Acylcarnitines are also involved in metabolic signaling, and in the absence of sufficient short-chain fatty acids may serve as an alternative energy source for the intestinal epithelium.
“Recently, we and others have shown that fecal acylcarnitines are increased in patients with IBD, especially during dysbiosis,” they noted. “However, the mechanism(s) responsible for the increase of fecal acylcarnitines in IBD and their biological function have not been elucidated.”
The present study aimed to address this knowledge gap by characterizing both carnitine and acylcarnitines in pediatric IBD.
First, the investigators confirmed that both carnitine and acylcarnitines were elevated in fecal samples from pediatric patients with IBD.
Next, they analyzed fecal samples from subjects in the Food and Resulting Microbiota and Metabolome (FARMM) study, which compared microbiota recovery after gut purge and antibiotics among participants eating an omnivorous diet, a vegan diet, or an exclusive enteral nutrition (EEN) diet lacking in fiber. After the antibiotics, levels of fecal carnitine and acylcarnitines increased significantly in all groups, suggesting that microbiota were consuming these molecules.
To clarify the relationship between inflammation and levels of carnitine and acylcarnitines in the absence of microbiota, Dr. Wu and colleagues employed a germ-free mouse model with dextran sodium sulfate (DSS)–induced colitis. Levels of both molecule types were significantly increased in bile and plasma of mice with colitis versus those that were not exposed to DSS.
“Because the gut microbiota consumes both carnitine and acylcarnitines, these results are consistent with the notion that the increase of these metabolites in the feces of patients with IBD is driven by increased biliary delivery of acylcarnitines to the lumen combined with the reduced number and function of mitochondria in the colonic epithelium as previously reported,” the investigators wrote.
Further experiments with plated cultures and mice revealed that various bacterial species consumed carnitine and acylcarnitines in distinct patterns. Enterobacteriaceae demonstrated a notable proclivity for consumption in vitro and within the murine gut.
“As a high-dimensional analytic feature, the pattern of fecal acylcarnitines, perhaps together with bacterial taxonomy, may have utility as a biomarker for the presence or prognosis of IBD,” Dr. Wu and colleagues concluded. “In addition, based on currently available information about the impact of carnitine on the biology of Enterobacteriaceae, acylcarnitines also may have an important functional effect on the biology of the gut microbiota that is relevant to the pathogenesis or course of disease in patients with IBD.”
The study was supported by the Crohn’s and Colitis Foundation, the PennCHOP Microbiome Program, the Penn Center for Nutritional Science and Medicine, and others. The investigators disclosed no conflicts of interest.
The description of noninvasive biomarkers for inflammatory bowel disease (IBD) is key to better characterizing the disease pathogenesis. In this new publication, Lemons et al. describe deleterious effects of gut luminal carnitine and acylcarnitine in pediatric IBD patients, showing that these metabolites can serve as energy substrates to the microbiota, especially Enterobacteriaceae, promoting the growth of pathobionts and contributing to the persistence of dysbiosis which, in turn, may foster the course of IBD. In fact, acylcarnitine had been highlighted as a potential new target for IBD during dysbiosis by a previous multi-omics study of the gut microbiome. Moreover, Dr. Gary Wu’s team has shown that the intestinal epithelium can uptake and use acylcarnitine as an alternative source for energy production. However, epithelial mitochondrial dysfunction triggered by inflammation reduces the capacity of colonocytes to consume long-chain fatty acids, thus enhancing the fecal levels of acylcarnitine as described in IBD patients.
The description of noninvasive biomarkers for inflammatory bowel disease (IBD) is key to better characterizing the disease pathogenesis. In this new publication, Lemons et al. describe deleterious effects of gut luminal carnitine and acylcarnitine in pediatric IBD patients, showing that these metabolites can serve as energy substrates to the microbiota, especially Enterobacteriaceae, promoting the growth of pathobionts and contributing to the persistence of dysbiosis which, in turn, may foster the course of IBD. In fact, acylcarnitine had been highlighted as a potential new target for IBD during dysbiosis by a previous multi-omics study of the gut microbiome. Moreover, Dr. Gary Wu’s team has shown that the intestinal epithelium can uptake and use acylcarnitine as an alternative source for energy production. However, epithelial mitochondrial dysfunction triggered by inflammation reduces the capacity of colonocytes to consume long-chain fatty acids, thus enhancing the fecal levels of acylcarnitine as described in IBD patients.
The description of noninvasive biomarkers for inflammatory bowel disease (IBD) is key to better characterizing the disease pathogenesis. In this new publication, Lemons et al. describe deleterious effects of gut luminal carnitine and acylcarnitine in pediatric IBD patients, showing that these metabolites can serve as energy substrates to the microbiota, especially Enterobacteriaceae, promoting the growth of pathobionts and contributing to the persistence of dysbiosis which, in turn, may foster the course of IBD. In fact, acylcarnitine had been highlighted as a potential new target for IBD during dysbiosis by a previous multi-omics study of the gut microbiome. Moreover, Dr. Gary Wu’s team has shown that the intestinal epithelium can uptake and use acylcarnitine as an alternative source for energy production. However, epithelial mitochondrial dysfunction triggered by inflammation reduces the capacity of colonocytes to consume long-chain fatty acids, thus enhancing the fecal levels of acylcarnitine as described in IBD patients.
These findings improve our understanding of IBD pathogenesis and disease course, and could prove valuable in biomarker research, reported lead author Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
In health, carnitine and acylcarnitines aid in fatty acid transport, the investigators wrote in September in Cellular and Molecular Gastroenterology and Hepatology. Acylcarnitines are also involved in metabolic signaling, and in the absence of sufficient short-chain fatty acids may serve as an alternative energy source for the intestinal epithelium.
“Recently, we and others have shown that fecal acylcarnitines are increased in patients with IBD, especially during dysbiosis,” they noted. “However, the mechanism(s) responsible for the increase of fecal acylcarnitines in IBD and their biological function have not been elucidated.”
The present study aimed to address this knowledge gap by characterizing both carnitine and acylcarnitines in pediatric IBD.
First, the investigators confirmed that both carnitine and acylcarnitines were elevated in fecal samples from pediatric patients with IBD.
Next, they analyzed fecal samples from subjects in the Food and Resulting Microbiota and Metabolome (FARMM) study, which compared microbiota recovery after gut purge and antibiotics among participants eating an omnivorous diet, a vegan diet, or an exclusive enteral nutrition (EEN) diet lacking in fiber. After the antibiotics, levels of fecal carnitine and acylcarnitines increased significantly in all groups, suggesting that microbiota were consuming these molecules.
To clarify the relationship between inflammation and levels of carnitine and acylcarnitines in the absence of microbiota, Dr. Wu and colleagues employed a germ-free mouse model with dextran sodium sulfate (DSS)–induced colitis. Levels of both molecule types were significantly increased in bile and plasma of mice with colitis versus those that were not exposed to DSS.
“Because the gut microbiota consumes both carnitine and acylcarnitines, these results are consistent with the notion that the increase of these metabolites in the feces of patients with IBD is driven by increased biliary delivery of acylcarnitines to the lumen combined with the reduced number and function of mitochondria in the colonic epithelium as previously reported,” the investigators wrote.
Further experiments with plated cultures and mice revealed that various bacterial species consumed carnitine and acylcarnitines in distinct patterns. Enterobacteriaceae demonstrated a notable proclivity for consumption in vitro and within the murine gut.
“As a high-dimensional analytic feature, the pattern of fecal acylcarnitines, perhaps together with bacterial taxonomy, may have utility as a biomarker for the presence or prognosis of IBD,” Dr. Wu and colleagues concluded. “In addition, based on currently available information about the impact of carnitine on the biology of Enterobacteriaceae, acylcarnitines also may have an important functional effect on the biology of the gut microbiota that is relevant to the pathogenesis or course of disease in patients with IBD.”
The study was supported by the Crohn’s and Colitis Foundation, the PennCHOP Microbiome Program, the Penn Center for Nutritional Science and Medicine, and others. The investigators disclosed no conflicts of interest.
These findings improve our understanding of IBD pathogenesis and disease course, and could prove valuable in biomarker research, reported lead author Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
In health, carnitine and acylcarnitines aid in fatty acid transport, the investigators wrote in September in Cellular and Molecular Gastroenterology and Hepatology. Acylcarnitines are also involved in metabolic signaling, and in the absence of sufficient short-chain fatty acids may serve as an alternative energy source for the intestinal epithelium.
“Recently, we and others have shown that fecal acylcarnitines are increased in patients with IBD, especially during dysbiosis,” they noted. “However, the mechanism(s) responsible for the increase of fecal acylcarnitines in IBD and their biological function have not been elucidated.”
The present study aimed to address this knowledge gap by characterizing both carnitine and acylcarnitines in pediatric IBD.
First, the investigators confirmed that both carnitine and acylcarnitines were elevated in fecal samples from pediatric patients with IBD.
Next, they analyzed fecal samples from subjects in the Food and Resulting Microbiota and Metabolome (FARMM) study, which compared microbiota recovery after gut purge and antibiotics among participants eating an omnivorous diet, a vegan diet, or an exclusive enteral nutrition (EEN) diet lacking in fiber. After the antibiotics, levels of fecal carnitine and acylcarnitines increased significantly in all groups, suggesting that microbiota were consuming these molecules.
To clarify the relationship between inflammation and levels of carnitine and acylcarnitines in the absence of microbiota, Dr. Wu and colleagues employed a germ-free mouse model with dextran sodium sulfate (DSS)–induced colitis. Levels of both molecule types were significantly increased in bile and plasma of mice with colitis versus those that were not exposed to DSS.
“Because the gut microbiota consumes both carnitine and acylcarnitines, these results are consistent with the notion that the increase of these metabolites in the feces of patients with IBD is driven by increased biliary delivery of acylcarnitines to the lumen combined with the reduced number and function of mitochondria in the colonic epithelium as previously reported,” the investigators wrote.
Further experiments with plated cultures and mice revealed that various bacterial species consumed carnitine and acylcarnitines in distinct patterns. Enterobacteriaceae demonstrated a notable proclivity for consumption in vitro and within the murine gut.
“As a high-dimensional analytic feature, the pattern of fecal acylcarnitines, perhaps together with bacterial taxonomy, may have utility as a biomarker for the presence or prognosis of IBD,” Dr. Wu and colleagues concluded. “In addition, based on currently available information about the impact of carnitine on the biology of Enterobacteriaceae, acylcarnitines also may have an important functional effect on the biology of the gut microbiota that is relevant to the pathogenesis or course of disease in patients with IBD.”
The study was supported by the Crohn’s and Colitis Foundation, the PennCHOP Microbiome Program, the Penn Center for Nutritional Science and Medicine, and others. The investigators disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Low-dose aspirin provokes no flares in patients with IBD during pregnancy
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
FROM ACG 2023
Study: CBD provides symptom relief and improvements in gastroparesis
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Even a short course of opioids could jeopardize IBD patient health
These findings amplify the safety signal from previous inpatient studies by showing that even a short course of opioids in an outpatient setting may increase risks of corticosteroid use and emergency department utilization, prompting caution among prescribers, reported Laura Telfer, MS, of Penn State College of Medicine, Hershey, Pa., and colleagues.
“Opioids are frequently prescribed to treat pain associated with IBD,” the investigators wrote in Gastro Hep Advances. “Unfortunately, they are associated with many problems in IBD, including increased risk of emergency room visits, hospitalization, surgery, and mortality. Chronic opioid use may also exacerbate symptoms and induce IBD flares, prompting discontinuation, thus increasing the risk of opioid withdrawal syndrome. Ironically, there is no published evidence that opioids even help to improve abdominal pain in IBD, particularly in the long term. Notably, most studies investigating opioid use in IBD have been limited to hospitalized patients, and few have directly evaluated the impact of opioid prescription length.”
To address this knowledge gap, Ms. Telfer and colleagues conducted a retrospective, population-based cohort study involving patients with IBD who were classified as either long-term opioid users, short-term opioid users, or nonusers. Drawing data from more than 80,000 patients in the TriNetX Diamond Network, the investigators evaluated relative, intergroup risks for corticosteroid use, emergency department utilization, mortality, and IBD-related surgery.
Comparing short-term opioid users and nonusers revealed that short-term use more than doubled the risk of corticosteroid prescription (relative risk [RR], 2.517; P less than .001), and increased the risk of an emergency department visit by approximately 32% (RR, 1.315; P less than .001). Long-term use was associated with a similar doubling in risk of corticosteroid prescription (RR, 2.383; P less than .001), and an even greater risk of emergency department utilization (RR, 2.083; P less than .001). Risks of death or IBD-related surgery did not differ for either of these comparisons.
Next, the investigators compared long-term opioid use versus short-term opioid use. This suggested a duration-related effect, as long-term users were 57% more likely than were short-term users to utilize emergency department services (RR, 1.572; P less than .001). No significant differences for the other outcomes were detected in this comparison.
“Unlike previous studies, we did not find an association between opioid use and IBD-related surgery or death,” the investigators wrote. “Notably, these [previously reported] associations utilized opioid dosage (e.g., morphine equivalent or number of prescriptions), rather than length of opioid prescription (as we did). We also focused on IBD outpatients, while prior studies evaluated (in part or completely) inpatient populations, who typically present with more severe illness.”
Still, they added, the present findings should serve as a warning to prescribers considering even a short course of opioids for patients with IBD.
“This study demonstrates that prescribing opioids to IBD outpatients carries significant, specific risks, regardless of prescription length,” Ms. Telfer and colleagues wrote. “Healthcare professionals should exercise caution before prescribing these agents.”
The study was supported by the Peter and Marshia Carlino Early Career Professorship in Inflammatory Bowel Disease, the Margot E. Walrath Career Development Professorship in Gastroenterology, and the National Institutes of Health. The investigators disclosed no conflicts of interest.
Given that objective control of inflammation does not always correlate with improvement in abdominal pain scores, the use of opioids in patients with inflammatory bowel diseases (IBD) remains a difficult area of clinical practice and research. In this study, Telfer and colleagues performed a retrospective analysis using the TriNetX Diamond Network to assess the impact of opioid use on health-associated outcomes and evaluate for a differential impact on outcomes depending on the length of opioid prescription. When compared to non–opioid users, both short- and long-term opioid users were more likely to utilize corticosteroids and emergency department services. However, in contrast to prior studies, there was no increased risk for mortality demonstrated among those patients with short- or long-term opioid use.
Edward L. Barnes, MD, MPH, is assistant professor of medicine at the University of North Carolina at Chapel Hill. He disclosed having served as a consultant for Target RWE (not relevant to this commentary).
Given that objective control of inflammation does not always correlate with improvement in abdominal pain scores, the use of opioids in patients with inflammatory bowel diseases (IBD) remains a difficult area of clinical practice and research. In this study, Telfer and colleagues performed a retrospective analysis using the TriNetX Diamond Network to assess the impact of opioid use on health-associated outcomes and evaluate for a differential impact on outcomes depending on the length of opioid prescription. When compared to non–opioid users, both short- and long-term opioid users were more likely to utilize corticosteroids and emergency department services. However, in contrast to prior studies, there was no increased risk for mortality demonstrated among those patients with short- or long-term opioid use.
Edward L. Barnes, MD, MPH, is assistant professor of medicine at the University of North Carolina at Chapel Hill. He disclosed having served as a consultant for Target RWE (not relevant to this commentary).
Given that objective control of inflammation does not always correlate with improvement in abdominal pain scores, the use of opioids in patients with inflammatory bowel diseases (IBD) remains a difficult area of clinical practice and research. In this study, Telfer and colleagues performed a retrospective analysis using the TriNetX Diamond Network to assess the impact of opioid use on health-associated outcomes and evaluate for a differential impact on outcomes depending on the length of opioid prescription. When compared to non–opioid users, both short- and long-term opioid users were more likely to utilize corticosteroids and emergency department services. However, in contrast to prior studies, there was no increased risk for mortality demonstrated among those patients with short- or long-term opioid use.
Edward L. Barnes, MD, MPH, is assistant professor of medicine at the University of North Carolina at Chapel Hill. He disclosed having served as a consultant for Target RWE (not relevant to this commentary).
These findings amplify the safety signal from previous inpatient studies by showing that even a short course of opioids in an outpatient setting may increase risks of corticosteroid use and emergency department utilization, prompting caution among prescribers, reported Laura Telfer, MS, of Penn State College of Medicine, Hershey, Pa., and colleagues.
“Opioids are frequently prescribed to treat pain associated with IBD,” the investigators wrote in Gastro Hep Advances. “Unfortunately, they are associated with many problems in IBD, including increased risk of emergency room visits, hospitalization, surgery, and mortality. Chronic opioid use may also exacerbate symptoms and induce IBD flares, prompting discontinuation, thus increasing the risk of opioid withdrawal syndrome. Ironically, there is no published evidence that opioids even help to improve abdominal pain in IBD, particularly in the long term. Notably, most studies investigating opioid use in IBD have been limited to hospitalized patients, and few have directly evaluated the impact of opioid prescription length.”
To address this knowledge gap, Ms. Telfer and colleagues conducted a retrospective, population-based cohort study involving patients with IBD who were classified as either long-term opioid users, short-term opioid users, or nonusers. Drawing data from more than 80,000 patients in the TriNetX Diamond Network, the investigators evaluated relative, intergroup risks for corticosteroid use, emergency department utilization, mortality, and IBD-related surgery.
Comparing short-term opioid users and nonusers revealed that short-term use more than doubled the risk of corticosteroid prescription (relative risk [RR], 2.517; P less than .001), and increased the risk of an emergency department visit by approximately 32% (RR, 1.315; P less than .001). Long-term use was associated with a similar doubling in risk of corticosteroid prescription (RR, 2.383; P less than .001), and an even greater risk of emergency department utilization (RR, 2.083; P less than .001). Risks of death or IBD-related surgery did not differ for either of these comparisons.
Next, the investigators compared long-term opioid use versus short-term opioid use. This suggested a duration-related effect, as long-term users were 57% more likely than were short-term users to utilize emergency department services (RR, 1.572; P less than .001). No significant differences for the other outcomes were detected in this comparison.
“Unlike previous studies, we did not find an association between opioid use and IBD-related surgery or death,” the investigators wrote. “Notably, these [previously reported] associations utilized opioid dosage (e.g., morphine equivalent or number of prescriptions), rather than length of opioid prescription (as we did). We also focused on IBD outpatients, while prior studies evaluated (in part or completely) inpatient populations, who typically present with more severe illness.”
Still, they added, the present findings should serve as a warning to prescribers considering even a short course of opioids for patients with IBD.
“This study demonstrates that prescribing opioids to IBD outpatients carries significant, specific risks, regardless of prescription length,” Ms. Telfer and colleagues wrote. “Healthcare professionals should exercise caution before prescribing these agents.”
The study was supported by the Peter and Marshia Carlino Early Career Professorship in Inflammatory Bowel Disease, the Margot E. Walrath Career Development Professorship in Gastroenterology, and the National Institutes of Health. The investigators disclosed no conflicts of interest.
These findings amplify the safety signal from previous inpatient studies by showing that even a short course of opioids in an outpatient setting may increase risks of corticosteroid use and emergency department utilization, prompting caution among prescribers, reported Laura Telfer, MS, of Penn State College of Medicine, Hershey, Pa., and colleagues.
“Opioids are frequently prescribed to treat pain associated with IBD,” the investigators wrote in Gastro Hep Advances. “Unfortunately, they are associated with many problems in IBD, including increased risk of emergency room visits, hospitalization, surgery, and mortality. Chronic opioid use may also exacerbate symptoms and induce IBD flares, prompting discontinuation, thus increasing the risk of opioid withdrawal syndrome. Ironically, there is no published evidence that opioids even help to improve abdominal pain in IBD, particularly in the long term. Notably, most studies investigating opioid use in IBD have been limited to hospitalized patients, and few have directly evaluated the impact of opioid prescription length.”
To address this knowledge gap, Ms. Telfer and colleagues conducted a retrospective, population-based cohort study involving patients with IBD who were classified as either long-term opioid users, short-term opioid users, or nonusers. Drawing data from more than 80,000 patients in the TriNetX Diamond Network, the investigators evaluated relative, intergroup risks for corticosteroid use, emergency department utilization, mortality, and IBD-related surgery.
Comparing short-term opioid users and nonusers revealed that short-term use more than doubled the risk of corticosteroid prescription (relative risk [RR], 2.517; P less than .001), and increased the risk of an emergency department visit by approximately 32% (RR, 1.315; P less than .001). Long-term use was associated with a similar doubling in risk of corticosteroid prescription (RR, 2.383; P less than .001), and an even greater risk of emergency department utilization (RR, 2.083; P less than .001). Risks of death or IBD-related surgery did not differ for either of these comparisons.
Next, the investigators compared long-term opioid use versus short-term opioid use. This suggested a duration-related effect, as long-term users were 57% more likely than were short-term users to utilize emergency department services (RR, 1.572; P less than .001). No significant differences for the other outcomes were detected in this comparison.
“Unlike previous studies, we did not find an association between opioid use and IBD-related surgery or death,” the investigators wrote. “Notably, these [previously reported] associations utilized opioid dosage (e.g., morphine equivalent or number of prescriptions), rather than length of opioid prescription (as we did). We also focused on IBD outpatients, while prior studies evaluated (in part or completely) inpatient populations, who typically present with more severe illness.”
Still, they added, the present findings should serve as a warning to prescribers considering even a short course of opioids for patients with IBD.
“This study demonstrates that prescribing opioids to IBD outpatients carries significant, specific risks, regardless of prescription length,” Ms. Telfer and colleagues wrote. “Healthcare professionals should exercise caution before prescribing these agents.”
The study was supported by the Peter and Marshia Carlino Early Career Professorship in Inflammatory Bowel Disease, the Margot E. Walrath Career Development Professorship in Gastroenterology, and the National Institutes of Health. The investigators disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
Advances in endoscopic therapies in inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16
Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
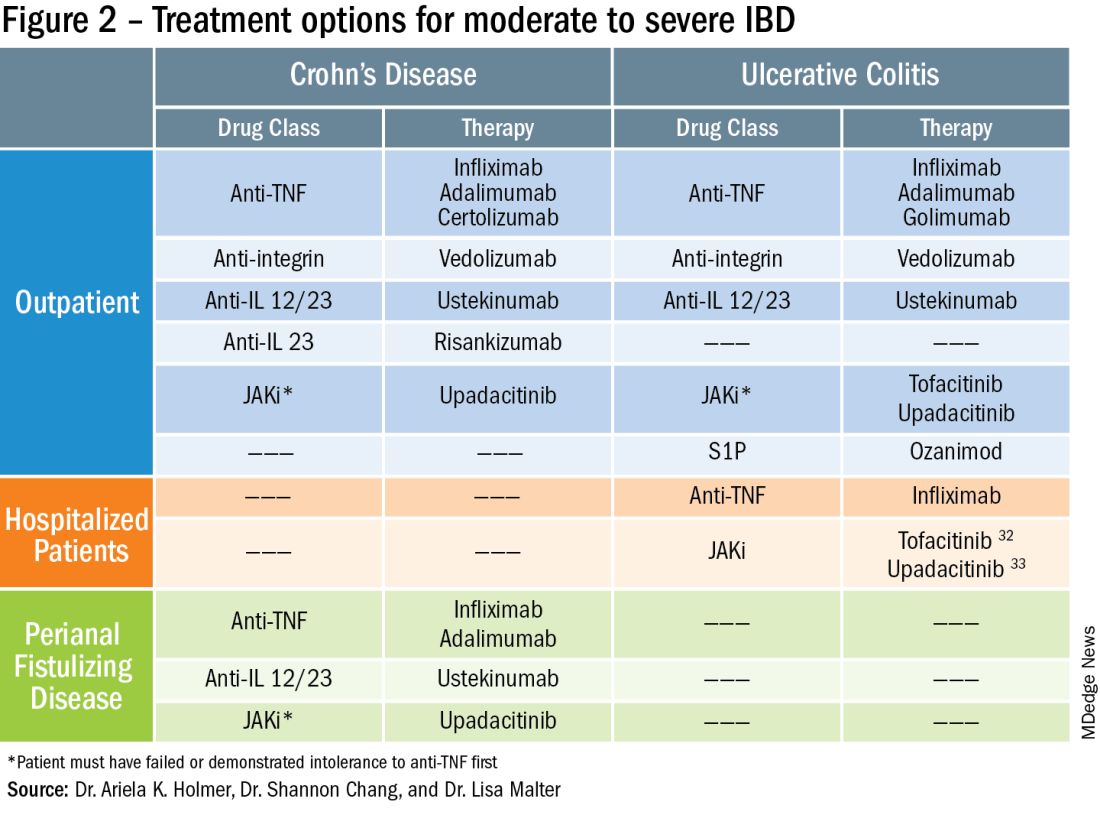
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.