User login
Tangled Truths: Unraveling the Link Between Frontal Fibrosing Alopecia and Allergic Contact Dermatitis
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
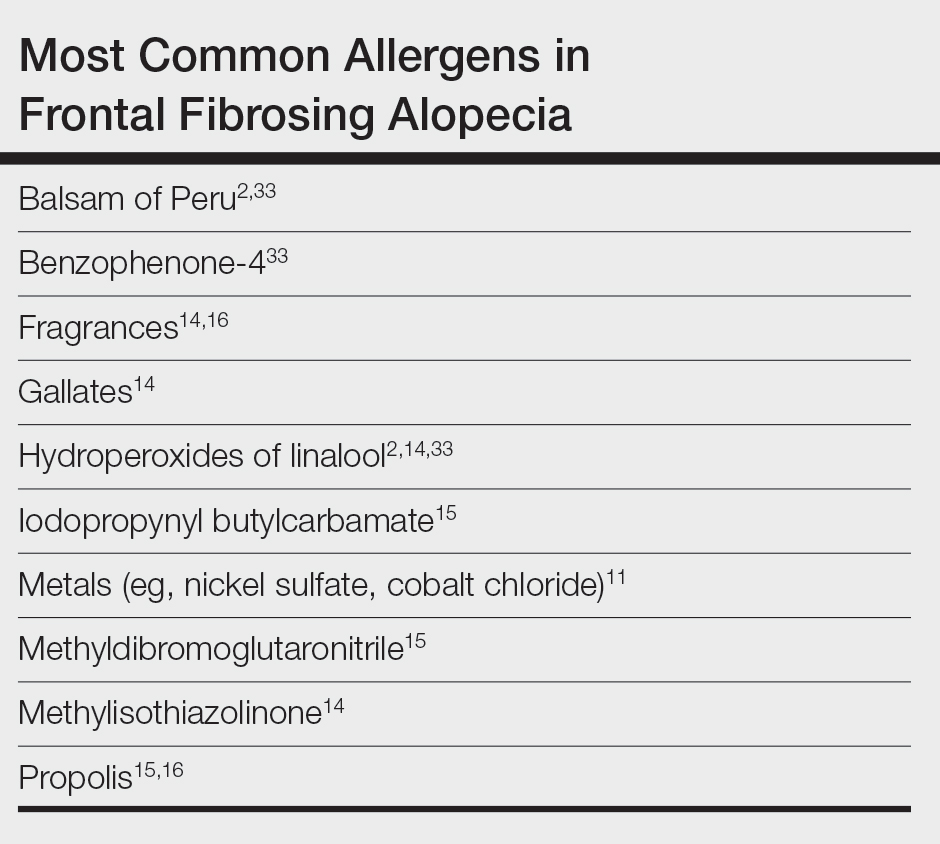
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Practice Points
- Frontal fibrosing alopecia (FFA), a variant of lichen planopilaris (LPP), is an increasingly prevalent type of scarring alopecia that may have a closer relationship to contact allergy than was previously understood. However, there is no evidence of a causal association to date.
- When evaluating for FFA/LPP, clinicians should assess for use of cosmetic products or sunscreens that may have a potential impact on the disease course.
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
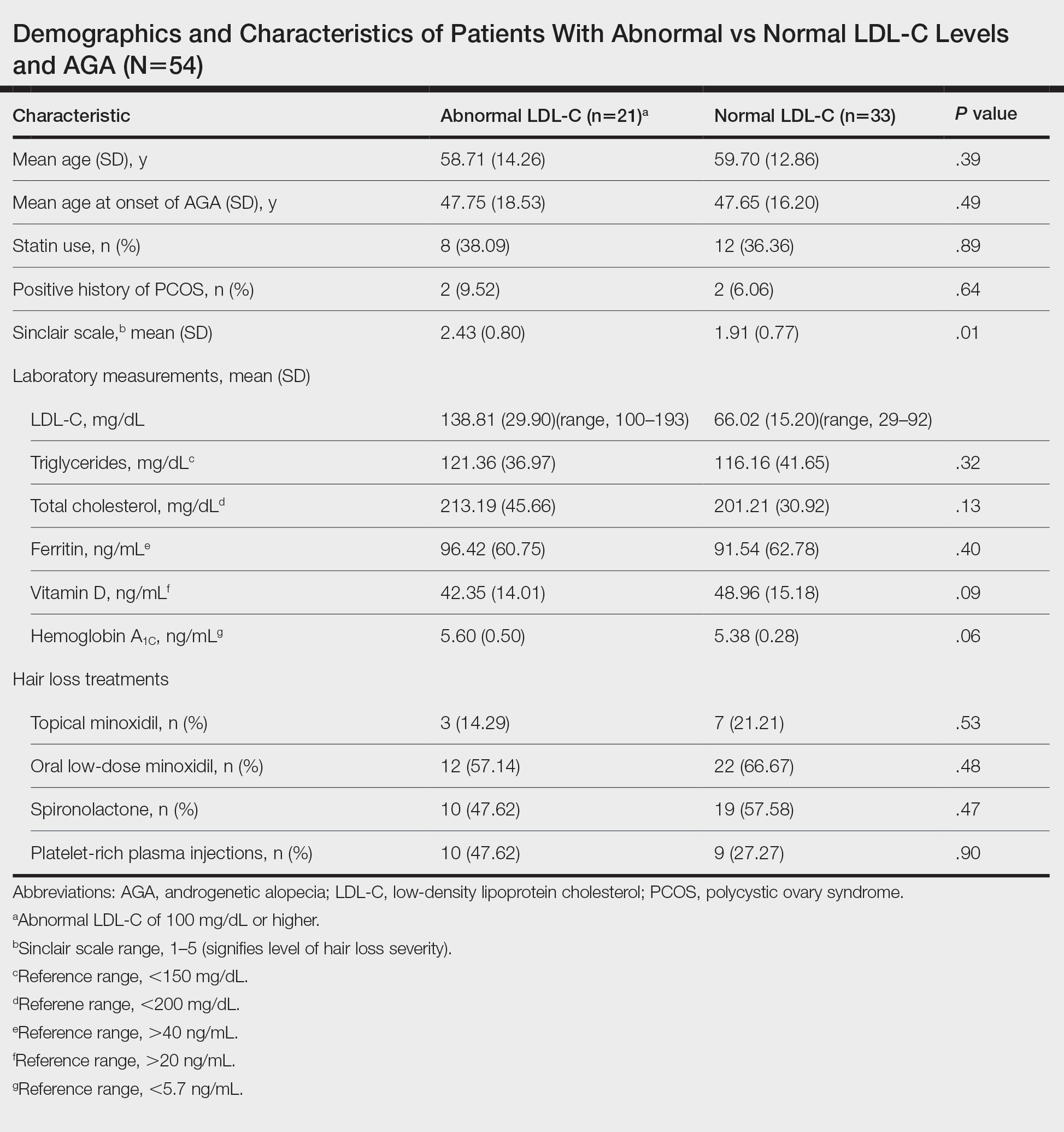
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.
Analysis of Nail Excision Practice Patterns in the Medicare Provider Utilization and Payment Database 2012-2017
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.
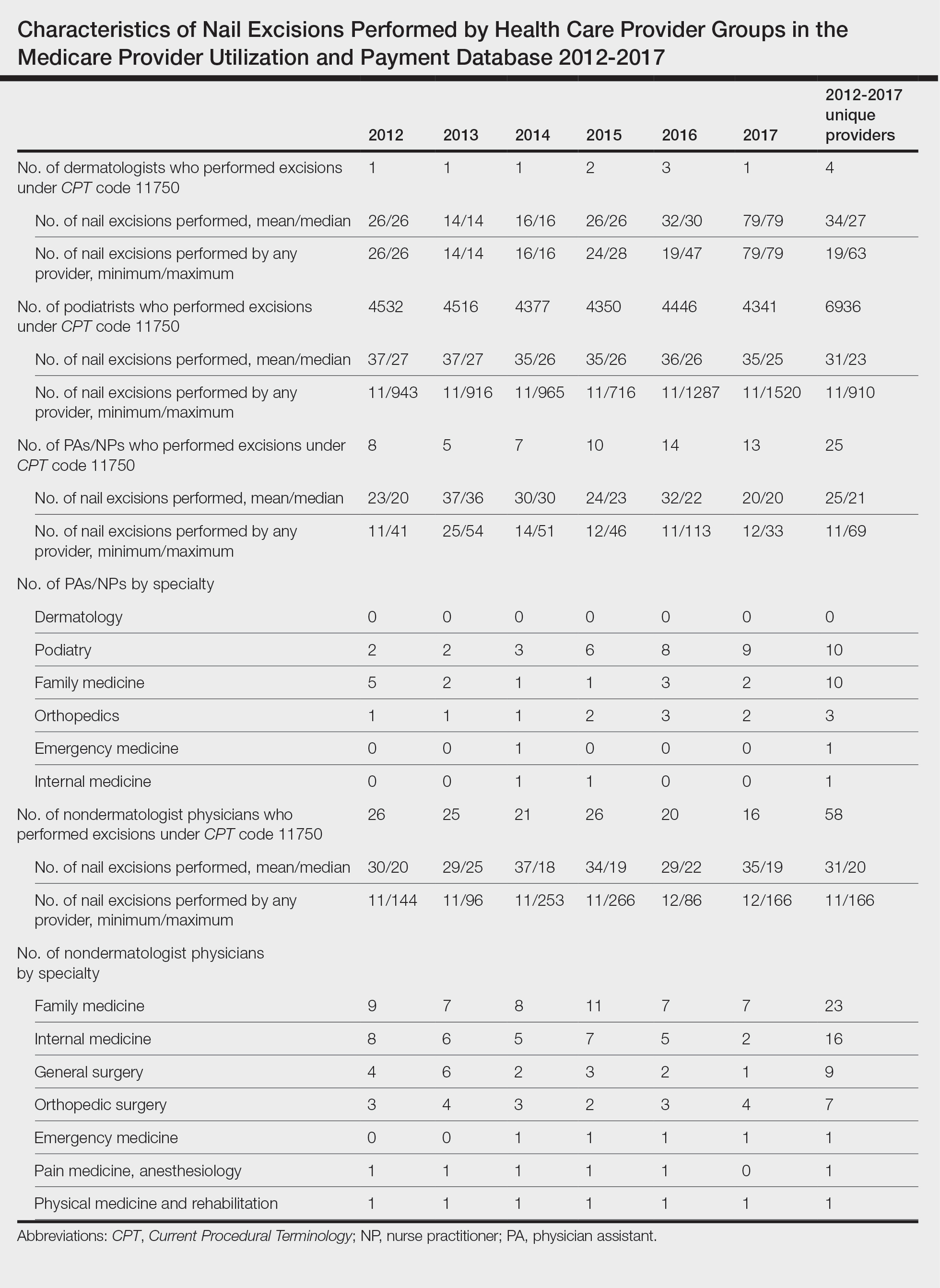
A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).
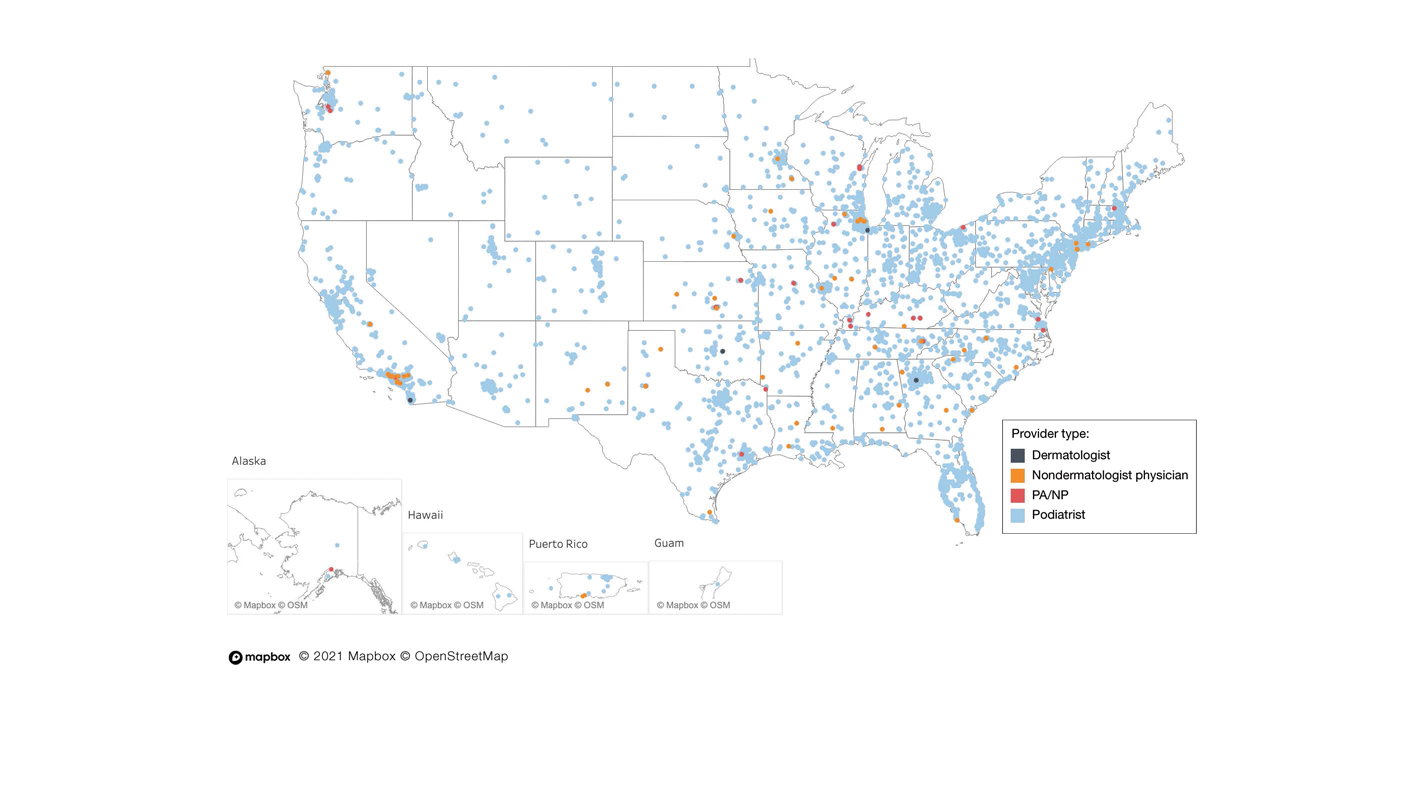
Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
Practice Points
- Dermatologists are considered nail experts but perform nail excisions less frequently than their podiatric counterparts and physicians in other specialties.
- Aspects of podiatric surgical training should be incorporated into dermatology residency to increase competency and comfort of dermatologists in nail excision procedures.
- Dermatologists may not be perceived as nail experts by the public, indicating a need for increased community education on the role of dermatologists in treating nail disease.
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Hair Creams: Do You Know the Health Risks?
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
Hair Loss in Children: How to Spot and Treat Different Causes
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT ODAC 2024
Smoking Associated With Increased Risk for Hair Loss Among Men
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Rosemary, Part 1
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
Culprits of Medication-Induced Telogen Effluvium, Part 2
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s) such as heparin and its derivatives.
- Infection, illness, or hospitalization around the time of initiation of the suspected culprit medication may complicate identification of the inciting cause and may contribute to TE.
- Angiotensin-converting enzyme inhibitors and β-blockers are unlikely culprits of medication-induced TE, and the benefits of discontinuing a suspected culprit medication should be weighed carefully against the risks of medication cessation.